Chitosan Biocomposites for the Adsorption and Release of H2S
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Materials Characterization
2.3. Hydrogen Sulphide Adsorption Studies
2.4. Hydrogen Sulphide Release in Aqueous Solution

2.5. In vitro Cytotoxicity Studies
3. Results
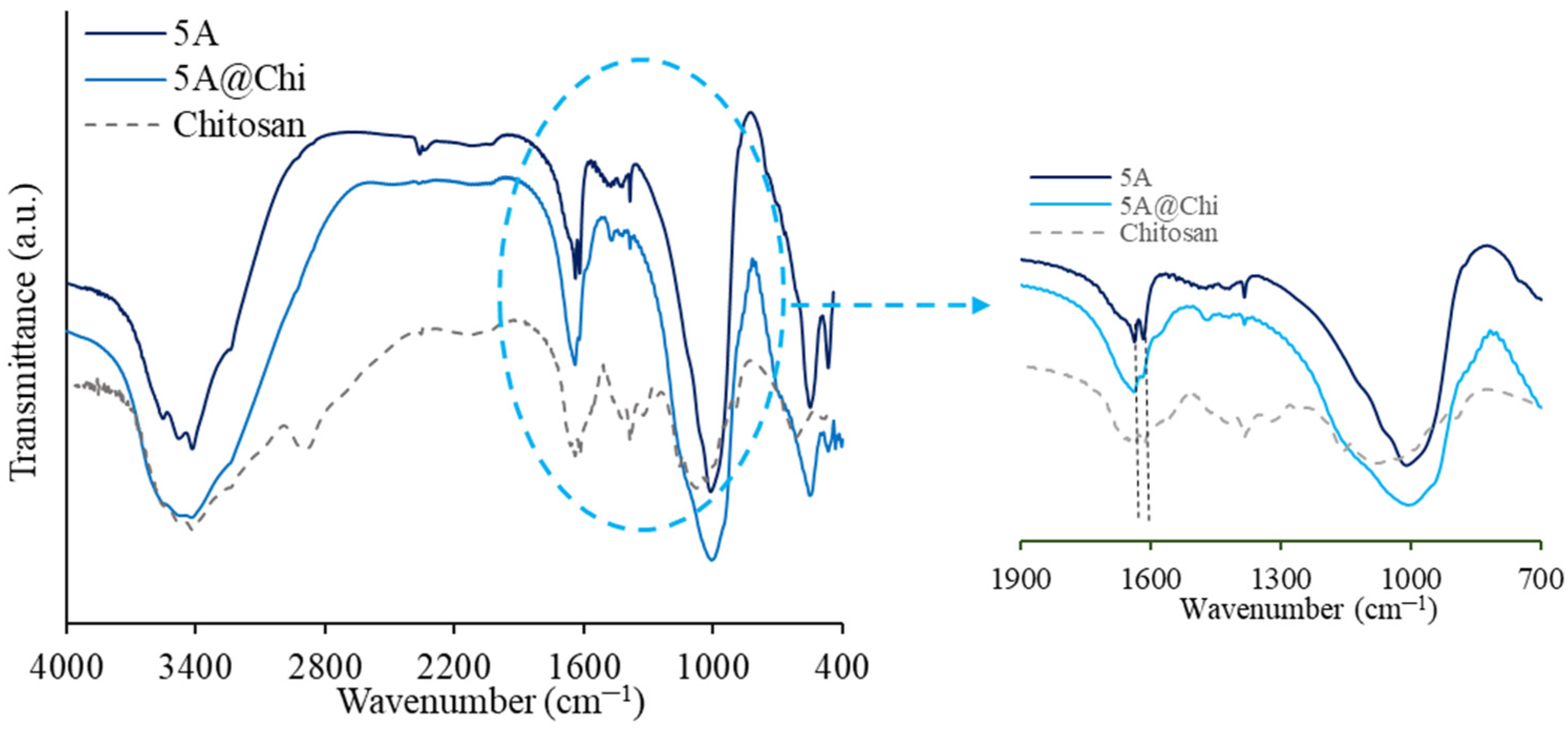
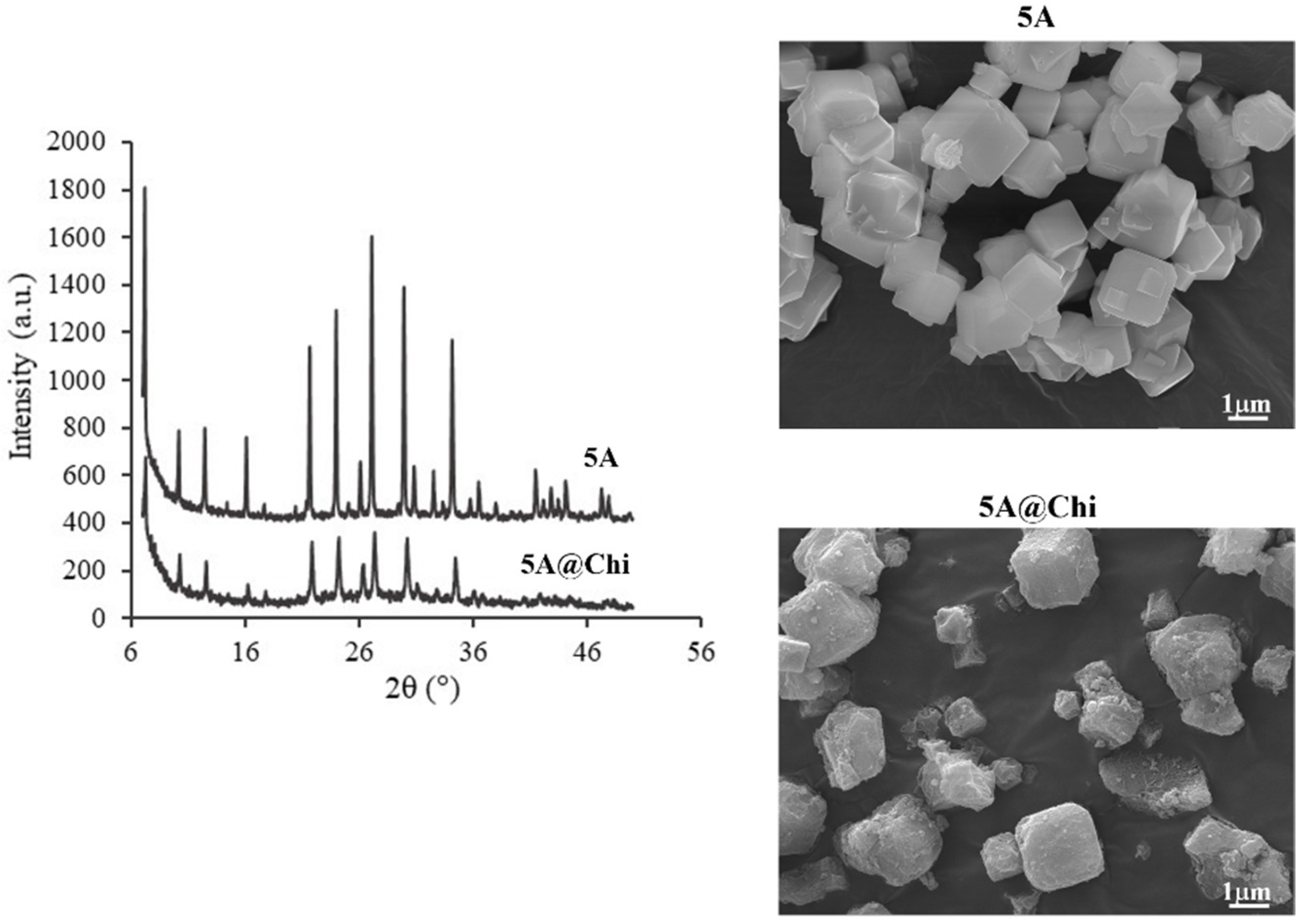
3.1. Characterization of the Materials
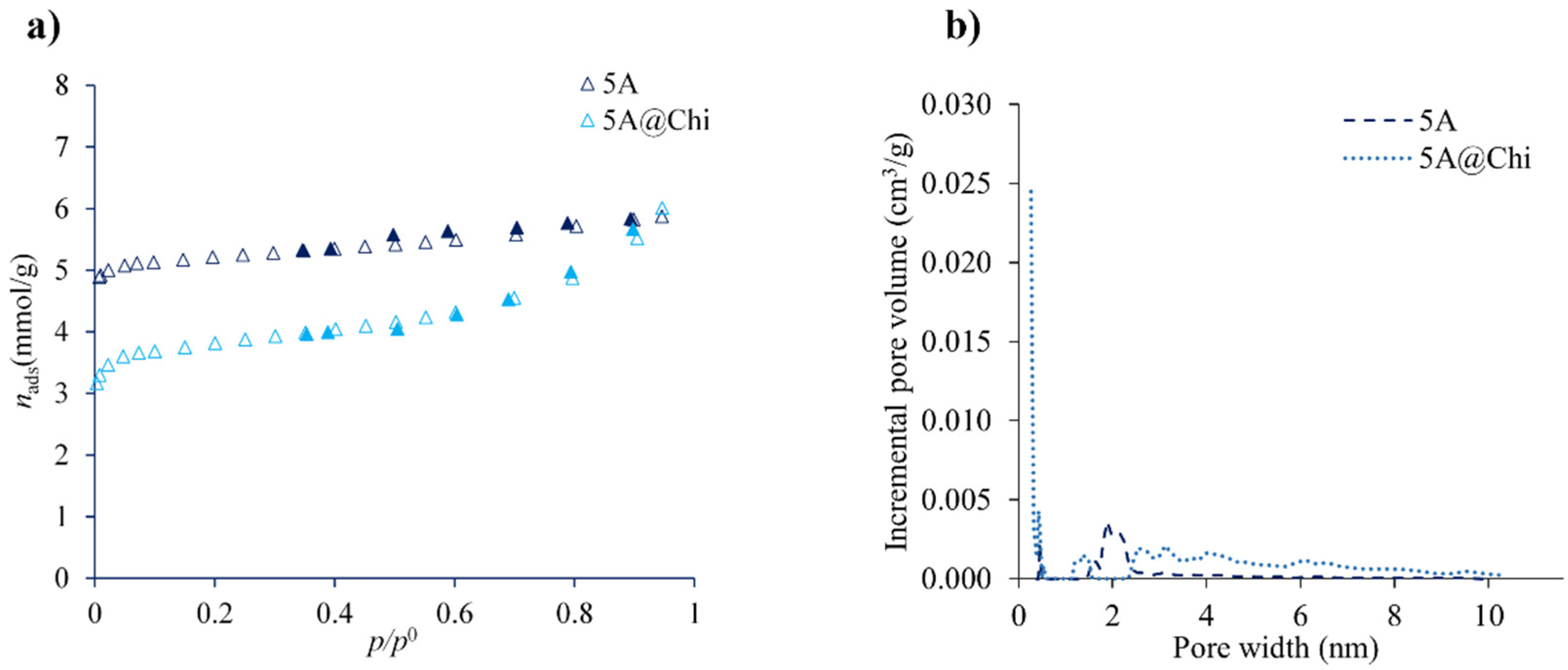
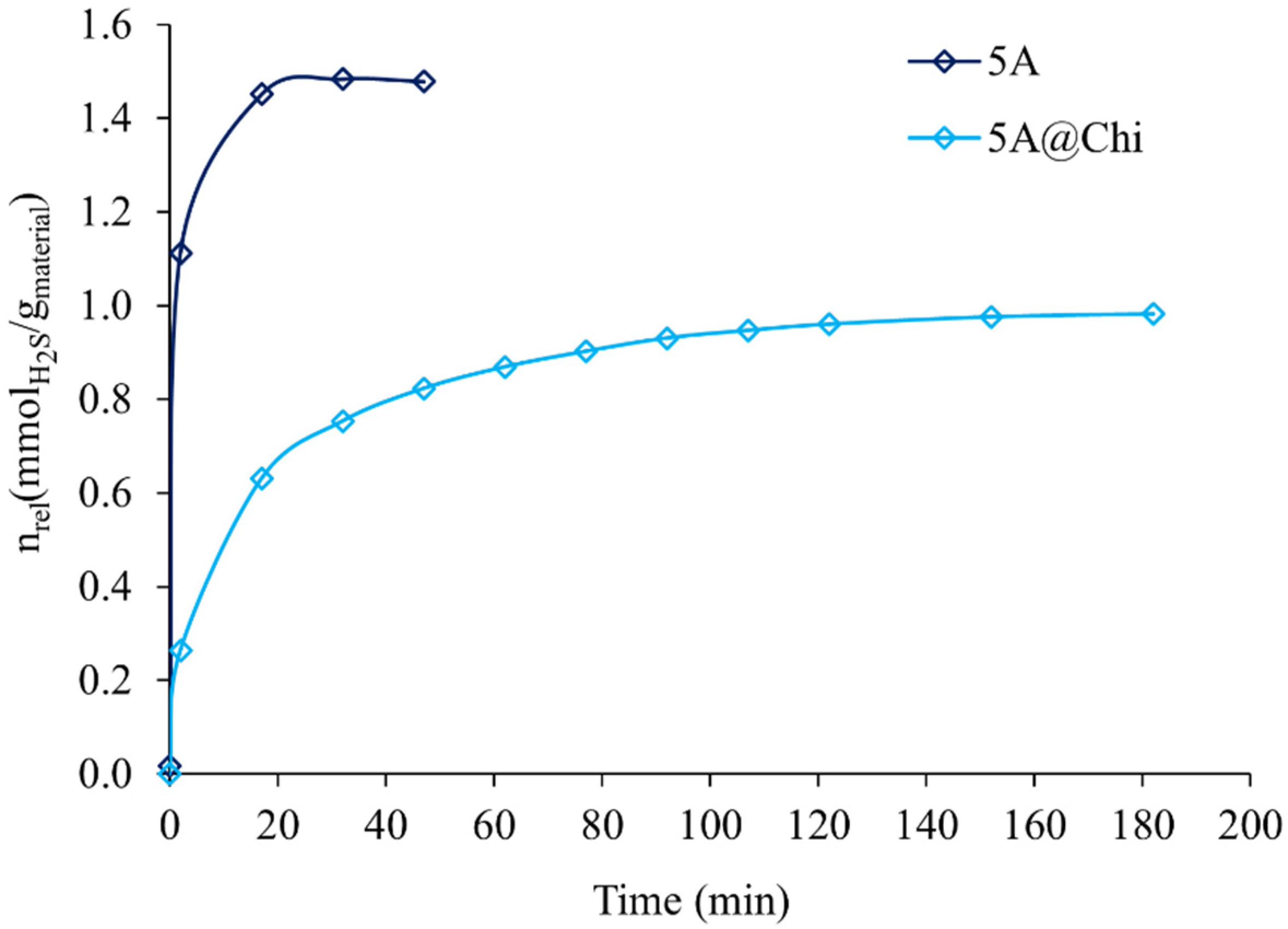
3.2. Hydrogen Sulphide Adsorption Isotherms
3.3. Hydrogen Sulphide Release in Aqueous Solution
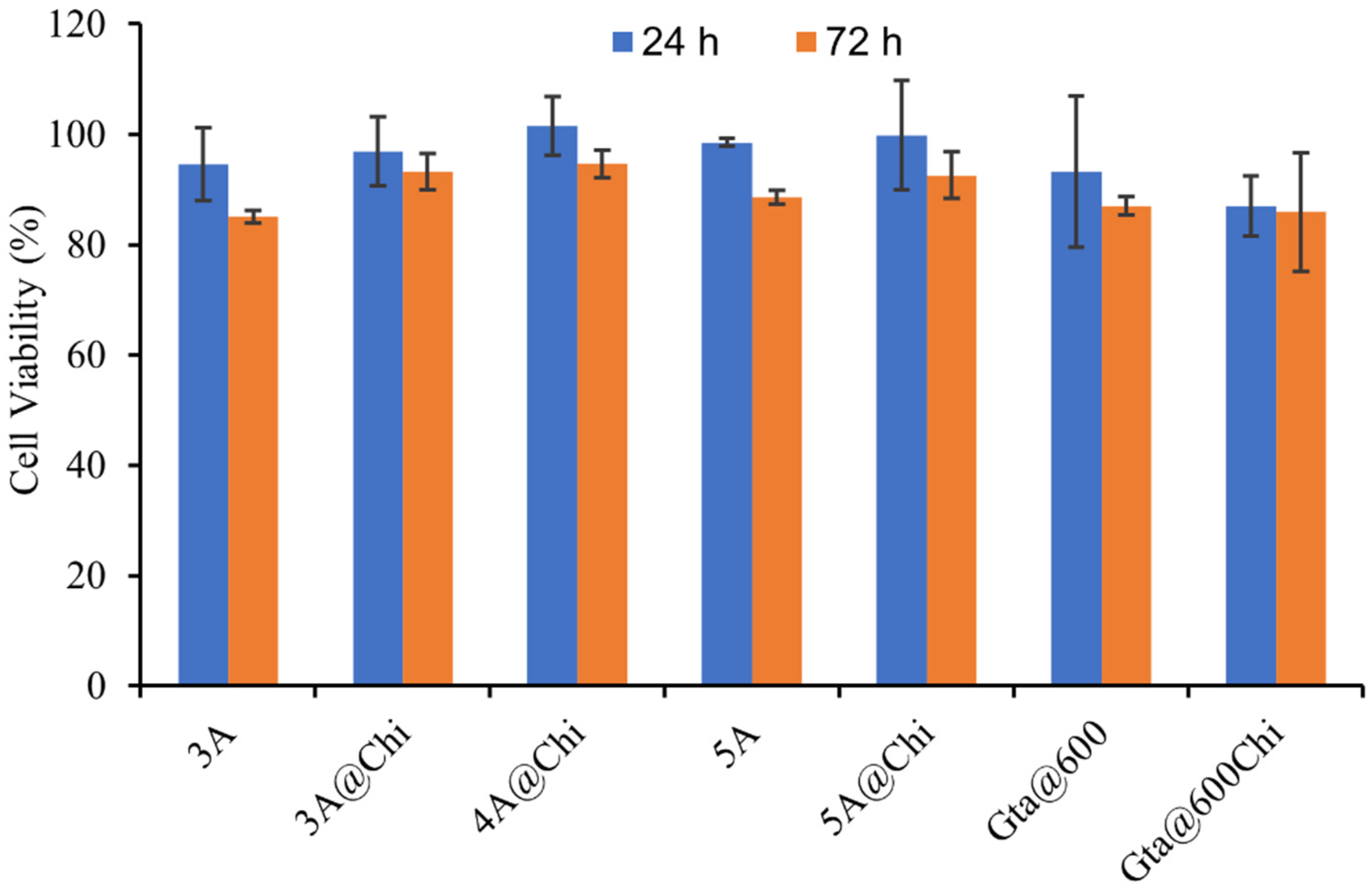
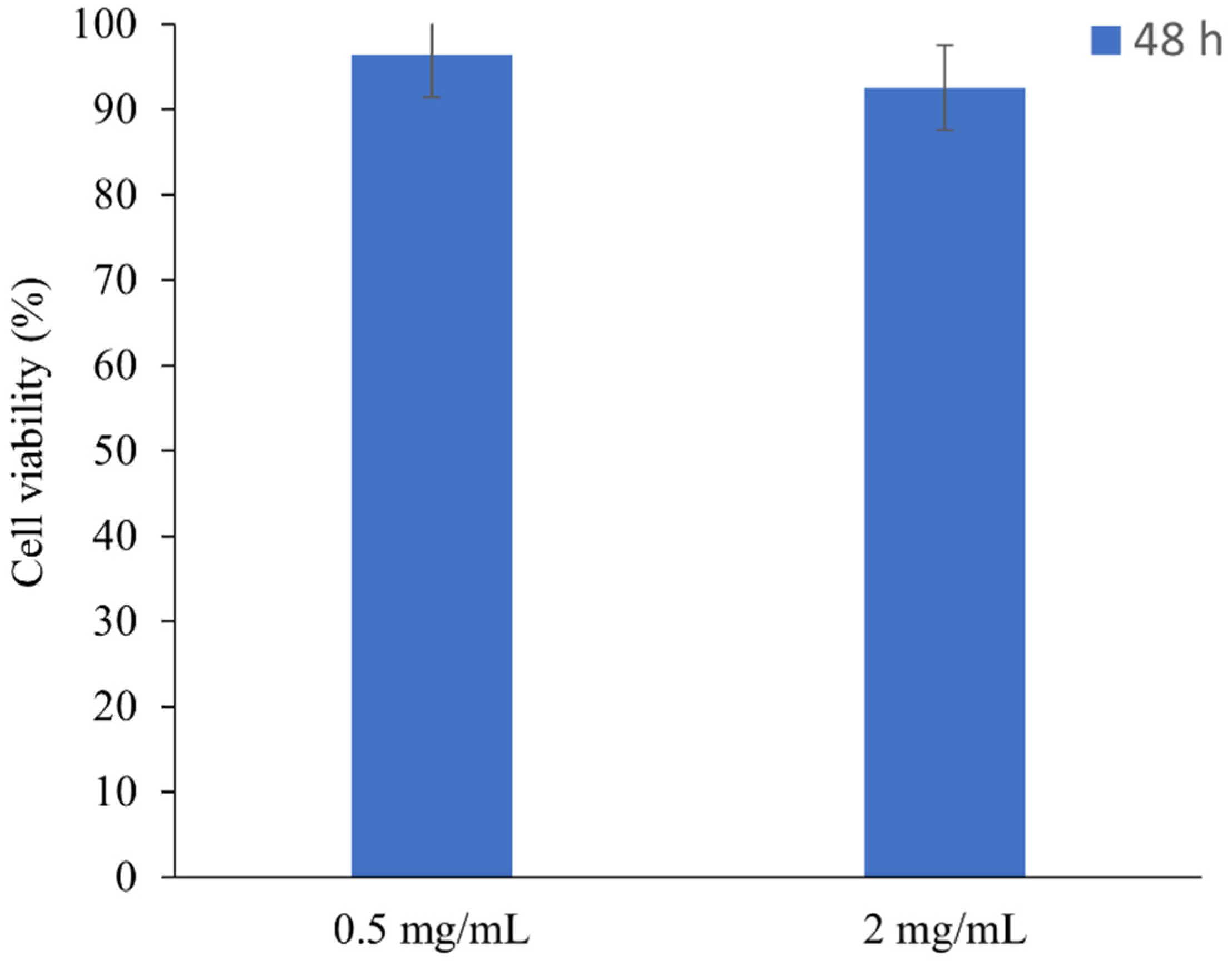
3.4. Cytotoxicity Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, R.P. Macroscope: A Short History of Hydrogen Sulfide. Am. Sci. 2010, 98, 6–9. [Google Scholar]
- Snyder, J.W.; Safir, E.F.; Summerville, G.P.; Middleberg, R.A. Occupational fatality and persistent neurological sequelae after mass exposure to hydrogen sulfide. Am. J. Emerg. Med. 1995, 13, 199–203. [Google Scholar] [CrossRef]
- Milby, T.H.; Baselt, R.C. Hydrogen Sulfide Poisoning: Clarification of Some Controversial Issues. J. Ind. Med. 1999, 35, 192–195. [Google Scholar] [CrossRef]
- Warenycia, M.W.; Goodwin, L.R.; Benishin, C.G.; Reiffenstein, R.J.; Francom, D.M.; Taylor, J.D.; Dieken, F.P. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem. Pharmacol. 1989, 38, 973–981. [Google Scholar] [CrossRef]
