Process Parameter Optimization of a Polymer Derived Ceramic Coatings for Producing Ultra-High Gas Barrier
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Barrier Coatings
2.3. Characterisation of Films
2.3.1. Spectroscopic Analysis
2.3.2. Permeability Measurements
3. Results and Discussion
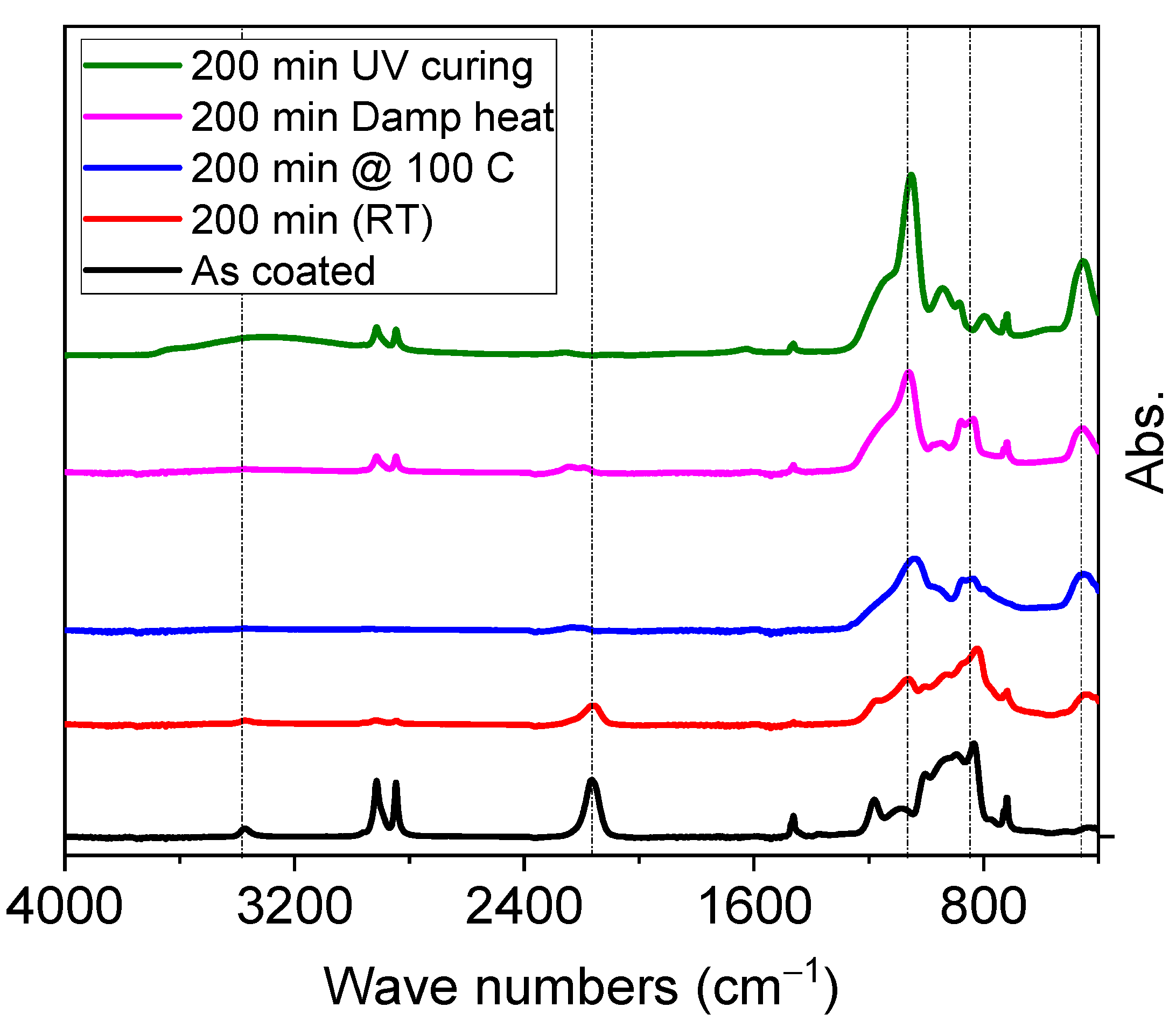
