3.1. The Electrochemical Behavior of Ni3+ on Mo Electrode
3.1.1. Cyclic Voltammetry
Figure 1 shows the cyclic voltammetry test results of NaCl-CaCl
2 molten salt and NaCl-CaCl
2-LiNiO
2 molten salt when the scanning speed is 0.25 V/s. The dotted line in the figure is the cyclic voltammetry curve of NaCl-CaCl
2 molten salt. It can be seen that there are only the reduction peaks of Ca
2+ and Na
+ and the oxidation peaks of Ca and Na, indicating that the NaCl-CaCl
2 molten salt system is between −1.68 V and 0.37 V. No oxidation–reduction reaction occurs, and this range can be used as the scanning range after adding LiNiO
2 to the NaCl-CaCl
2 molten salt.
The theoretical decomposition voltages of NaCl and CaCl
2 at 1023 K are calculated from the data obtained from HSC Chemistry6.0 thermodynamic calculation software (Outokumpu, Finland). The theoretical decomposition voltages are −3.238 V and −3.325 V, respectively. Since the theoretical decomposition voltage difference between the two is relatively small, peak D and peak D′ are the overlapping reduction peak and oxidation peak of Na and Ca, respectively. Since Na is volatile, the peak D′ is smaller than the reduced peak D. Since CaCl
2 also has a certain degree of water absorption in molten salt, the formation of CaO causes the peak F to be relatively gentle. The formation of the peak F′ is mainly due to the oxidation of the elemental Ca [
22]. Peak E′ corresponds to the oxidation of Cl
−. The generation of Cl
2 changes the ion concentration around the molten salt and increases the reduction rate. The increase in the reduction rate increases the current to form peak E. The cyclic voltammetry curve shown by the solid line in the figure has multiple peaks, indicating that the electrochemical process of Ni
3+ on the Mo electrode is a multi-step reduction process.
Konishi et al. [
23] studied the process of preparing Fe-Dy alloy thin films by the molten salt electrochemical method. In this process, iron and dysprosium can form alloys. It can be seen from the electrochemical cyclic voltammetry curve that the reduction peak potential during the reaction is more positive than the deposition potential of dysprosium metal, so this peak is considered as an alloy peak. Kubota et al. [
24] studied the formation mechanism of Co-Gd alloy films by regulating the electrochemical reaction process of molten salt. Since cobalt and gadolinium can form an alloy, it is judged that the peak corresponding to the reduction current in the cyclic voltammetry curve is the alloy peak. The objects of this study, Ni and Mo, will also form an alloy. When using a Mo electrode, there are three reduction peaks in the cyclic voltammetry curve of Ni. The peak potential of peak A is more positive than the deposition potential of Ni
3+. It is judged that peak A is an alloy peak based on literature; there is no electron transfer and metal ion reduction in the process. Therefore, the reduction process of Ni
3+ when using a Mo electrode is a two-step reduction.
The cyclic voltammetry curve results of the Mo electrode measured at different sweep speeds in the NaCl-CaCl
2-LiNiO
2 system at 750 °C are shown in
Figure 2. Under certain sweep speed conditions, when the potential reverse scanning process reaches the first chemical reaction reduction potential, the current gradually increases with the change in the potential. With the continuous negative shift of the scanning potential, Ni
3+ is gradually enriched at the cathode. In this process, the charge transfer speed is slow, which causes the current to not continue to increase, and finally forms the limiting current.
As the electrochemical reaction progresses, the reduction products at the cathode diffuse into the molten salt slowly, occupying the reduction sites at the cathode. The lower concentration of reactive ions reduces the number of charge transfers and reduces the current, eventually forming peak C. The electrolysis process is mainly controlled by diffusion. When the reduction potential of the second chemical reaction is reached, the reduction process changes from the charge transfer process to the diffusion process, and peak B is formed. During the forward sweep of the potential, the Ni elemental substance is re-oxidized to form the peak B′. It can be seen from
Figure 2 that the peak potentials
Epc and
Epa of peaks B and B′ are affected by the scanning speed
v. The increase in the scanning speed makes the electrochemical reaction speed faster, promotes the rapid transfer of electric charge and increases the limiting current.