- Tabe-Mohammadi, A. A Review of the Applications of Membrane Separation Technology in Natural Gas Treatment. Sep. Sci. Technol. 1999, 34, 2095–2111. [Google Scholar] [CrossRef]
- Savage, J.C.; Gould, D.H. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1990, 526, 540–545. [Google Scholar] [CrossRef]
- Goodwin, L.R.; Francom, D.; Dieken, F.P.; Taylor, J.D.; Warenycia, M.W.; Reiffenstein, R.J.; Dowling, G. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: Postmortem studies and two case reports. J. Anal. Toxicol. 1989, 13, 105–109. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Yu, B.; De La Cruz, L.K.; Choudhury, M.R.; Anifowose, A.; Wang, B. Toward hydrogen sulfide based therapeutics: Critical drug delivery and developability issues. Med. Res. Rev. 2018, 38, 57–100. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Matson, J.B. Gasotransmitter delivery via self-assembling peptides: Treating diseases with natural signaling gases. Adv. Drug Deliv. Rev. 2017, 110–111, 137–156. [Google Scholar] [CrossRef]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Carné-Sánchez, A.; Carmona, F.J.; Kim, C.; Furukawa, S. Porous materials as carriers of gasotransmitters towards gas biology and therapeutic applications. Chem. Commun. 2020, 56, 9750–9766. [Google Scholar] [CrossRef]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen sulfide (H2S) releasing agents: Chemistry and biological applications. Chem. Commun. 