3.1. Various Treatment Methods for Curing Polysilazanes
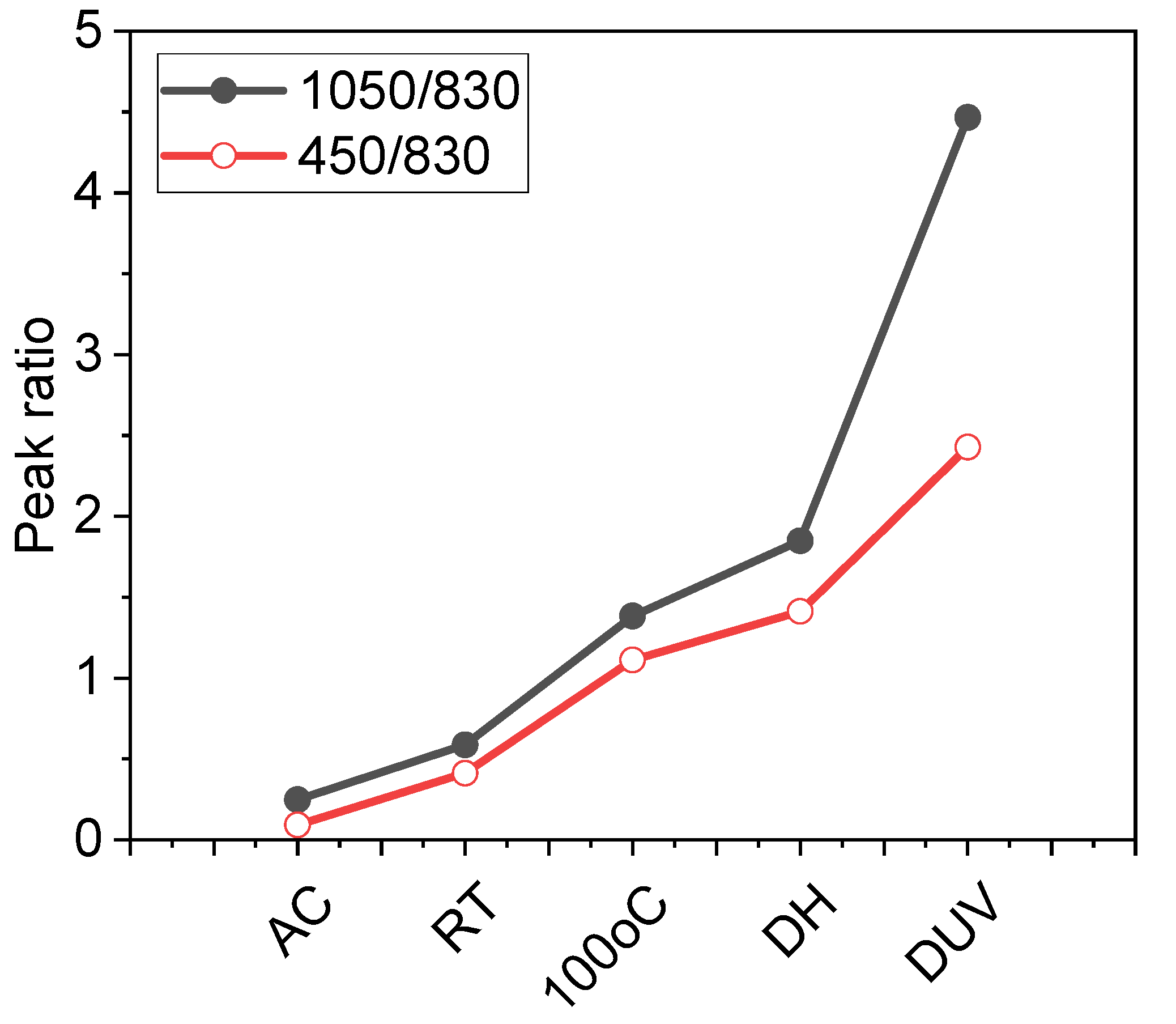
3.1.1. PHPS Treatment with Deep UV under Different Environments
