Chloride Ions’ Penetration of Fly Ash and Ground Granulated Blast Furnace Slags-Based Alkali-Activated Mortars
Abstract
:1. Introduction
2. Tested Materials and Methods
- Water to binder (w/b) = 0.30;
- Alkaline solution to binder = 0.45;
- Sand to binder = 1.50.
3. Test Methods
3.1. Physical and Transport Properties
3.2. Gas Permeability—The Nitrogen Cembureau Method
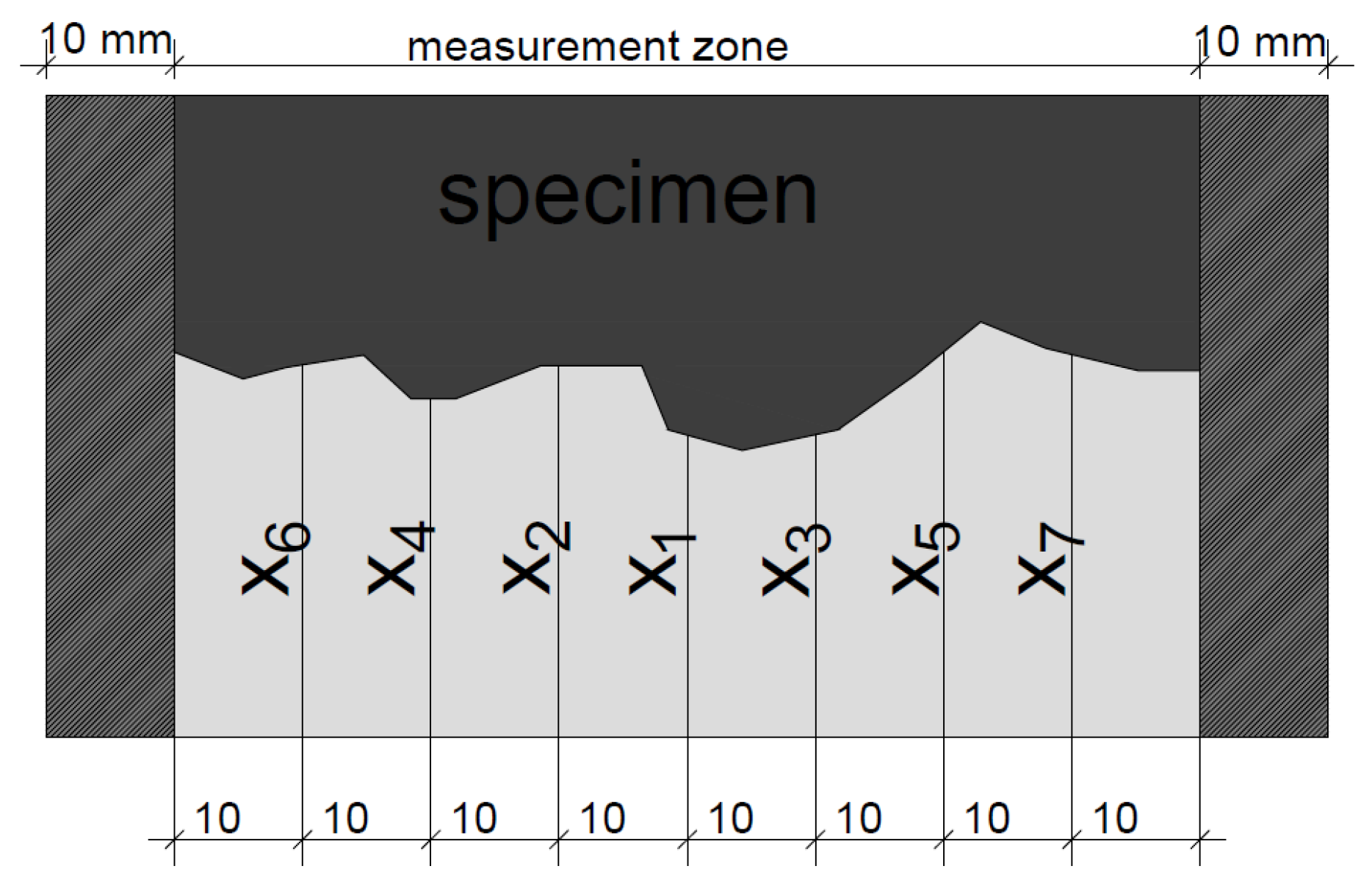
3.3. Chloride Transport—Modified NT BUILD 492 Migration Test
4. Results and Discussion
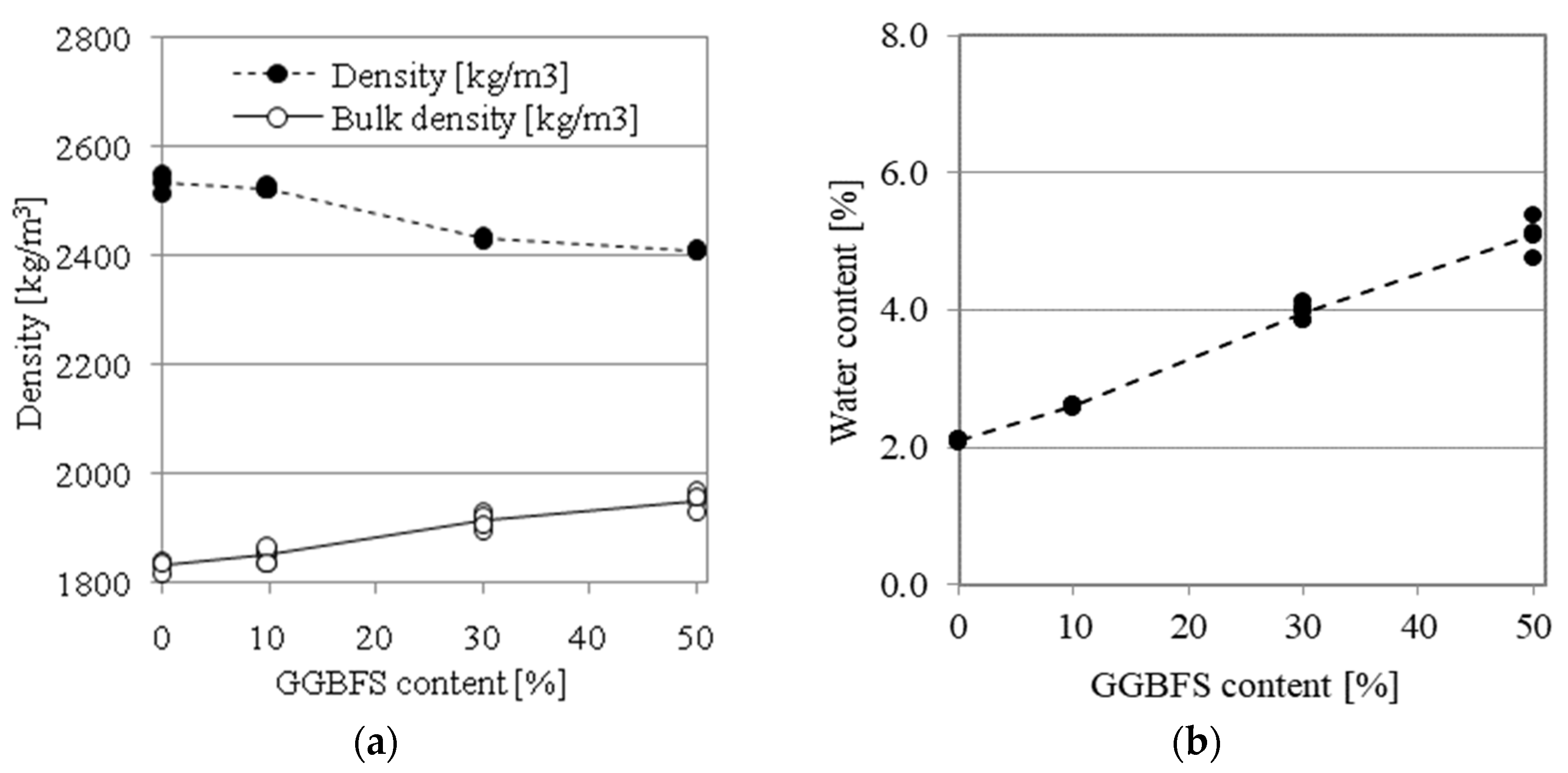
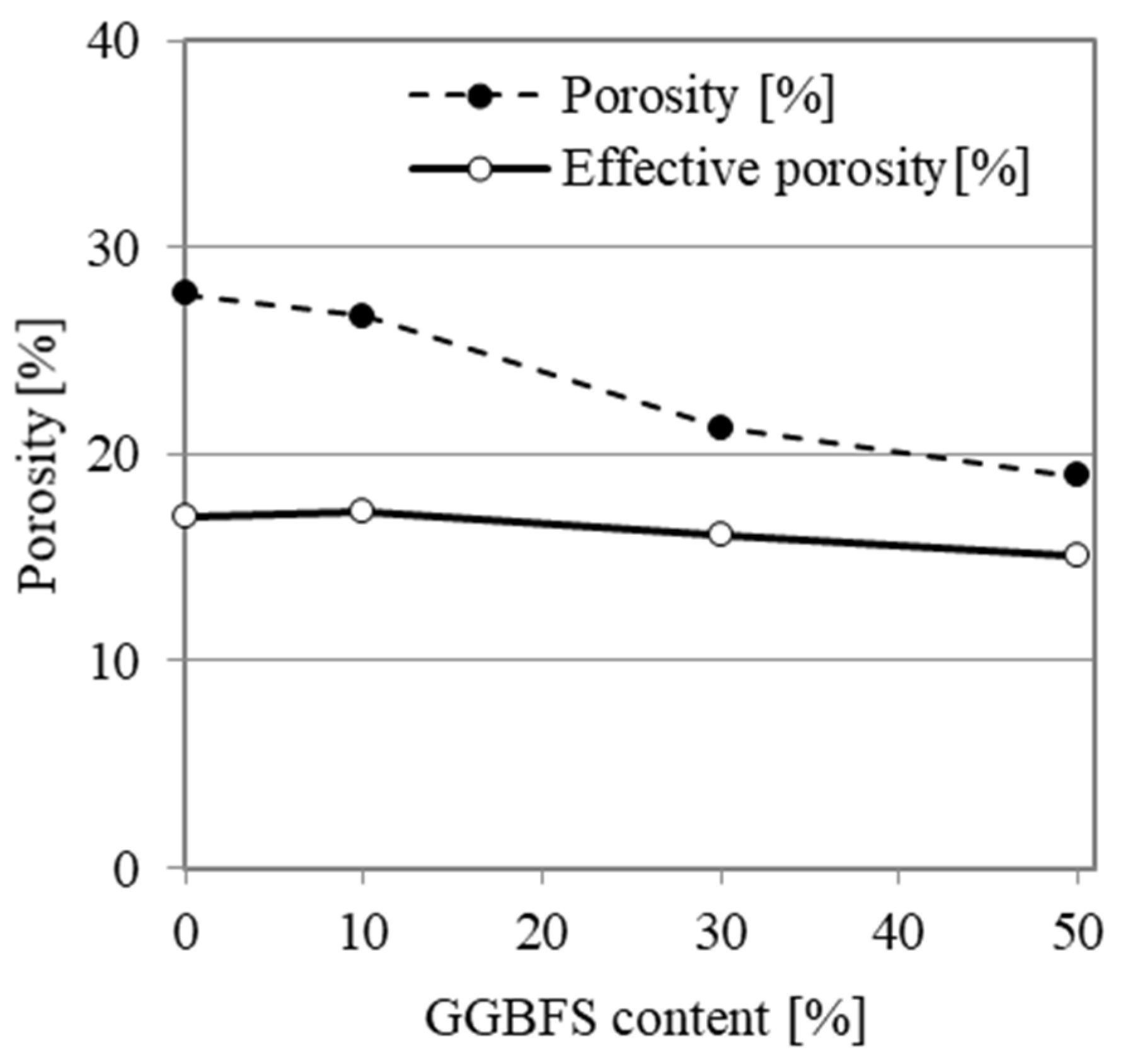
4.1. Physical Properties
4.2. Gas Permeability
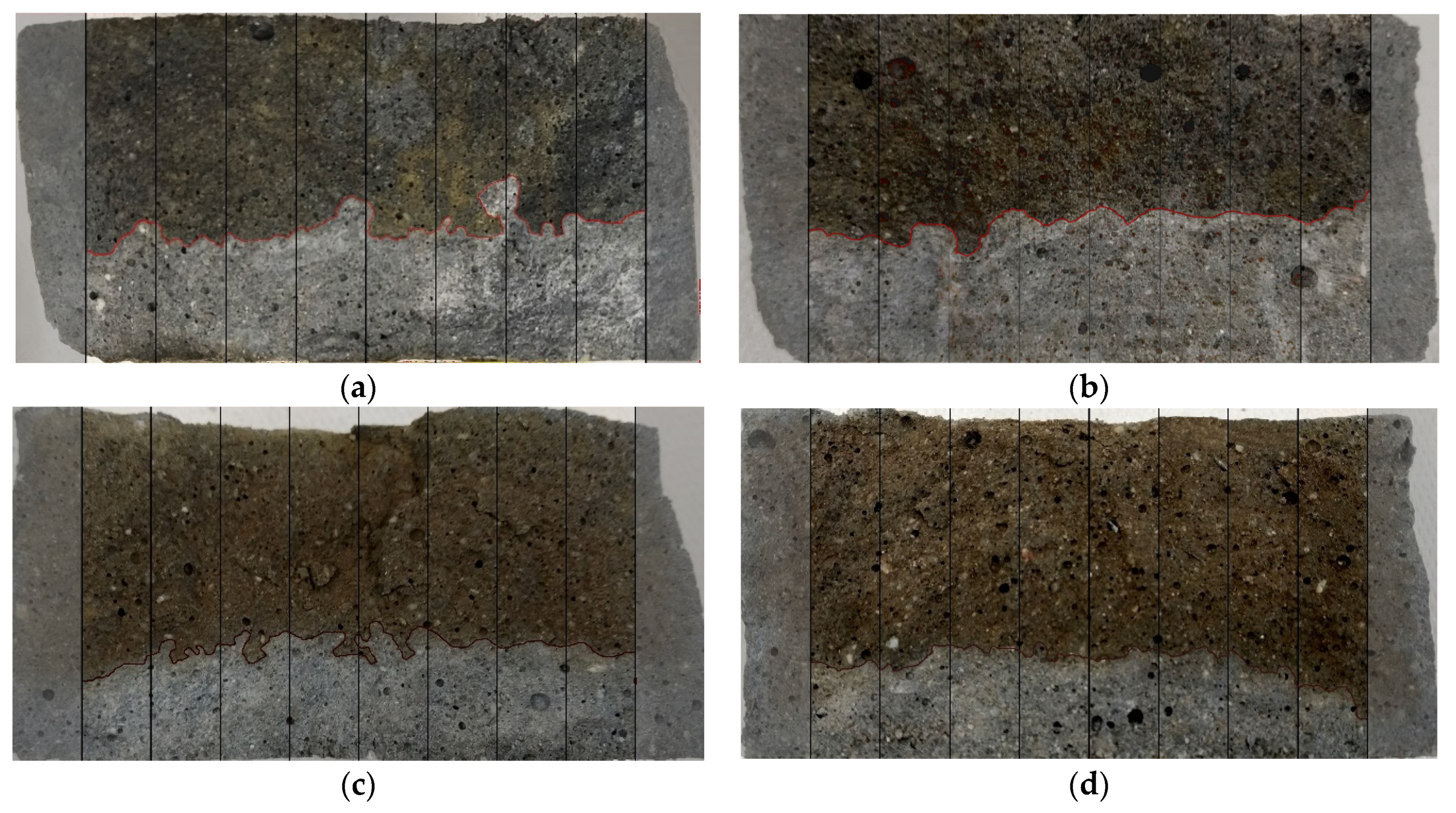
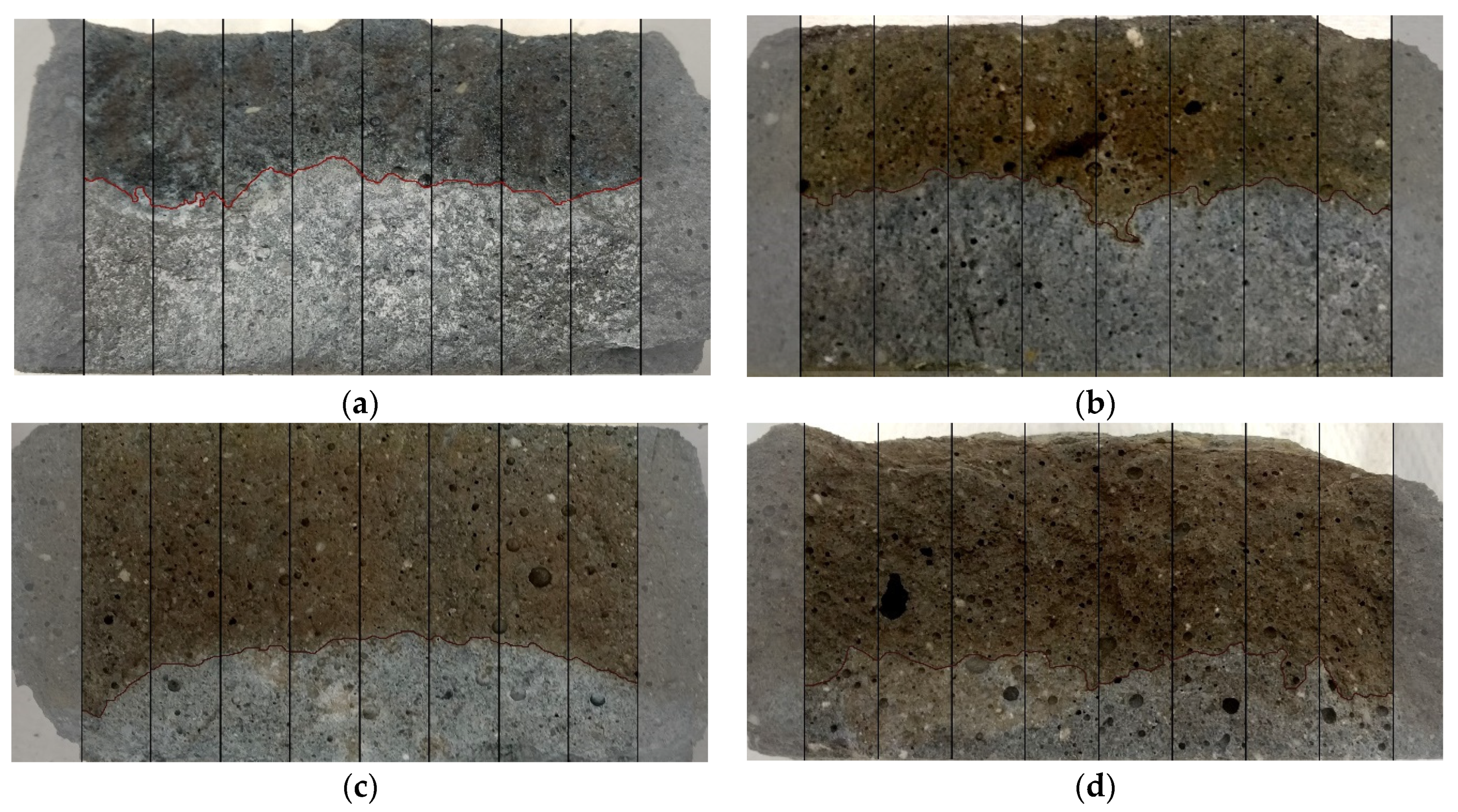
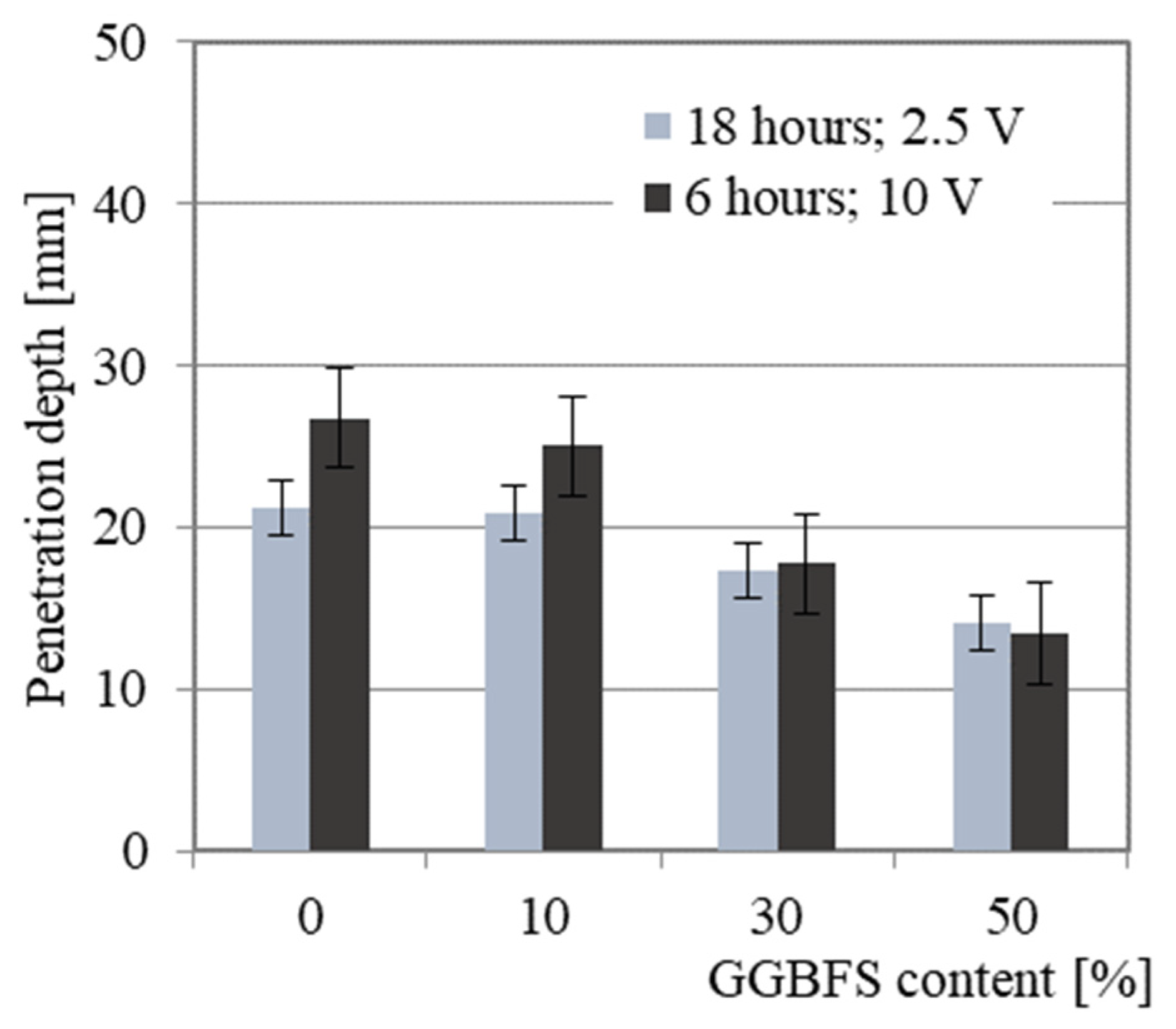
4.3. Chloride Penetration
5. Conclusions
