The Role of Blood Clot in Guided Bone Regeneration: Biological Considerations and Clinical Applications with Titanium Foil
Abstract
:1. Introduction
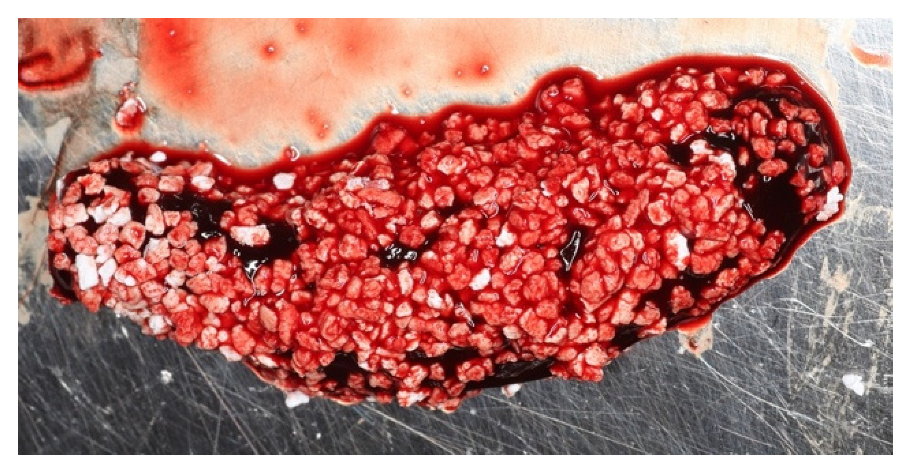
2. The Blood Clot
3. Clot Management and Titanium Foil: Biological Principles
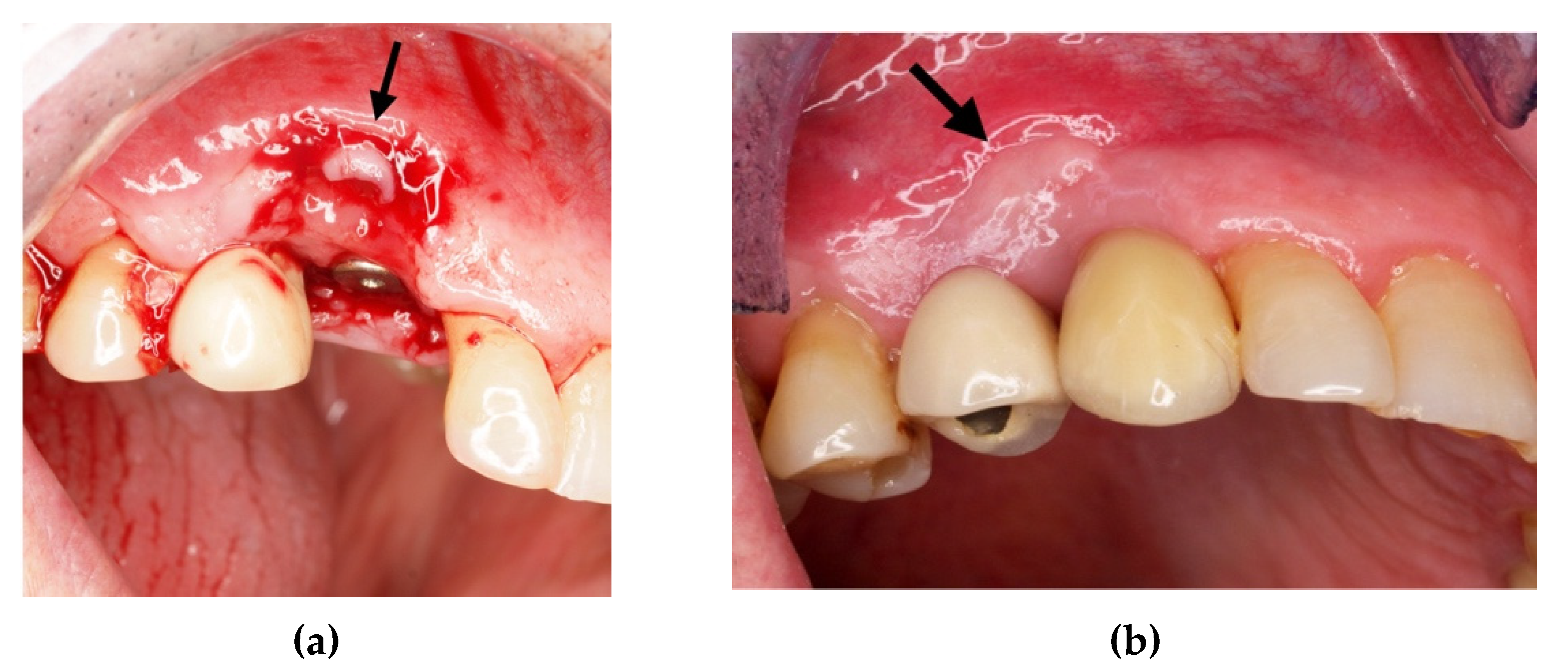
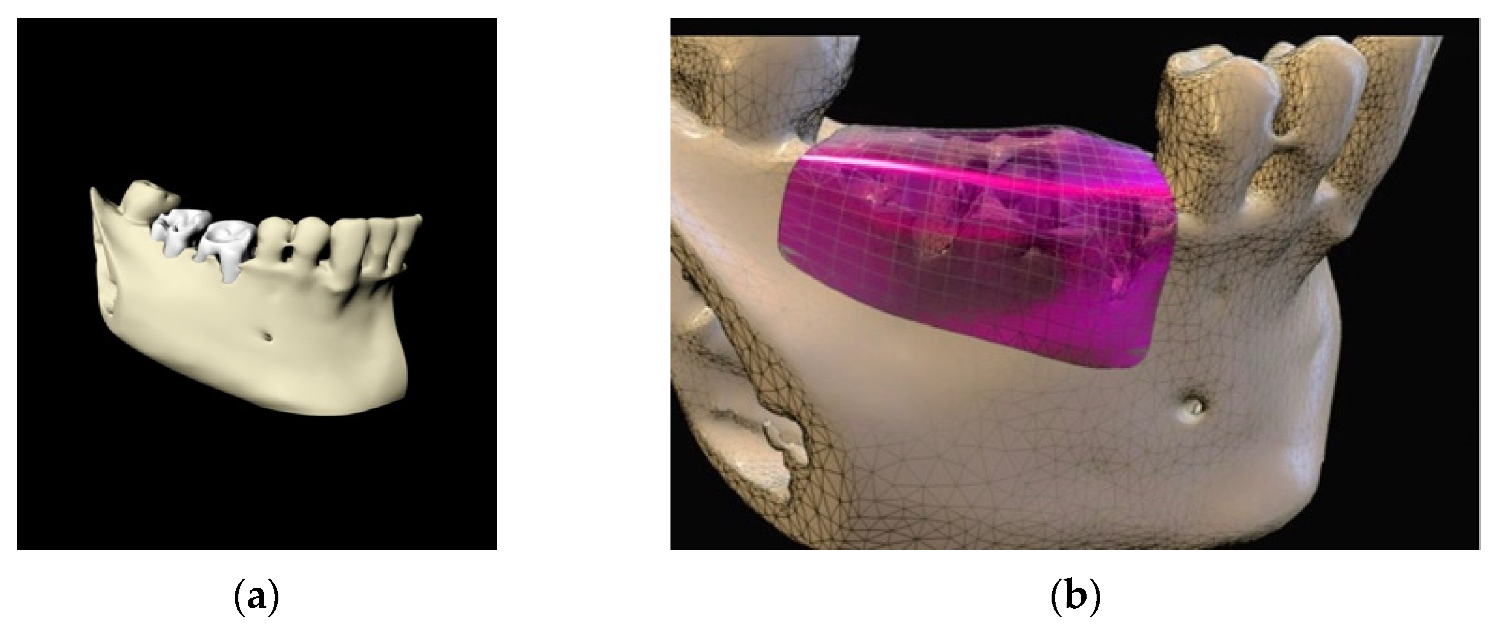
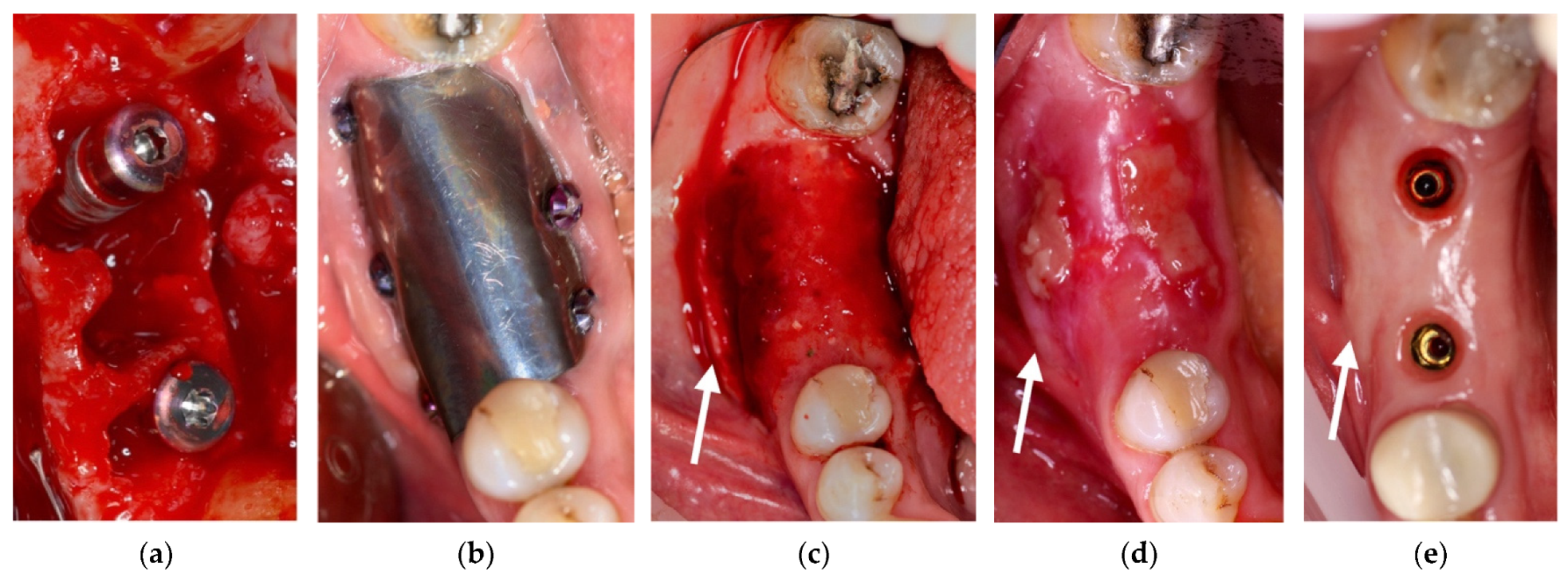
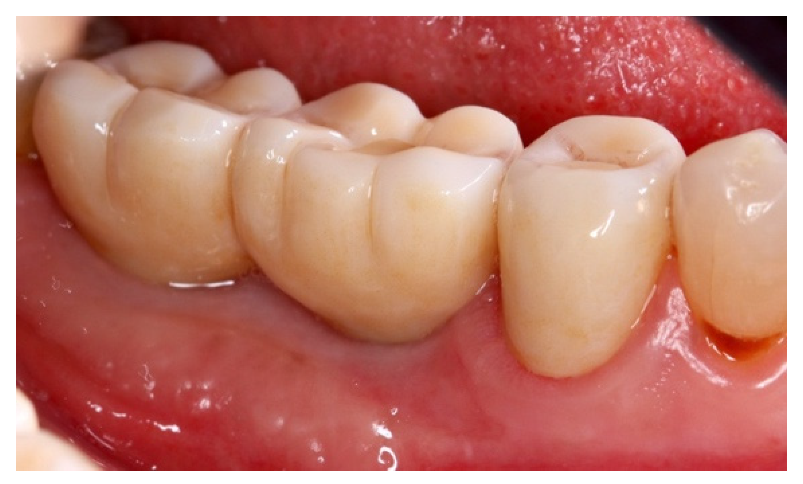
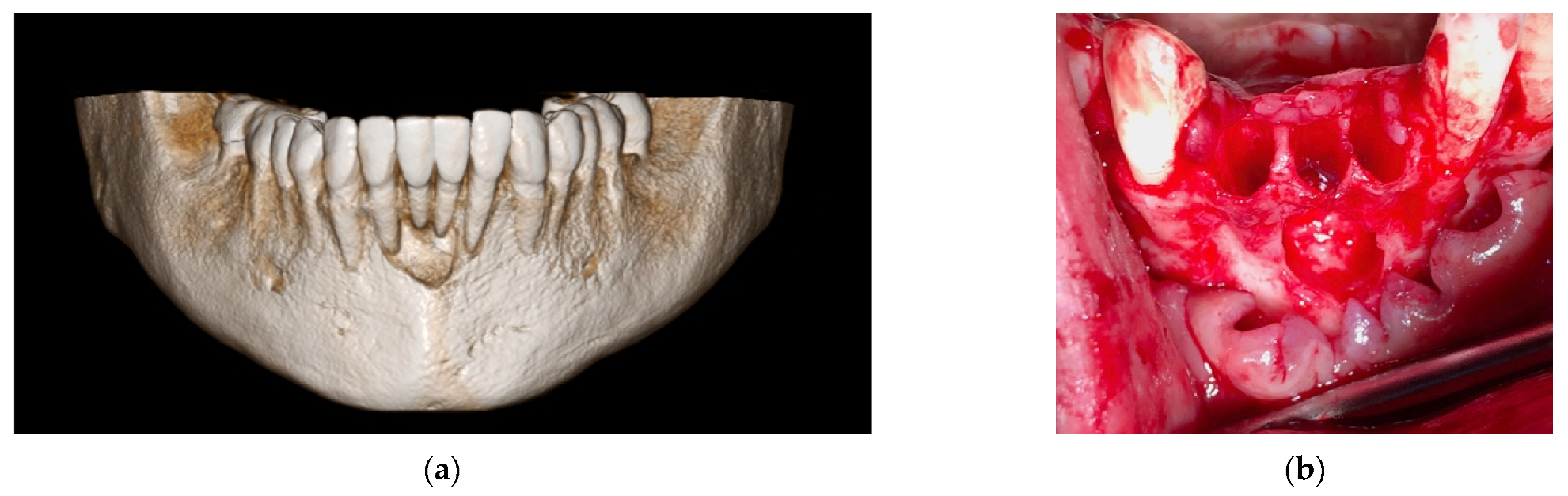
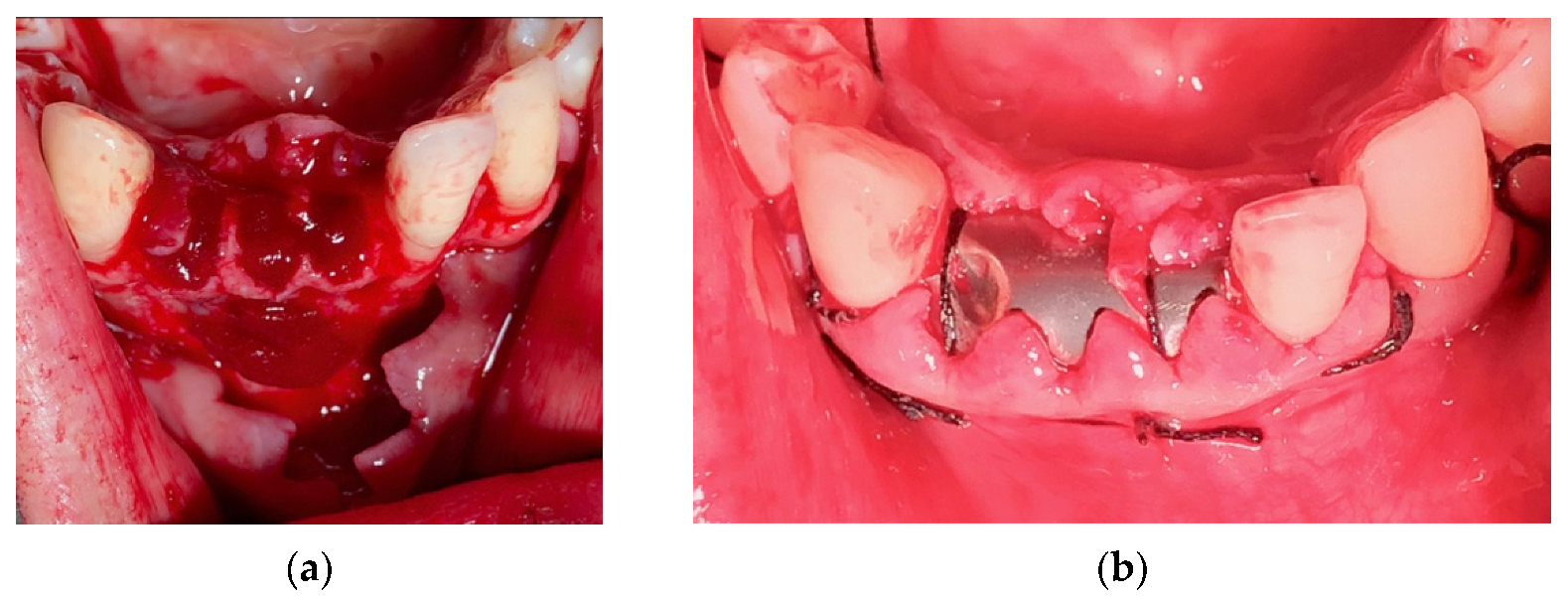
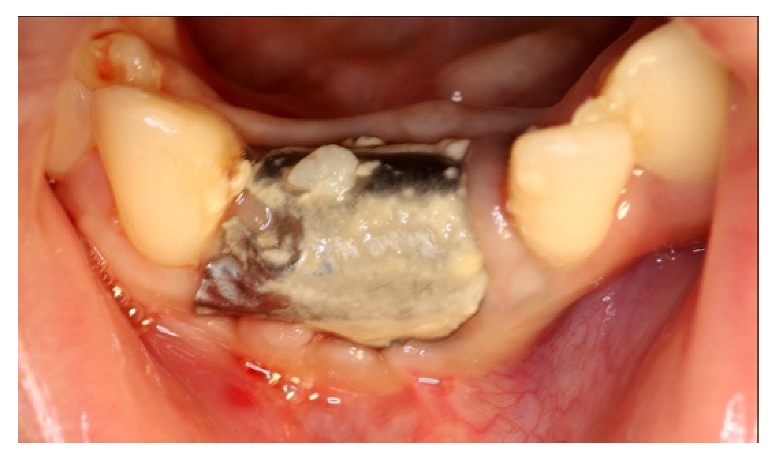
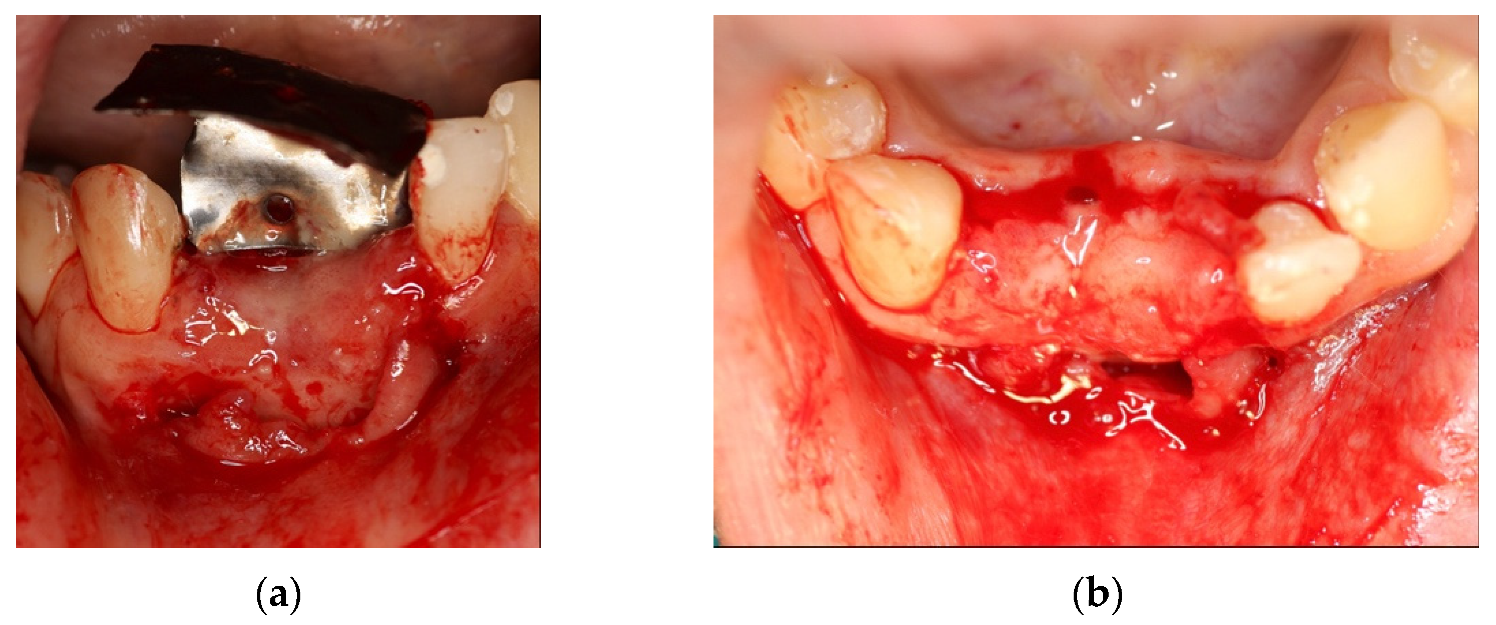
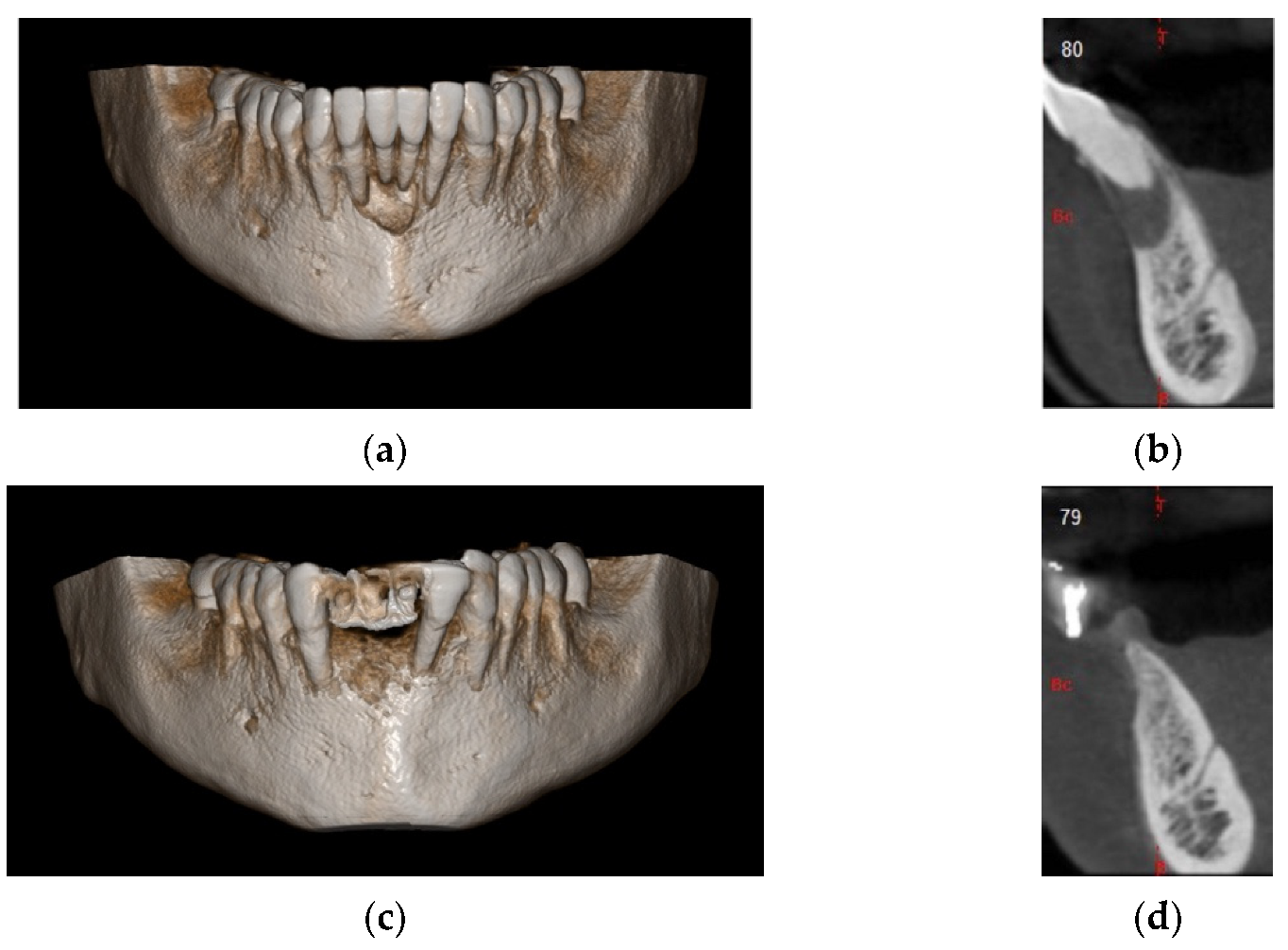
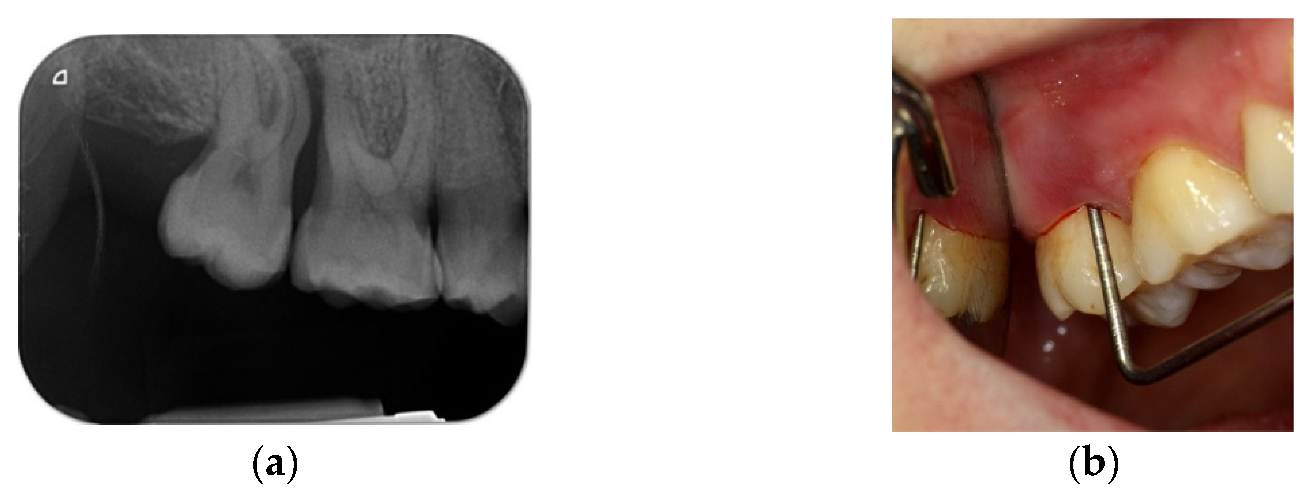
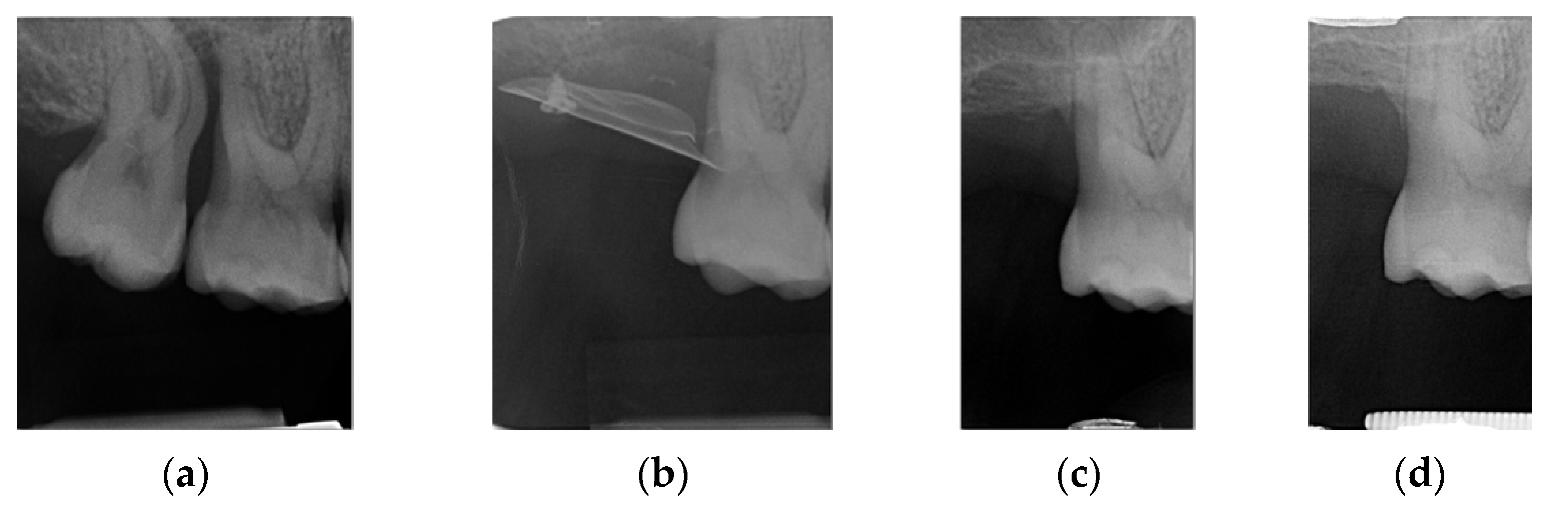
4. Case Series
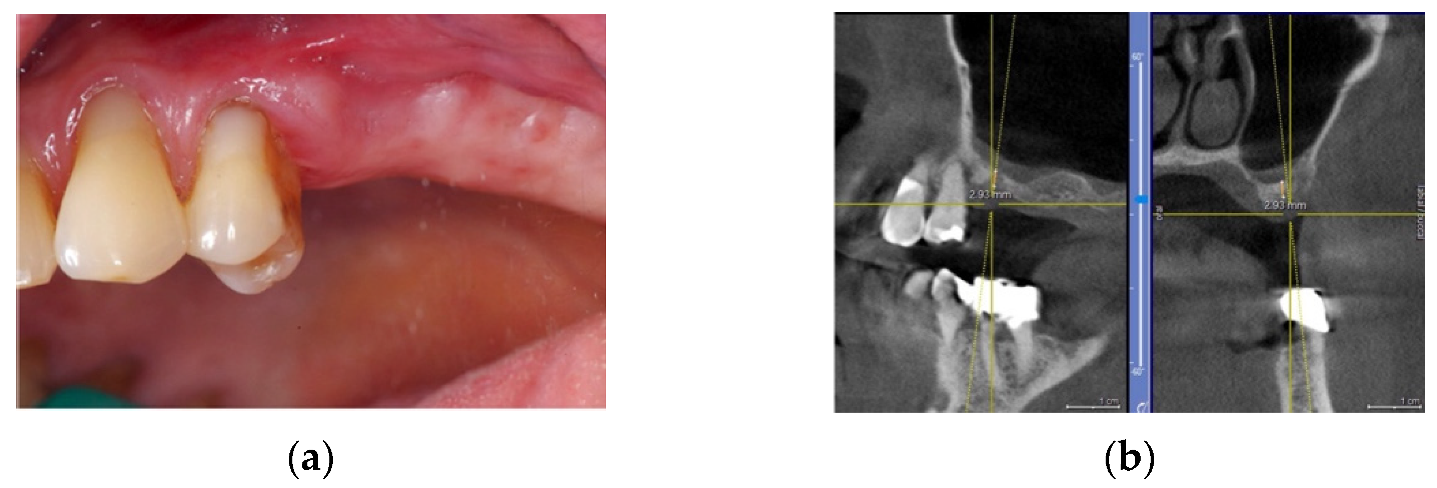
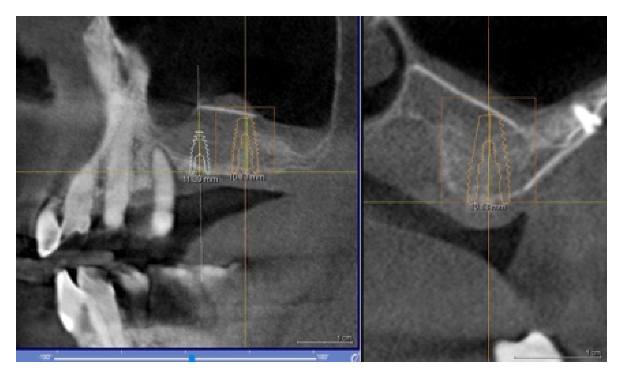
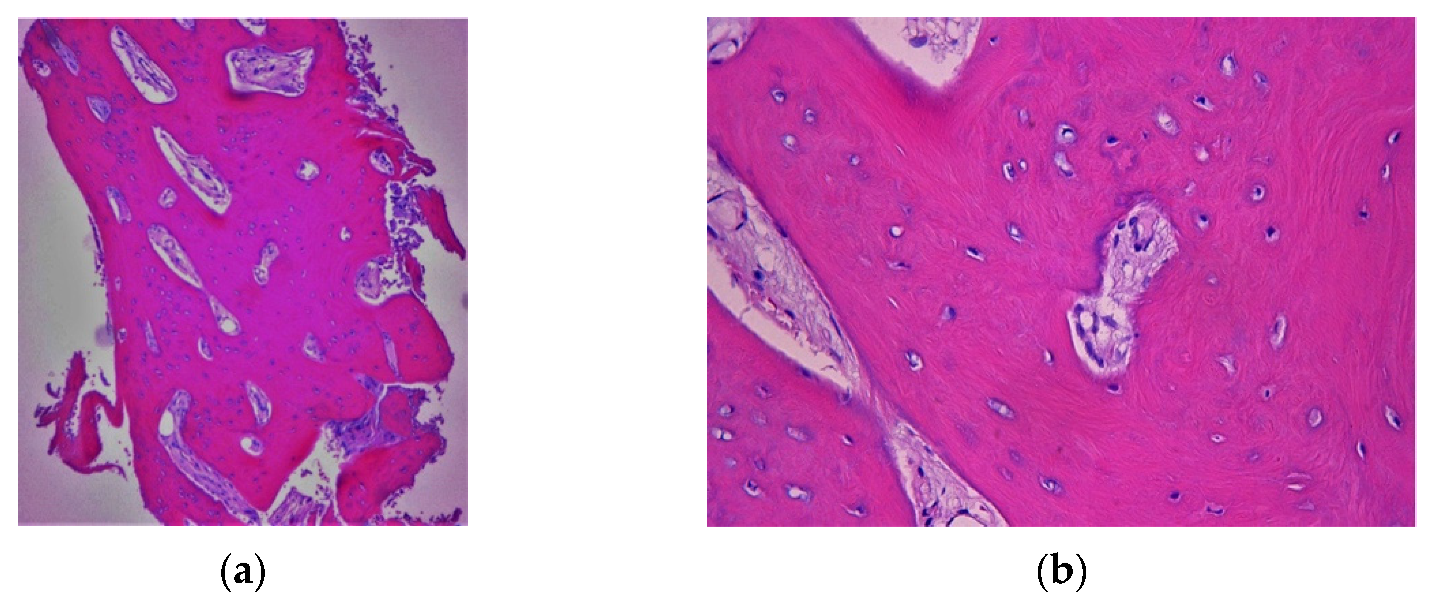
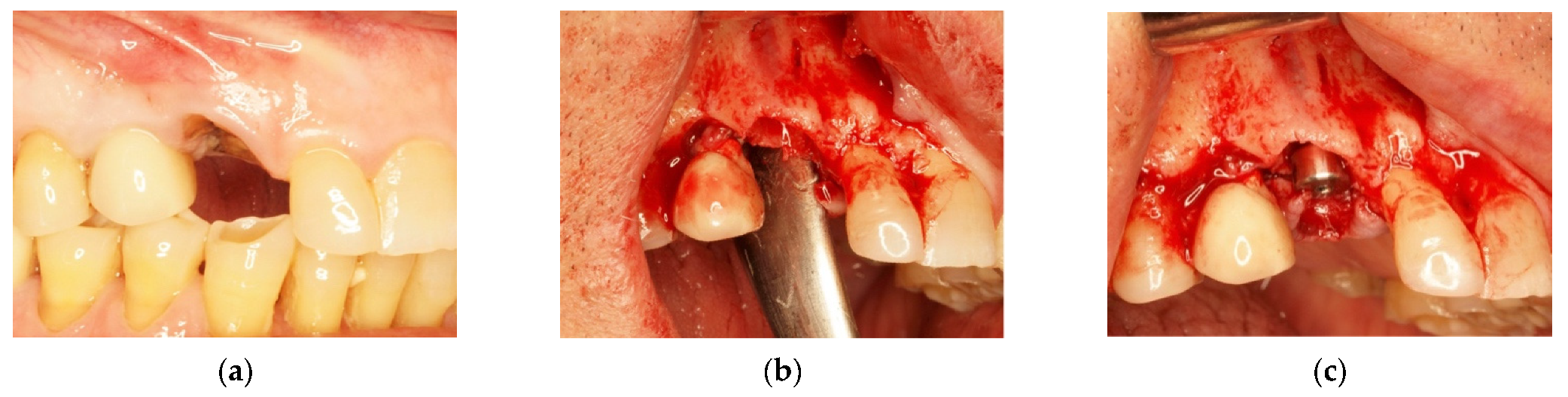
4.1. Clinical Case n.1.
4.2. Clinical Case n.2.
4.3. Clinical Case n.3.
4.4. Clinical Case n.4.
4.5. Clinical Case n.5.
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gultekin, B.A.; Bedeloglu, E.; Kose, T.E.; Mijiritsky, E. Comparison of Bone Resorption Rates after Intraoral Block Bone and Guided Bone Regeneration Augmentation for the Reconstruction of Horizontally Deficient Maxillary Alveolar Ridges. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pistilli, R.; Checchi, V.; Sammartino, G.; Simion, M.; Felice, P. Safe New Approach to the Lingual Flap Management in Mandibular Augmentation Procedures: The Digitoclastic Technique. Implant Dent. 2017, 26, 790–795. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Ronda, M.; Stacchi, C. A Novel Approach for the Coronal Advancement of the Buccal Flap. Int. J. Periodontics Restor. Dent. 2015, 35, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 103–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windisch, P.; Martin, A.; Shahbazi, A.; Molnar, B. Reconstruction of horizontovertical alveolar defects. Presentation of a novel split-thickness flap design for guided bone regeneration: A case report with 5-year follow-up. Quintessence Int. 2017, 48, 535–547. [Google Scholar] [CrossRef]