The Ni2+ in the molten salt is transported to the surface of the cathode through liquid phase mass transfer, and electrons are obtained between the two-phase interface of the cathode and the molten salt and, finally, metallic Ni is formed on the surface of the cathode. In the above process, the formation speed of metal Ni is faster, and the speed of mass transfer and electron transfer is slow, which leads to the phenomenon of electrode polarization. This phenomenon causes the electrode potential to move in a direction deviating from the equilibrium potential, which is manifested in that the peak potential of peak B moves in the negative direction, and the peak potential of peak B′ moves in the positive direction. According to the characteristics of the cyclic voltammetry curve of peak B, it is judged that peak B corresponds to a quasi-reversible reaction. The peak current of peak C increases with the increase in the scanning speed, there is no corresponding oxidation peak and the electrode reaction is judged to be an irreversible reaction.
In order to judge the control steps of the electrochemical reaction process, the
ipc-
v1/2 and
Epc-
v1/2 curves of peak C and peak B are drawn according to
Figure 2, as shown in
Figure 3. The current density value
ipc of peak C has a linear relationship with the square root of the scan rate
v1/2, and this process is mainly controlled by diffusion transfer. The current density value
ipc and peak potential of peak B are linearly related to the square root of the scan rate
v1/2, and the process is jointly controlled by diffusion and electron transfer.
3.1.2. Square Wave Voltammetry
In order to study the electrochemical reduction behavior of Ni
3+ in the NaCl-CaCl
2-LiNiO
2 molten salt system, the square wave voltammetry with higher resolution and sensitivity was used to measure at different frequencies. This method can effectively suppress the capacitive background current that often occurs in cyclic voltammetry at relatively high scanning speeds. The selected test temperature is 750 °C, and the frequency is 10–30 Hz. The curve is shown in
Figure 4.
There are three peaks A, C and B in the square wave voltammetry curve, and peak A is the alloy peak. The presence of peaks B and C indicates that Ni3+ forms a Ni elementary substance through a two-step electrochemical reduction process, which is consistent with the results of the cyclic voltammetry test.
In the square wave volt–ampere curve, the relationship between the half-width
W1/2 and the number of transferred electrons
n is expressed by Formula (1).
In the formula: R—molar gas constant (8.3145 J/mol/K), T—temperature (K), n—electron transfer number, F—Faraday’s constant (96,485 C/mol).
Generally speaking, this method is only effective for reversible reactions, and the reversibility can be judged by the linear relationship between the square root of frequency and the peak current density. Draw the relationship between the peak current density and the square root of frequency according to the square wave volt-ampere data in
Figure 4, as shown in
Figure 5. It can be seen from the figure that for peak B,
ipc,
Epc and
f1/2 are linear, but for peak C,
Epc and
f1/2 are not linear. At the same time, it is judged that the electrochemical reduction process corresponding to this peak is irreversible, so the number of transferred electrons can be calculated for peak B by Formula (1).
Peak B was fitted according to the square wave voltammetry, as shown in
Figure 6. The half-width of peak B is
W1/2 = 0.15, and the number of electrons transferred from peak B is
n = 2.06, calculated from Formula (1), which is about equal to
n = 2, and the number of electrons transferred from peak C is judged to be 1. Therefore, the reaction of Ni
3+ on the Mo electrode is shown in Equations (2) and (3):
It can be seen from the above analysis that the electrochemical reduction process of Ni3+ on the Mo electrode is divided into two steps, which is consistent with the results of the cyclic voltammetry curve test.
3.1.3. Chronoamperometry
Figure 7 shows the
I-t curve of the Mo electrode in the molten salt system of NaCl-CaCl
2-LiNiO
2 at 750 °C under different potential tests. Due to the electric double layer charging behavior between the electrode and the electrolyte, the initial current value in the figure is relatively large. Under different potential conditions, the initial current is proportional to the potential.
In the potential range of −0.6–−0.7 V, no obvious potential transition occurs. The current step A at −0.8 V indicates that Ni3+ has electrochemical reduction behavior in the potential range of −0.7–−0.8 V, corresponding to the reduction step of Ni3+ → Ni2+. With the change in potential, the current step B at −1.0 V indicates that Ni2+ has electrochemical reduction behavior in the range of −0.9–−1.0 V, corresponding to the reduction step of Ni2+ → Ni.
3.1.4. Open Circuit Chronopotentiometry
In order to further study the electrochemical reduction behavior of Ni
3+ on the Mo electrode in the NaCl-CaCl
2-LiNiO
2 molten salt system, the open-circuit chronopotentiometry was used for determination. This method records the potential difference between the working electrode and the reference electrode after the electrode is charged for a short period of time, and the result is shown in
Figure 8.