- The moisture content of the samples stored in ambient conditions is higher for AAMs with a higher GGBFS content;
- Gas permeability is strongly dependent on the water content of the test specimen. For the specimens stored in ambient humidity a reduction in permeability with higher GGBFS content was observed;
- For oven-dried samples, the results of permeability do not differ significantly along with the GGBFS content. It is possible that the geopolymerization process was triggered by heat and for materials with lower GGBFS contents. Therefore, it is not recommended to use this drying procedure in AAM investigations;
- The addition of GGBFS reduces total porosity and, to a lesser extent, effective porosity (defined as the volume of pores that are available for water saturation);
- A linear relationship between the penetration depth and porosity was found;
- Chloride ion penetration is reduced by blending FS with GGBFS, resulting in a positive effect on the overall durability of the AAM.
- The effect of the addition of GGBFS on the chloride penetration rate shows a similar trend as that of changes in total porosity and effective porosity.
- The modified NT BUILD 492 uses an initial current voltage of 10 V instead of 60 V, allowing research to be performed on AAMs. The addition of at least 30% GGBFS reduces the sensitivity of the samples in the various tested conditions and provides a reliable evaluation of chloride ion penetration.
Author Contributions
Funding
Conflicts of Interest
References
- Law, D.W.; Adam, A.A.; Molyneaux, T.K.; Patnaikuni, I.; Wardhono, A. Long term durability properties of class F fly ash geopolymer concrete. Mater. Struct. 2014, 48, 721–731. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Bakharev, T. Geopolymeric materials prepared using Class F fly ash and elevated temperature curing. Cem. Concr. Res. 2005, 35, 1224–1232. [Google Scholar] [CrossRef]
- Hager, I.; Sitarz, M.; Mróz, K. Fly-ash based geopolymer mortar for high-temperature application—Effect of slag addition. J. Clean. Prod. 2021, 316, 128168. [Google Scholar] [CrossRef]
- Provis, J.L.; Van Deventer, J.S.J. Alkali Activated Materials, RILEM State Art Reports, RILEM TC 224-AAM; Springer: Berlin, Germany, 2014; Volume 13. [Google Scholar]