- Windisch, P.; Orban, K.; Salvi, G.E.; Sculean, A.; Molnar, B. Vertical-guided bone regeneration with a titanium-reinforced d-PTFE membrane utilizing a novel split-thickness flap design: A prospective case series. Clin. Oral Investig. 2021, 25, 2969–2980. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, S.; Schwarz, F.; Cordaro, L.; Derks, J.; Hämmerle, C.H.F.; Heitz-Mayfield, L.J.; Hernández-Alfaro, F.; Meijer, H.J.A.; Naenni, N.; Ortiz-Vigón, A.; et al. Regeneration of alveolar ridge defects. Consensus report of group 4 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46 (Suppl. 21), 277–286. [Google Scholar] [CrossRef]
- Patterson, J.; Martino, M.; Hubbell, J.A. Biomimetic materials in tissue engineering. Mater. Today 2010, 13, 14–22. [Google Scholar] [CrossRef]
- Grayson, W.L.; Martens, T.P.; Eng, G.M.; Radisic, M.; Vunjak-Novakovic, G. Biomimetic approach to tissue engineering. Semin. Cell Dev. Biol. 2009, 20, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, M.; Spiekermann, H.; Biesterfeld, S.; Edelhoff, D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss® in combination with venous blood: A histologic and histomorphometric study in humans. Clin. Oral Implant. Res. 2000, 11, 217–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traini, T.; Valentini, P.; Iezzi, G.; Piattelli, A. A Histologic and Histomorphometric Evaluation of Anorganic Bovine Bone Retrieved 9 Years After a Sinus Augmentation Procedure. J. Periodontol. 2007, 78, 955–961. [Google Scholar] [CrossRef]
- Guarnieri, R.; Belleggia, F.; DeVillier, P.; Testarelli, L. Histologic and Histomorphometric Analysis of Bone Regeneration with Bovine Grafting Material after 24 Months of Healing. A Case Report. J. Funct. Biomater. 2018, 9, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyström, E.; Nilson, H.; Gunne, J.; Lundgren, S. A 9–14 year follow-up of onlay bone grafting in the atrophic maxilla. Int. J. Oral Maxillofac. Surg. 2009, 38, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, H. The Biology of Bone Graft Repair. Clin. Orthop. Relat. Res. 1983, 174, 28–42. [Google Scholar] [CrossRef]
- Widmark, G.; Andersson, B.; Ivanoff, C.-J. Mandibular bone graft in the anterior maxilla for single-tooth implants: Presentation of a surgical method. Int. J. Oral Maxillofac. Surg. 1997, 26, 106–109. [Google Scholar] [CrossRef]
- Stricker, A.; Jacobs, R.; Maes, F.; Fluegge, T.; Vach, K.; Fleiner, J. Resorption of retromolar bone grafts after alveolar ridge augmentation—Volumetric changes after 12 months assessed by CBCT analysis. Int. J. Implant. Dent. 2021, 7, 1–7. [Google Scholar] [CrossRef]