2014, 50, 11788–11805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Pugliesi, I.; Barresi, E.; Nesi, G.; Rapposelli, S.; Taliana, S.; Da Settimo, F.; et al. Arylthioamides as H2S Donors: L-Cysteine-Activated Releasing Properties and Vascular Effects in Vitro and in Vivo. ACS Med. Chem. Lett. 2013, 4, 904–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, J.L.; Vong, L.; McKnight, W.; Dicay, M.; Martin, G.R. Endogenous and Exogenous Hydrogen Sulfide Promotes Resolution of Colitis in Rats. Gastroenterology 2009, 137, 569–578.e1. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.L.; Pinto, R.V.; Carvalho, S.; Antunes, F.; Pires, J. Emerging nitric oxide and hydrogen sulphide releasing carriers for skin wound healing therapy. ChemMedChem 2021. [Google Scholar] [CrossRef]
- Mahmodi, G.; Zarrintaj, P.; Taghizadeh, A.; Taghizadeh, M.; Manouchehri, S.; Dangwal, S.; Ronte, A.; Ganjali, M.R.; Ramsey, J.D.; Kim, S.J.; et al. From microporous to mesoporous mineral frameworks: An alliance between zeolite and chitosan. Carbohydr. Res. 2020, 489, 107930. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Ouyang, J.; Yang, H.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312. [Google Scholar] [CrossRef]