- Noushini, A.; Castel, A. Performance-based criteria to assess the suitability of geopolymer concrete in marine environments using modified ASTM C1202 and ASTM C1556 methods. Mater. Struct. 2018, 51, 146. [Google Scholar] [CrossRef]
- Balcikanli, M.; Ozbay, E. Optimum design of alkali activated slag concretes for the low oxygen/chloride ion permeability and thermal conductivity. Compos. Part B Eng. 2016, 91, 243–256. [Google Scholar] [CrossRef]
- Sitarz, M.; Hager, I.; Choińska, M. Evolution of Mechanical Properties with Time of Fly-Ash-Based Geopolymer Mortars under the Effect of Granulated Ground Blast Furnace Slag Addition. Energies 2020, 13, 1135. [Google Scholar] [CrossRef] [Green Version]
- ASTM C1556-11a. Standard Test Method for Determining the Apparent Chloride Diffusion Coefficient of Cementitious Mixtures by Bulk Diffusion; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM C1202-19. Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- NT BUILD 492. Concrete, Mortar and Cement-Based Repair Materials: Chloride Migration Coefficient from Non-Steady State Migration Experiments; Nordtest Method: Espoo, Finland, 1999. [Google Scholar]
- Stanish, K.D.; Hooton, R.D.; Thomas, M.D. Testing the Chloride Penetration Resistance of Concrete: A Literature Review. FHWA Contract DTFH61-97-R-00022 Prediction of Chloride Penetration in Concrete. 2001. Available online: https://www.fhwa.dot.gov (accessed on 14 February 2020).
- Sitarz, M.; Choińska, M.; Hager, I.; Khelidj, A. Mechanical Behaviour and Permeability of Geopolymer Mortars. Available online: https://www.matec-conferences.org/articles/matecconf/pdf/2020/18/matecconf_matbud2020_01043.pdf (accessed on 7 September 2021).