According to the chronocurrent curve shown in
Figure 7, the potential corresponding to the two-step oxidation process of nickel can be roughly judged. In the chronopotential curve shown in
Figure 8, the plateau corresponding to −0.9 V is more obvious. For differentiation, consider part of the data in the figure. The result is shown in
Figure 8a. When
t = 16.5, the slope is the smallest, indicating that the curve has reached the smoothest stage, and the corresponding potential is −0.76 V. This process is consistent with the appearance mechanism of peak A in the cyclic voltammetry curve. The plateau near −0.90 V exists for a short time, indicating that as the potential increases negatively, the reduction rate increases, and the Ni
2+ concentration in the molten salt decreases with the rapid progress of the reaction. This process is consistent with the appearance mechanism of peak B in the cyclic voltammetry curve. According to the above analysis, in the NaCl-CaCl
2-LiNiO
2 molten salt system, the electrochemical reduction process of Ni
2+ on the Mo electrode is Ni
3+ → Ni
2+ → Ni.
3.3. Preparation of Nickel by Electrolysis of LiNiO2 with Molten Salt Constant Current
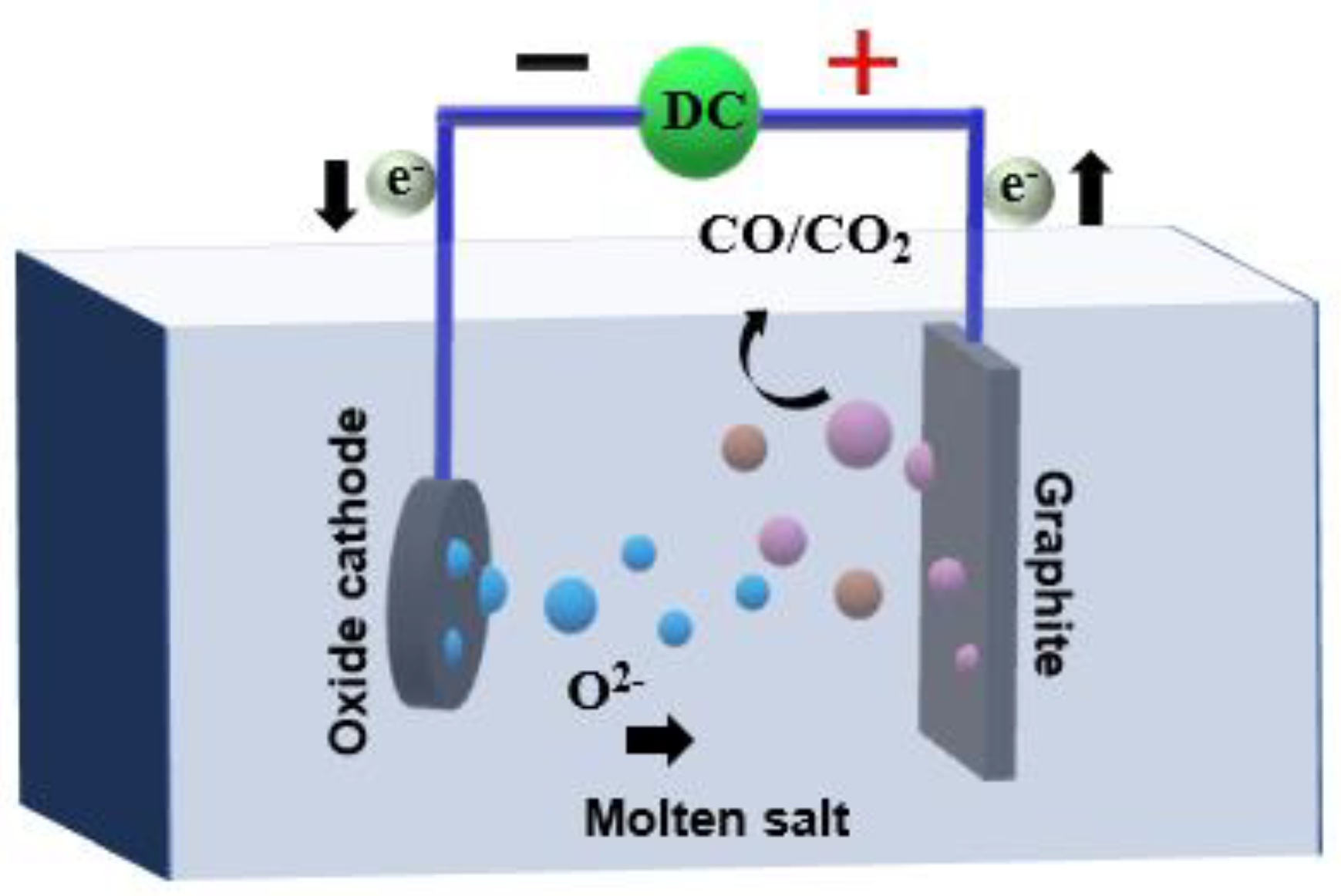
Constant current electrolysis is used to study the reduction process of Ni, and its intermediate products, combined with the reaction process, the interface diffusion and transfer of Ni3+ reduction, are analyzed, and the reduction mechanism of Ni electrolysis is explored. The electrolysis mechanism diagram is shown in
Figure 13. I have confirmed.