- Chiapasco, M.; Casentini, P.; Zaniboni, M. Bone augmentation procedures in implant dentistry. Int. J. Oral Maxillofac. Implant. 2009, 24, 237–260. [Google Scholar]
- Chiapasco, M.; Zaniboni, M.; Rimondini, L. Autogenous onlay bone grafts vs. alveolar distraction osteogenesis for the correction of vertically deficient edentulous ridges: A 2–4-year prospective study on humans. Clin. Oral Implant. Res. 2007, 18, 432–440. [Google Scholar] [CrossRef]
- Murray, G.; Holden, R.; Roschlau, W. Experimental and clinical study of new growth of bone in a cavity. Am. J. Surg. 1957, 93, 385–387. [Google Scholar] [CrossRef]
- Melcher, A.H.; Dreyer, C.J. Protection of the Blood Clot in Healing Circumscribed Bone Defects. J. Bone Jt. Surg. Br. Vol. 1962, 44, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Friis, T.; Glatt, V.; Crawford, R.; Xiao, Y. Structural properties of fracture haematoma: Current status and future clinical implications. J. Tissue Eng. Regen. Med. 2017, 11, 2864–2875. [Google Scholar] [CrossRef]
- Lundgren, S.; Andersson, S.; Sennerby, L. Spontaneous Bone Formation in the Maxillary Sinus after Removal of a Cyst: Coincidence or Consequence? Clin. Implant Dent. Relat. Res. 2003, 5, 78–81. [Google Scholar] [CrossRef]
- Van Steenberghe, D.; Johansson, C.; Quirynen, M.; Molly, L.; Albrektsson, T.; Naert, I. Bone augmentation by means of a stiff occlusive titanium barrier. Clin. Oral Implant. Res. 2003, 14, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chipaila, N.; Marini, R.; Sfasciotti, G.L.; Cielo, A.; Bonanome, L.; Monaco, A. Graftless sinus augmentation technique with contextual placement of implants: A case report. J. Med. Case Rep. 2014, 8, 437. [Google Scholar] [CrossRef] [Green Version]
- Gomes, P.D.S.; Daugela, P.; Poskevicius, L.; Mariano, L.; Fernandes, M.H. Molecular and Cellular Aspects of Socket Healing in the Absence and Presence of Graft Materials and Autologous Platelet Concentrates: A Focused Review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar] [CrossRef]
- Kolar, P.; Schmidt-Bleek, K.; Schell, H.; Gaber, T.; Toben, D.; Schmidmaier, G.; Perka, C.; Buttgereit, F.; Duda, G.N. The Early Fracture Hematoma and Its Potential Role in Fracture Healing. Tissue Eng. Part B Rev. 2010, 16, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Lang, N.P.; Schweikert, M.T.; De Oliveira, J.A.; Rangel-Garcia, I.; Botticelli, D. Sequential healing of open extraction sockets. An experimental study in monkeys. Clin. Oral Implant. Res. 2013, 25, 288–295. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Chen, M.; Wei, X.; Hao, Y.; Wang, J. Evaluation of 3D-Printed Polycaprolactone Scaffolds Coated with Freeze-Dried Platelet-Rich Plasma for Bone Regeneration. Materials 2017, 10, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batas, L.; Tsalikis, L.; Stavropoulos, A. PRGF as adjunct to DBB in maxillary sinus floor augmentation: Histological results of a pilot split-mouth study. Int. J. Implant. Dent. 2019, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Medina, T.; Vaquette, C.; Ivanovski, S. Systematic Comparison of the Effect of Four Clinical-Grade Platelet Rich Hemoderivatives on Osteoblast Behaviour. Int. J. Mol. Sci. 2019, 20, 6243. [Google Scholar] [CrossRef] [Green Version]
- Shiu, H.T.; Goss, B.; Lutton, C.; Crawford, R.; Xiao, Y. Formation of Blood Clot on Biomaterial Implants Influences Bone Healing. Tissue Eng. Part B Rev. 2014, 20, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Gersh, K.C.; Nagaswami, C.; Weisel, J.W. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb. Haemost. 2009, 102, 1169–1175. [Google Scholar] [CrossRef] [Green Version]
- Thor, A.; Rasmusson, L.; Wennerberg, A.; Thomsen, P.; Hirsch, J.-M.; Nilsson, B.; Hong, J. The role of whole blood in thrombin generation in contact with various titanium surfaces. Biomaterials 2007, 28, 966–974. [Google Scholar] [CrossRef]
- Lundgren, S.; Andersson, S.; Gualini, F.; Sennerby, L. Bone reformation with sinus membrane elevation: A new surgical technique for maxillary sinus floor augmentation. Clin. Implant. Dent. Relat. Res. 2004, 6, 165–173. [Google Scholar] [CrossRef]
- Lundgren, S.; Cricchio, G.; Palma, V.C.; Salata, L.A.; Sennerby, L. Sinus membrane elevation and simultaneous insertion of dental implants: A new surgical technique in maxillary sinus floor augmentation. Periodontol. 2000 2008, 47, 193–205. [Google Scholar] [CrossRef]
- Riben, C.; Thor, A. Follow-Up of the Sinus Membrane Elevation Technique for Maxillary Sinus Implants without the Use of Graft Material. Clin. Implant. Dent. Relat. Res. 2015, 18, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Lambert, F.; Leonard, A.; Drion, P.; Sourice, S.; Layrolle, P.; Rompen, E. Influence of space-filling materials in subantral bone augmentation: Blood clot vs. autogenous bone chips vs. bovine hydroxyapatite. Clin. Oral Implant. Res. 2010, 22, 538–545. [Google Scholar] [CrossRef]