- Pargar, F.; Koleva, D.A.; Van Breugel, K. Determination of Chloride Content in Cementitious Materials: From Fundamental Aspects to Application of Ag/AgCl Chloride Sensors. Sensors 2017, 17, 2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Long, K.; Liu, W.; Li, L.; Long, W.-J. Carbonation and Chloride Ions’ Penetration of Alkali-Activated Materials: A Review. Molecules 2020, 25, 5074. [Google Scholar] [CrossRef] [PubMed]
- Sadok, A.H.; Courard, L. Chloride diffusion and oxygen permeability of mortars with low active blast furnace slag. Constr. Build. Mater. 2018, 181, 319–324. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, Z.; Zhu, Y.; Tian, L. Durability of alkali-activated fly ash concrete: Chloride penetration in pastes and mortars. Constr. Build. Mater. 2014, 65, 51–59. [Google Scholar] [CrossRef]
- Sitarz, M.; Zdeb, T.; Castro Gomes, J.; Grünhäuser Soares, E.; Hager, I. The Immobilisation of Heavy Metals from Sewage Sludge Ash in Geopolymer Mortars. Available online: https://www.matec-conferences.org/articles/matecconf/pdf/2020/18/matecconf_matbud2020_01026.pdf (accessed on 7 September 2021).
- Sitarz, M.; Urban, M.; Hager, I. Rheology and Mechanical Properties of Fly Ash-Based Geopolymer Mortars with Ground Granulated Blast Furnace Slag Addition. Energies 2020, 13, 2639. [Google Scholar] [CrossRef]
- Dudek, M.; Sitarz, M. Analysis of Changes in the Microstructure of Geopolymer Mortar after Exposure to High Temperatures. Materials 2020, 13, 4263. [Google Scholar] [CrossRef] [PubMed]
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Geopolymer Institute: Saint-Quentin, France, 2015. [Google Scholar]
- Osio-Norgaard, J.; Gevaudan, J.P.; Srubar, W.V. A review of chloride transport in alkali-activated cement paste, mortar, and concrete. Constr. Build. Mater. 2018, 186, 191–206. [Google Scholar] [CrossRef]
- Kropp, J. Permeability of Concrete as a Criterion of its Durability Measurement of the gas permeability of concrete by the RILEM-Cembureau method. Mater. Struct. Matériaux Constr. 1999, 32, 5. [Google Scholar]