Under the condition of 750 °C and 10 h of electric deoxidation time, this study selects different currents to recover and prepare metallic nickel. The products of 10 h deoxygenation at different currents are shown in
Figure 14a. Under the conditions of 0.01 A and 10 h, Ni and NiO were mainly formed on the surface of the product. Although Ni and NiO also existed inside the product, there was more NiO than the surface. The above phenomenon shows that under this current condition, the electrochemical reaction continuously extends from the outside to the inside along the three-phase interface (3 PIs), and this behavior causes part of the NiO inside the product to fail to continue to be reduced.
In addition, the presence of NiO also proves again that there is a two-step electrochemical reduction behavior of Ni
3+ in LiNiO
2. When the current is greater than or equal to 0.03 A, the products after 10 h are all Ni elemental. It can be seen that under the same deoxygenation time condition, a larger current can promote the reduction reaction, so that NiO can continue to be reduced to a Ni elementary substance. It can be seen that under the same deoxygenation time condition, a larger current can promote the reduction reaction, so that NiO can continue to be reduced to a Ni elementary substance. It can be seen from
Figure 14b that different currents cause differences in the degree of electron accumulation at the electrodes, resulting in different starting positions of the curve. In addition, comparing the time range corresponding to each reaction platform on the three curves in the figure, the first platform in the
U-
t curve measured under the condition of 0.07 A current corresponds to about 4 h, while the second platform corresponds to the duration of “shorter”. The above phenomenon shows that in the first electrochemical reduction process, more NiO and a small amount of Ni element are mainly formed. Therefore, in the second reduction process, the Ni elementary substance is formed faster. The current condition of 0.15 A promotes the progress of electrochemical reduction, so that the reduction reaction is concentrated in the second step of the reaction process. Under the condition of 0.13 A, the whole reaction process is relatively stable, and there is a good transition time. Under the conditions of an electrolysis time of 10 h and currents of 0.07 A, 0.13 A and 0.15 A, this study calculates the actual charge and the theoretical charge according to Equations (8) and (9), and finally calculates the current efficiency of the three, as shown in the
Table 1 shown.
In the formula, n is the amount of substance in the product under theoretical conditions, z is the number of transferred electrons, t is time (s), and F is Faraday’s constant (96,485 C/mol).
It can be seen from the table that as the current increases, the current efficiency decreases. This situation is because the increase in current causes the actual charge to increase, while the theoretical charge has a relatively small change, which eventually leads to a gradual decrease in current efficiency. At 0.13 A and 750 °C, 0.7 g lithium nickelate was subjected to constant current deoxygenation studies for 0.5 h, 4 h and 6 h, respectively. Further study on the influence of time on the electrochemical reduction process through product analysis is needed. The result is shown in
Figure 15.
The
U-
t curve is shown in
Figure 15a. In the reduction process of Ni
3+, different reduction stages correspond to different potential platforms. According to the XRD pattern of the 0.5 h product shown in
Figure 15b, it can be seen that the inside of the particles is mainly Ni and NiO, and most of the intermediate products Li
0.28Ni
0.72O, which is only caused by the slow extension of the three-phase interface during the reduction process. At this time, the black product inside the product is NiO and Li
0.28Ni
0.72O, and the steel gray product appears at the three-phase interface, which is metallic Ni. As the reaction progresses, O
2− continuously ionizes from the LiNiO
2 matrix and concentrates near the cathode. At the same time, the produced Ni element adheres to the surface of the substrate as a conductor and further hinders the ionization of O
2− and the transfer of electrons. The above phenomena cause electrons to accumulate in the electric double layer, and eventually lead to a negative increase in potential. When the second step reduction potential is reached, a second platform corresponding to 4 h is formed in the figure. Combined with the XRD test results, it can be seen that the surface of the 4 h product is mainly Ni, and the internal components are mainly Ni and NiO. The black substance inside the product gradually decreases, indicating that, as time goes by, O
2− in Li
0.28Ni
0.72O is continuously removed and, finally, NiO is formed. This phenomenon also further confirms that Ni
3+ conducts electricity from the outside to the inside of the electrode surface along the three-phase interface. When the time was increased to 6 h, the NiO inside the product was completely deoxidized, and Ni
2+ was reduced to elemental Ni. As time continues to extend, there are no other oxidation–reduction reactions in the molten salt, and the potential ultimately remains in a relatively stable state. The atomic structure diagram is shown in
Figure 16.