- Leghissa, G.C.; Zaffe, D.; Assenza, B.; Botticelli, A.R. Guided bone regeneration using titanium grids: Report of 10 cases. Clin. Oral Implant. Res. 1999, 10, 62–68. [Google Scholar] [CrossRef]
- Diès, F.; Etienne, D.; Abboud, N.B.; Ouhayoun, J.P. Bone regeneration in extraction sites after immediate placement of an e-PTFE membrane with or without a biomaterial. A report on 12 consecutive cases. Clin. Oral Implant. Res. 1996, 7, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Mattout, P.; Nowzari, H. Clinical evaluation of guided bone regeneration at exposed parts of Branemark dental implants with and without bone allograft. Clin. Oral Implant. Res. 1995, 6, 189–195. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Gaggl, A.; Schultes, G. Titanium Foil–Guided Tissue Regeneration in the Treatment of Periimplant Bone Defects. Implant. Dent. 1999, 8, 368–375. [Google Scholar] [CrossRef]
- Molly, L.; Quirynen, M.; Michiels, K.; Van Steenberghe, D. Comparison between jaw bone augmentation by means of a stiff occlusive titanium membrane or an autologous hip graft: A retrospective clinical assessment. Clin. Oral Implant. Res. 2006, 17, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, V.; Engelke, W.; Fuentes, R.; Decco, O.; Prieto, R.; Wilckens, M.; Borie, E. Bone Augmentation with Occlusive Barriers and Cortical Particulate Allograft in Transverse Maxillary Defects: A Pilot Study. Int. J. Morphol. 2014, 32, 364–368. [Google Scholar] [CrossRef] [Green Version]
- Engelke, W.; Deccó, O.; Cura, A.C.; Borie, E.; Beltrán, V. Rigid occlusive titanium barriers for alveolar bone augmentation: Two reports with 24-month follow-up. Int. J. Clin. Exp. Med. 2014, 7, 1160–1165. [Google Scholar]
- Bassi, M.A.; Andrisani, C.; Lico, S.; Ormanier, Z.; Ottria, L.; Gargari, M. Guided bone regeneration via a preformed titanium foil: Clinical, histological and histomorphometric outcome of a case series. ORAL Implant. 2016, 9, 164–174. [Google Scholar] [CrossRef]