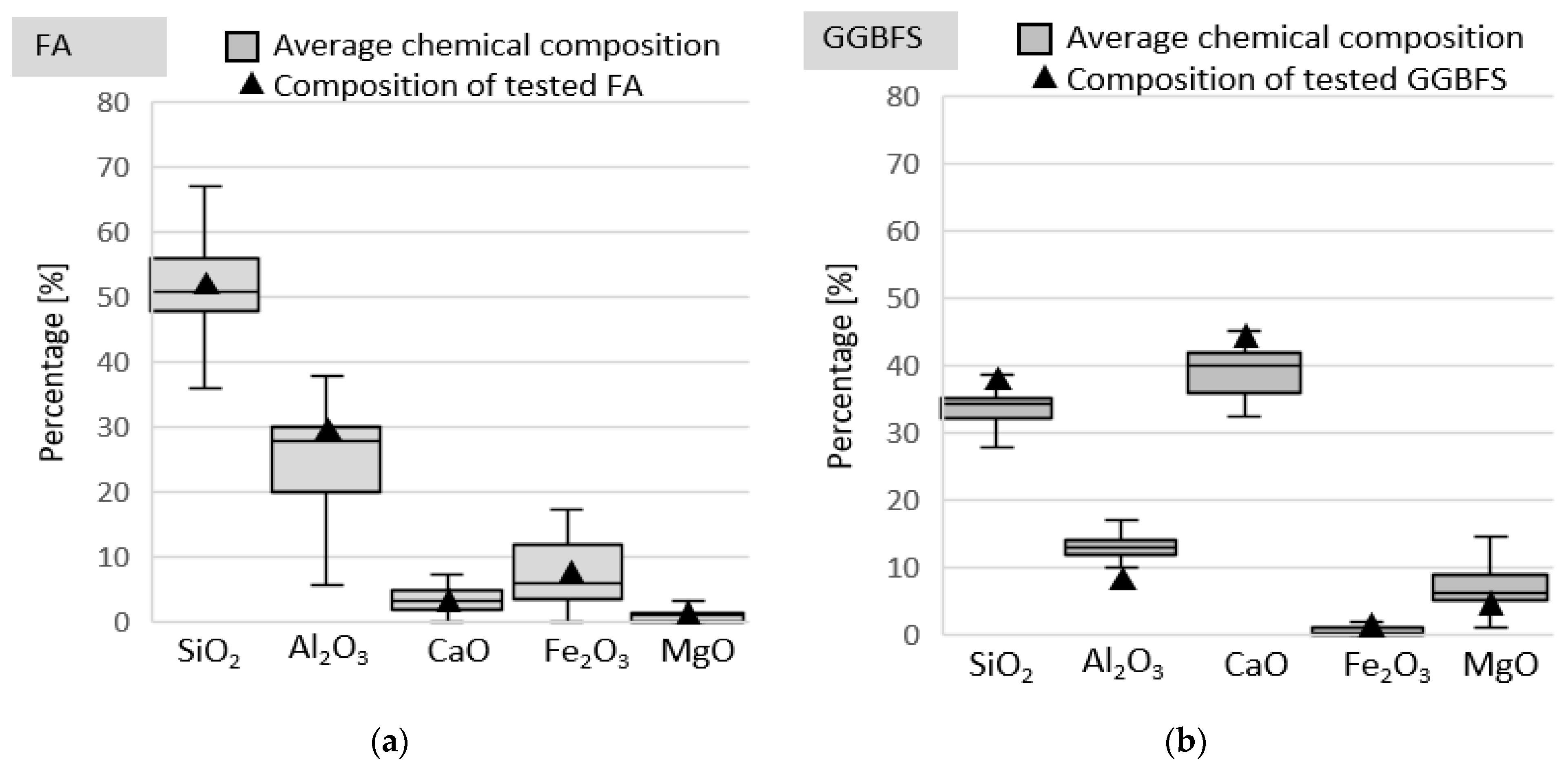




| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | P2O5 | TiO2 | Mn3O4 |
| 52.30 | 28.05 | 6.32 | 3.05 | 1.71 | 0.28 | 2.51 | 0.76 | 0.69 | 1.35 | 0.07 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | Cl¯ | Na2Oeq | Blaine |
| 39.31 | 7.61 | 1.49 | 43.90 | 4.15 | 0.51 | 0.356 | 0.468 | 0.038 | 0.702 | 3904 |
| Components | AAM0 (kg/m³) | AAM10 (kg/m³) | AAM30 (kg/m³) | AAM50 (kg/m³) |
|---|---|---|---|---|
| Alkaline solution | 330.70 | 333.90 | 340.60 | 347.50 |
| FA | 734.90 | 667.90 | 529.80 | 386.10 |
| GGBFS | 0.00 | 74.20 | 227.10 | 386.10 |
| Sand (0/2 mm) | 1102.42 | 1113.2 | 1135.30 | 1158.30 |
| Blend | Compressive Strength (MPa) | Flexural Tensile Strength (MPa) | ||||
|---|---|---|---|---|---|---|
| 3 Days | 14 Days | 28 Days | 3 Days | 14 Days | 28 Days | |
| AAM10 | 14.5 | 30.0 | 35.0 | 1.8 | 4.2 | 4.2 |
| AAM30 | 18.4 | 38.0 | 44.5 | 2.7 | 4.9 | 5.5 |
| AAM50 | 44.5 | 60.0 | 63.0 | 3.8 | 5.8 | 6.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duży, P.; Sitarz, M.; Adamczyk, M.; Choińska, M.; Hager, I. Chloride Ions’ Penetration of Fly Ash and Ground Granulated Blast Furnace Slags-Based Alkali-Activated Mortars. Materials 2021, 14, 6583. https://doi.org/10.3390/ma14216583
Duży P, Sitarz M, Adamczyk M, Choińska M, Hager I. Chloride Ions’ Penetration of Fly Ash and Ground Granulated Blast Furnace Slags-Based Alkali-Activated Mortars. Materials. 2021; 14(21):6583. https://doi.org/10.3390/ma14216583
Chicago/Turabian StyleDuży, Patrycja, Mateusz Sitarz, Marcin Adamczyk, Marta Choińska, and Izabela Hager. 2021. "Chloride Ions’ Penetration of Fly Ash and Ground Granulated Blast Furnace Slags-Based Alkali-Activated Mortars" Materials 14, no. 21: 6583. https://doi.org/10.3390/ma14216583
APA StyleDuży, P., Sitarz, M., Adamczyk, M., Choińska, M., & Hager, I. (2021). Chloride Ions’ Penetration of Fly Ash and Ground Granulated Blast Furnace Slags-Based Alkali-Activated Mortars. Materials, 14(21), 6583. https://doi.org/10.3390/ma14216583