- Płaza, A.; Kołodyńska, D.; Hałas, P.; Gęca, M.; Franus, M.; Hubicki, Z. The zeolite modified by chitosan as an adsorbent for environmental applications. Adsorpt. Sci. Technol. 2017, 35, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.C.; Pinto, R.; Carvalho, S.; Mafra, L.; Rocha, J.; Pinto, M.; Antunes, F.; Pires, J. Storage and delivery of H2S by microporous and mesoporous materials. Microporous Mesoporous Mater. 2021, 320, 111093. [Google Scholar] [CrossRef]
- Batista, M.K.S.; Mestre, A.S.; Matos, I.; Fonseca, I.M.; Carvalho, A.P. Biodiesel production waste as promising biomass precursor of reusable activated carbons for caffeine removal. RSC Adv. 2016, 6, 45419–45427. [Google Scholar] [CrossRef]
- Kumar, S.; Bera, R.; Das, N.; Koh, J. Chitosan-based zeolite-Y and ZSM-5 porous biocomposites for H2 and CO2 storage. Carbohydr. Polym. 2020, 232, 115808. [Google Scholar] [CrossRef] [PubMed]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids—Principles, Methodology and Applications, 1st ed.; Academic Press: London, UK, 1999; ISBN 9780080526010. [Google Scholar]
- ISO [International Organization for Standardization]. Determination of the Specific Surface Area of Solids by Gas Adsorption—BET Method (ISO 9277:2010(E)); ISO: Geneva, Switzerland, 2010; Volume 9277, 30p. [Google Scholar]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications: Second Edition; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780080970356. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, K. The Virial Coefficients of Gases. A Critical Compilation; Von Dymond, J.H., Smith, E.B., Eds.; (Oxford Science Research Papers No. 2); Clarendon Press; Oxford University Press: London, UK, 1969. [Google Scholar]