- Perret, F.; Romano, F.; Ferrarotti, F.; Aimetti, M. Occlusive Titanium Barrier for Immediate Bone Augmentation of Severely Resorbed Alveolar Sockets with Secondary Soft Tissue Healing: A 2-Year Case Series. Int. J. Periodontics Restor. Dent. 2019, 39, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Dodge, A.; Luepke, P.; Wang, H.-L.; Kapila, Y.; Lin, G.-H. Effect of membrane exposure on guided bone regeneration: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 328–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, G.; Lin, G.-H.; Monje, A.; Chan, H.-L.; Wang, H.-L. Wound Healing Complications Following Guided Bone Regeneration for Ridge Augmentation: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Ku, J.-K. Guided bone regeneration. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 361–366. [Google Scholar] [CrossRef]
- Laurito, D.; Lollobrigida, M.; Gianno, F.; Bosco, S.; Lamazza, L.; De Biase, A. Alveolar Ridge Preservation with nc-HA and d-PTFE Membrane: A Clinical, Histologic, and Histomorphometric Study. Int. J. Periodontics Restor. Dent. 2017, 37, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Iorio-Siciliano, V.; Blasi, A.; Nicolò, M.; Iorio-Siciliano, A.; Riccitiello, F.; Ramaglia, L. Clinical Outcomes of Socket Preservation Using Bovine-Derived Xenograft Collagen and Collagen Membrane Post–Tooth Extraction: A 6-Month Randomized Controlled Clinical Trial. Int. J. Periodontics Restor. Dent. 2017, 37, e290–e296. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-Y.; Kim, Y.-K.; Kim, H.-S.; Yun, P.-Y.; Kim, S.-G.; Choi, Y.-H. Extraction socket sealing using palatal gingival grafts and resorbable collagen membranes. Maxillofac. Plast. Reconstr. Surg. 2017, 39, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathnayake, N.; Trajkovski, B.; Rahman, B.; Zafiropoulos, G.G. Clinical Applications and Outcomes of non-resorbable Polytetrafluoroethylene (PTFE) Membranes in Guided Bone Regeneration. J. Int. Dent. Med. Res. 2019, 12, 1626–1635. [Google Scholar]
- Roca-Millan, E.; Jané-Salas, E.; Estrugo-Devesa, A.; López-López, J. Evaluation of Bone Gain and Complication Rates after Guided Bone Regeneration with Titanium Foils: A Systematic Review. Materials 2020, 13, 5346. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Fukase, Y.; Goke, E.; Yamada, Y.; Sato, S.; Nishiyama, M.; Ito, K. Three-dimensional evaluation for augmented bone using guided bone regeneration. J. Periodontal Res. 2005, 40, 269–276. [Google Scholar] [CrossRef]