Figure 17 is the SEM, EDS and surface scan detection spectrum of the 10 h electrolysis product under different multiple conditions. It can be seen that the metallic nickel has a honeycomb skeleton structure, and its content reaches 96.39%. The electrolytic metallic nickel is silver-white with metallic luster and magnetic.
XPS detection was performed on the electrolysis products after 4 h and 10 h, and the results are shown in
Figure 18 and
Figure 19, respectively. It can be seen from the XPS spectrum that elemental nickel appears in the product of electrolysis at 4 h. As the electrolysis time increases, more Ni
2+ is reduced to elemental Ni, and the peaks corresponding to elemental Ni in the XPS spectrum increase. Compared with the XPS spectrum after 4 h of electrolysis, the 2p peak of Ni after 10 h of electrolysis is weaker and wider, while the 2p peak of part of Ni
2+ is strengthened. This phenomenon is caused by the increase in oxides on the surface of the sample [
26].
3.4. The Behavior of Lithium under Constant Current Conditions
LiNiO
2 belongs to the trigonal crystal system. The oxygen atoms at position 6c are closely packed in cubic form to form a crystal framework, which occupy positions 3a and 3b, respectively. Li atoms and Ni atoms are located in alternate octahedral positions in the framework, with obvious layered structure, and the space group is R/3m. Due to the special electronic arrangement of Ni
2+, part of Ni
2+ existing in LiNiO
2 will occupy the position of Ni
3+. In order to maintain charge balance and the similar atomic radii of Ni
2+ and Li
+, the position of Li
+ will also be occupied by Ni
2+, and at the same time, during the electrolysis process, Ni
2+ will transform to Ni
3+ with a smaller radius, causing the structure to change, and the position of Li
+ will further lose the ability to insert lithium [
27,
28]. In addition, under the layered structure, van der Waals force and ionic bonds are used to maintain the interlayer and the inner layer, respectively. Because the van der Waals force is weak, the Li
+ in the layer is required to maintain the stability of the structure through electrostatic action. However, there is a big difference between the Li
+(1s
2) energy level and the O
2− energy level, while the Ni
3+(3d
7) and O
2−(2p
6) energy levels are relatively similar, which makes the electron cloud overlap between Li-O more than that between Ni–O. If it was smaller, the Li-O bond energy would eventually be weaker. Therefore, the Li–O bond will be preferentially broken during the electrolysis process, and Li
+ will escape from the Ni-O layer [
29].
In order to further study the electrochemical behavior of Li
+ in molten salt or Li
+ in cathode reduction particles, thermodynamic calculations of related reactions are carried out. Equation (10) shows that the Gibbs free energy of the reaction is negative. Therefore, the reduction in Li
+ will proceed with more difficulty. During the electrochemical scanning process, the redox peak of Li did not appear, which is consistent with the analysis of the cyclic voltammogram.
In this study, 0.7 g of LiNiO
2 powder was made into the cathode and smooth graphite flakes were used as the anode, and they were put into NaCl-CaCl
2 molten salt and electrolyzed at 750 °C with a constant current of 0.18 A for 10 h. After the electrolysis, XRD analysis was performed on the molten salt and the product near the cathode, and the result is shown in
Figure 20. It can be seen from
Figure 20 that the product is elemental Ni after electrolysis for 10 h under the condition of 0.18 A current. After electrolysis, there is LiCl in the XRD of the molten salt near the cathode. The above phenomenon shows that Li
+ in LiNiO
2 finally enters the molten salt. The constant cell voltage electrolysis in the two-electrode system further supports the conclusions obtained by cyclic voltammetry, square wave voltammetry, open circuit potential and chronoamperometry.