- Li, Z.G. Quantification of hydrogen sulfide concentration using methylene blue and 5,5′-dithiobis(2-nitrobenzoic acid) methods in plants. Methods Enzymol. 2015, 554, 101–110. [Google Scholar] [CrossRef]
- Wheatley, P.S.; Butler, A.R.; Crane, M.S.; Fox, S.; Xiao, B.; Rossi, A.G.; Megson, I.L.; Morris, R.E. NO-releasing zeolites and their antithrombotic properties. J. Am. Chem. Soc. 2006, 128, 502–509. [Google Scholar] [CrossRef]
- Menazea, A.A.; Eid, M.M.; Ahmed, M.K. Synthesis, characterization, and evaluation of antimicrobial activity of novel Chitosan/Tigecycline composite. Int. J. Biol. Macromol. 2020, 147, 194–199. [Google Scholar] [CrossRef]
- Sarwar, A.; Katas, H.; Samsudin, S.N.; Zin, N.M. Regioselective sequential modification of chitosan via azide-alkyne click reaction: Synthesis, characterization, and antimicrobial activity of chitosan derivatives and nanoparticles. PLoS ONE 2015, 10, e0123084. [Google Scholar] [CrossRef] [Green Version]
- Hong, P.-Z.; Li, S.-D.; Ou, C.-Y.; Li, C.-P.; Yang, L.; Zhang, C.-H. Thermogravimetric analysis of chitosan. J. Appl. Polym. Sci. 2007, 105, 547–551. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H.; Kaczmarek-Kędziera, A. Effect of side substituents on thermal stability of the modified chitosan and its nanocomposites with magnetite. J. Therm. Anal. Calorim. 2016, 124, 1267–1280. [Google Scholar] [CrossRef] [Green Version]