- Yamada, Y.; Nanba, K.; Ito, K. Effects of occlusiveness of a titanium cap on bone generation beyond the skeletal envelope in the rabbit calvarium. Clin. Oral Implant. Res. 2003, 14, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Ezirganlı, Ş.; Polat, S.; Barış, E.; Tatar, İ.; Çelik, H.H. Comparative investigation of the effects of different materials used with a titanium barrier on new bone formation. Clin. Oral Implant. Res. 2013, 24, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Arbez, B.; Manero, F.; Mabilleau, G.; Libouban, H.; Chappard, D. Human macrophages and osteoclasts resorb β-tricalcium phosphate in vitro but not mouse macrophages. Micron 2019, 125, 102730. [Google Scholar] [CrossRef] [PubMed]
- Pascaretti-Grizon, F.; Guillaume, B.; Terranova, L.; Arbez, B.; Libouban, H.; Chappard, D. Maxillary Sinus Lift with Beta-Tricalcium Phosphate (β-TCP) in Edentulous Patients: A Nanotomographic and Raman Study. Calcif. Tissue Int. 2017, 101, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Murai, M.; Sato, S.; Fukase, Y.; Yamada, Y.; Komiyama, K.; Ito, K. Effects of Different Sizes of β-tricalcium Phosphate Particles on Bone Augmentation within a Titanium Cap in Rabbit Calvarium. Dent. Mater. J. 2006, 25, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjo, U.M.E. Biology and principles of periodontal wound healing/regeneration. Periodontol. 2000 2006, 41, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Robertson, P.B. Fundamental principles affecting the outcomes of therapy for osseous lesions. Periodontol. 2000 2000, 22, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Wikesjö, U.M.E.; Lim, W.H.; Thomson, R.C.; Hardwick, W.R. Periodontal repair in dogs: Gingival tissue occlusion, a critical requirement for GTR? J. Clin. Periodontol. 2003, 30, 655–664. [Google Scholar] [CrossRef]
- Luo, J.; Xu, J.; Cai, J.; Wang, L.; Sun, Q.; Yang, P. The In Vitro and In Vivo Osteogenic Capability of the Extraction Socket-Derived Early Healing Tissue. J. Periodontol. 2016, 87, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Naung, N.Y.; Shehata, E.; Van Sickels, J.E. Resorbable Versus Nonresorbable Membranes: When and Why? Dent. Clin. N. Am. 2019, 63, 419–431. [Google Scholar] [CrossRef]
- Kudo, A. Periostin in Bone Biology. Adv. Exp. Med. Biol. 2019, 1132, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Groeneveldt, L.C.; Herpelinck, T.; Maréchal, M.; Politis, C.; Van Ijcken, W.F.J.; Huylebroeck, D.; Geris, L.; Mulugeta, E.; Luyten, F.P. The Bone-Forming Properties of Periosteum-Derived Cells Differ Between Harvest Sites. Front. Cell Dev. Biol. 2020, Nov 25;8, 554984. [Google Scholar] [CrossRef]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef]
- Ivanovski, S.; Lee, R. Comparison of peri-implant and periodontal marginal soft tissues in health and disease. Periodontol. 2000 2018, 76, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Bunk, D.; Eisenburger, M.; Häckl, S.; Eberhard, J.; Stiesch, M.; Grischke, J. The effect of adjuvant oral irrigation on self-administered oral care in the management of peri-implant mucositis: A randomized controlled clinical trial. Clin. Oral Implant. Res. 2020, 31, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Tutwiler, V.; Wang, H.; Litvinov, R.I.; Weisel, J.W.; Shenoy, V.B. Interplay of Platelet Contractility and Elasticity of Fibrin/Erythrocytes in Blood Clot Retraction. Biophys. J. 2017, 112, 714–723. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.V.; Litvinov, R.I.; Alber, M.S.; Weisel, J.W. Quantitative structural mechanobiology of platelet-driven blood clot contraction. Nat. Commun. 2017, 8, 1274. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Brown, A.C.; Myers, D.; Sakurai, Y.; Mannino, R.G.; Tran, R.; Ahn, B.; Hardy, E.T.; Kee, M.F.; Kumar, S.; et al. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc. Natl. Acad. Sci. USA 2014, 111, 14430–14435. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milillo, L.; Cinone, F.; Lo Presti, F.; Lauritano, D.; Petruzzi, M. The Role of Blood Clot in Guided Bone Regeneration: Biological Considerations and Clinical Applications with Titanium Foil. Materials 2021, 14, 6642. https://doi.org/10.3390/ma14216642
Milillo L, Cinone F, Lo Presti F, Lauritano D, Petruzzi M. The Role of Blood Clot in Guided Bone Regeneration: Biological Considerations and Clinical Applications with Titanium Foil. Materials. 2021; 14(21):6642. https://doi.org/10.3390/ma14216642
Chicago/Turabian StyleMilillo, Lucio, Fabrizio Cinone, Federico Lo Presti, Dorina Lauritano, and Massimo Petruzzi. 2021. "The Role of Blood Clot in Guided Bone Regeneration: Biological Considerations and Clinical Applications with Titanium Foil" Materials 14, no. 21: 6642. https://doi.org/10.3390/ma14216642
APA StyleMilillo, L., Cinone, F., Lo Presti, F., Lauritano, D., & Petruzzi, M. (2021). The Role of Blood Clot in Guided Bone Regeneration: Biological Considerations and Clinical Applications with Titanium Foil. Materials, 14(21), 6642. https://doi.org/10.3390/ma14216642