- Moussout, H.; Aazza, M.; Ahlafi, H. Handbook of Chitin and Chitosan; Gopi, S., Thomas, A.P.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 139–174. ISBN 9780128179680. [Google Scholar]
- Kusumaningtyas, R.D. Effect of zeolite 4A on water concentration in the system of the esterefication reaction of acetic acid with 1-butanol. Indones. J. Chem. 2006, 6, 132–137. [Google Scholar] [CrossRef]
- Yotani, T.K.; Himotsuma, N.S.; Akui, S.K.; Nanotech, B. FTIR-ATR Study of the Surface of a Tubular Zeolite NaA Membrane. Anal. Chem. 2006, 22, 325–327. [Google Scholar] [CrossRef] [Green Version]
- Asokan, V.; Kosinski, P.; Skodvin, T.; Myrseth, V. Characterization of carbon black modified by maleic acid. Front. Mater. Sci. 2013, 7, 302–307. [Google Scholar] [CrossRef]
- Cheung, O.; Hedin, N. Zeolites and related sorbents with narrow pores for CO2 separation from flue gas. RSC Adv. 2014, 4, 14480–14494. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.S.; Tsapatsis, M.; Siepmann, J.I. Hydrogen Sulfide Capture: From Absorption in Polar Liquids to Oxide, Zeolite, and Metal-Organic Framework Adsorbents and Membranes. Chem. Rev. 2017, 117, 9755–9803. [Google Scholar] [CrossRef] [PubMed]
- CRC. Handbook of Chemistry and Physics, 97th ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1498754287. [Google Scholar]
- Starke, A.; Pasel, C.; Bläker, C.; Eckardt, T.; Zimmermann, J.; Bathen, D. Impact of Na+ and Ca2+ Cations on the Adsorption of H2S on Binder-Free LTA Zeolites. Adsorpt. Sci. Technol. 2021, 2021, 5531974. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, Y.; Xian, M.; Wang, Q. Biological thiols-triggered hydrogen sulfide releasing microfibers for tissue engineering applications. Acta Biomater. 2015, 27, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Yi, L.; Chaochao, H.; Jianming, K.; Jingjing, Y.; Zecong, X.; Jingjing, Z.; Anqi, C.; Xiaokun, L.; Jian, X.; et al. Novel H2S Releasing Nanofibrous Coating for In Vivo Dermal Wound Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 27474–27481. [Google Scholar] [CrossRef]
- Peluso, A.; Gargiulo, N.; Aprea, P.; Pepe, F.; Caputo, D. Nanoporous Materials as H2S Adsorbents for Biogas Purification: A Review. Sep. Purif. Rev. 2019, 48, 78–89. [Google Scholar] [CrossRef]


| Sample | N (%) | C (%) | H (%) | S (%) | O (%) |
|---|---|---|---|---|---|
| 3A@Chi | n.d. | 1.0 | 2.2 | n.d. | n.d |
| 4A@Chi | n.d. | 1.9 | 2.4 | n.d. | n.d |
| 5A@Chi | n.d. | 1.4 | 2.3 | n.d. | n.d |
| Gta@600Chi | 1.0 | 75 | 2.1 | 7 | 14.9 |
| Gta@600 | 0.9 | 80 | n.d. | 7 | 12.1 |
| Sample | Parent Material | Material with Chitosan | Chitosan (%) | ||
|---|---|---|---|---|---|
| 225 °C | 525 °C | 225 °C | 525 °C | ||
| 3A | 16.5 | 20.7 | 16.9 | 26.1 | 5.4 |
| 4A | 17 | 21.6 | 17.9 | 28.4 | 10.5 |
| 5A | 17.3 | 22.7 | 19.8 | 28.9 | 9.1 |
| Gta@600 | 9.1 | 53.1 | 8.3 | 75.6 | 23.3 |
| Sample | H2Sreleased (mmolH2S/gsample) | H2Sreleased (%) | tmax (min) |
|---|---|---|---|
| 3A | 1.60 | - | 17 |
| 3A@Chi | 0.02 | - | 17 |
| 4A | 1.35 | 43.8 | 17 |
| 4A@Chi | 0.03 | 18.8 | 17 |
| 5A | 1.48 | 28.3 | 17 |
| 5A@Chi | 0.98 | 31.8 | 120 |
| Gta@600 | 0.02 | 0.94 | 17 |
| Gta@600Chi | 0.012 | 2.28 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, M.; Pinto, M.L.; Antunes, F.; Pires, J.; Carvalho, S. Chitosan Biocomposites for the Adsorption and Release of H2S. Materials 2021, 14, 6701. https://doi.org/10.3390/ma14216701
Batista M, Pinto ML, Antunes F, Pires J, Carvalho S. Chitosan Biocomposites for the Adsorption and Release of H2S. Materials. 2021; 14(21):6701. https://doi.org/10.3390/ma14216701
Chicago/Turabian StyleBatista, Mary, Moisés L. Pinto, Fernando Antunes, João Pires, and Silvia Carvalho. 2021. "Chitosan Biocomposites for the Adsorption and Release of H2S" Materials 14, no. 21: 6701. https://doi.org/10.3390/ma14216701
APA StyleBatista, M., Pinto, M. L., Antunes, F., Pires, J., & Carvalho, S. (2021). Chitosan Biocomposites for the Adsorption and Release of H2S. Materials, 14(21), 6701. https://doi.org/10.3390/ma14216701