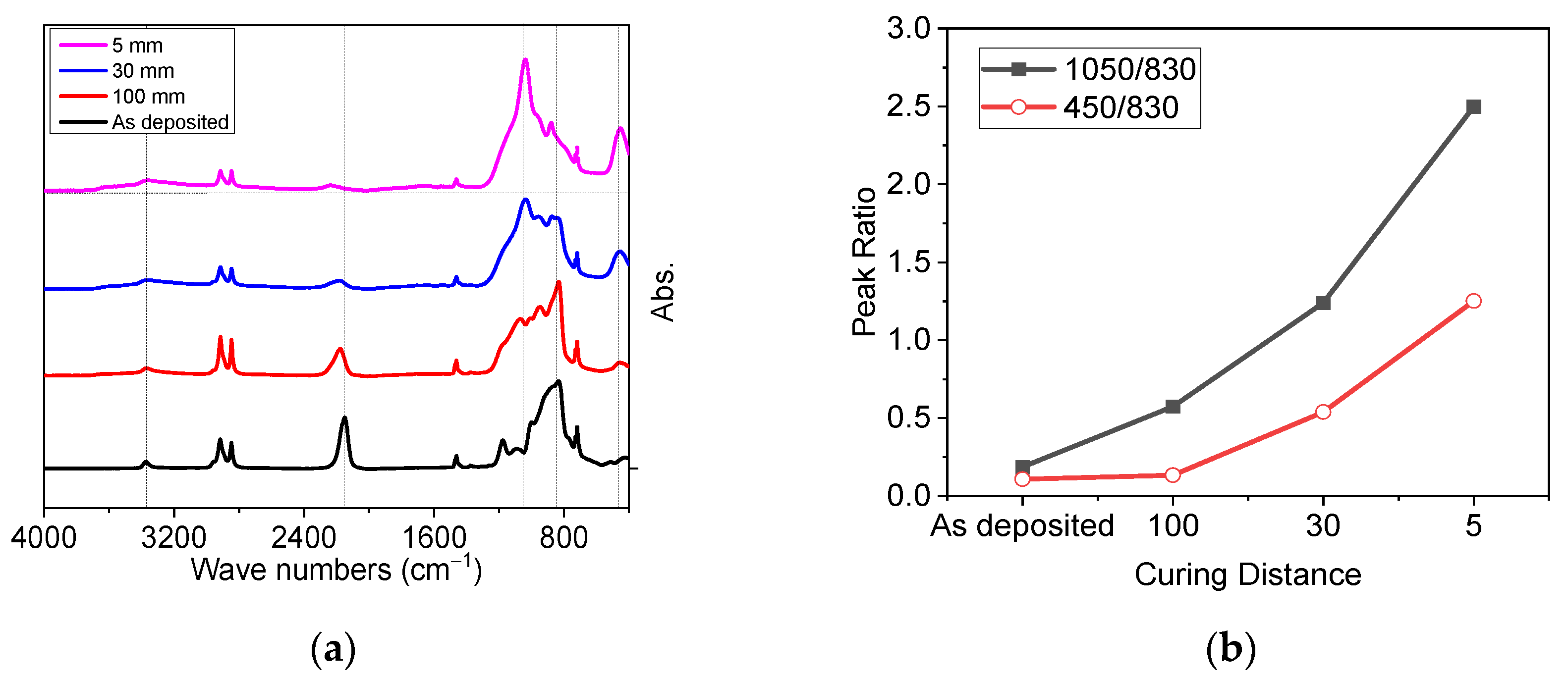
3.1.2. Optimizing the Distance for Faster Transformation of PHPS into Silica
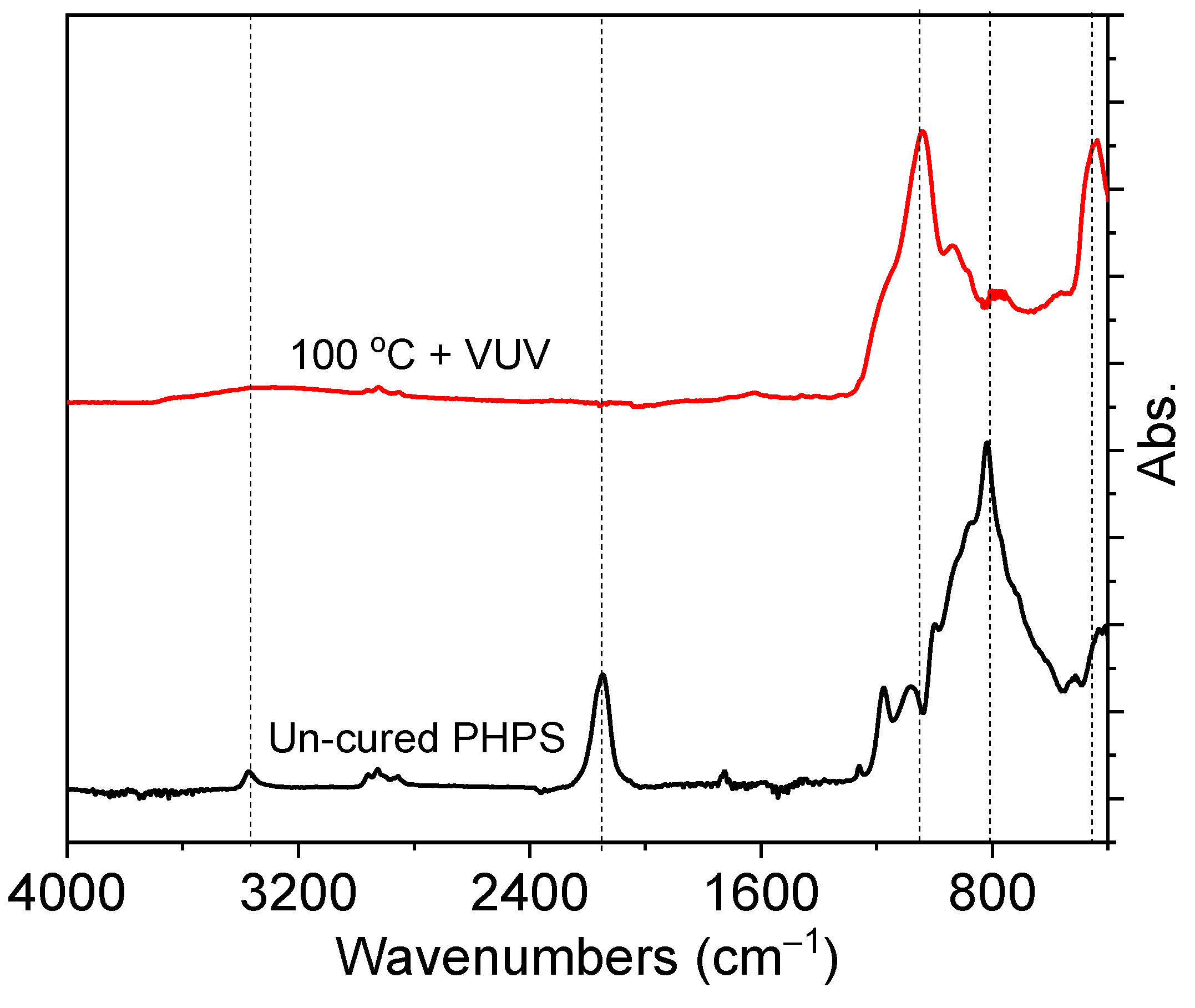
3.1.3. Fastest Curing
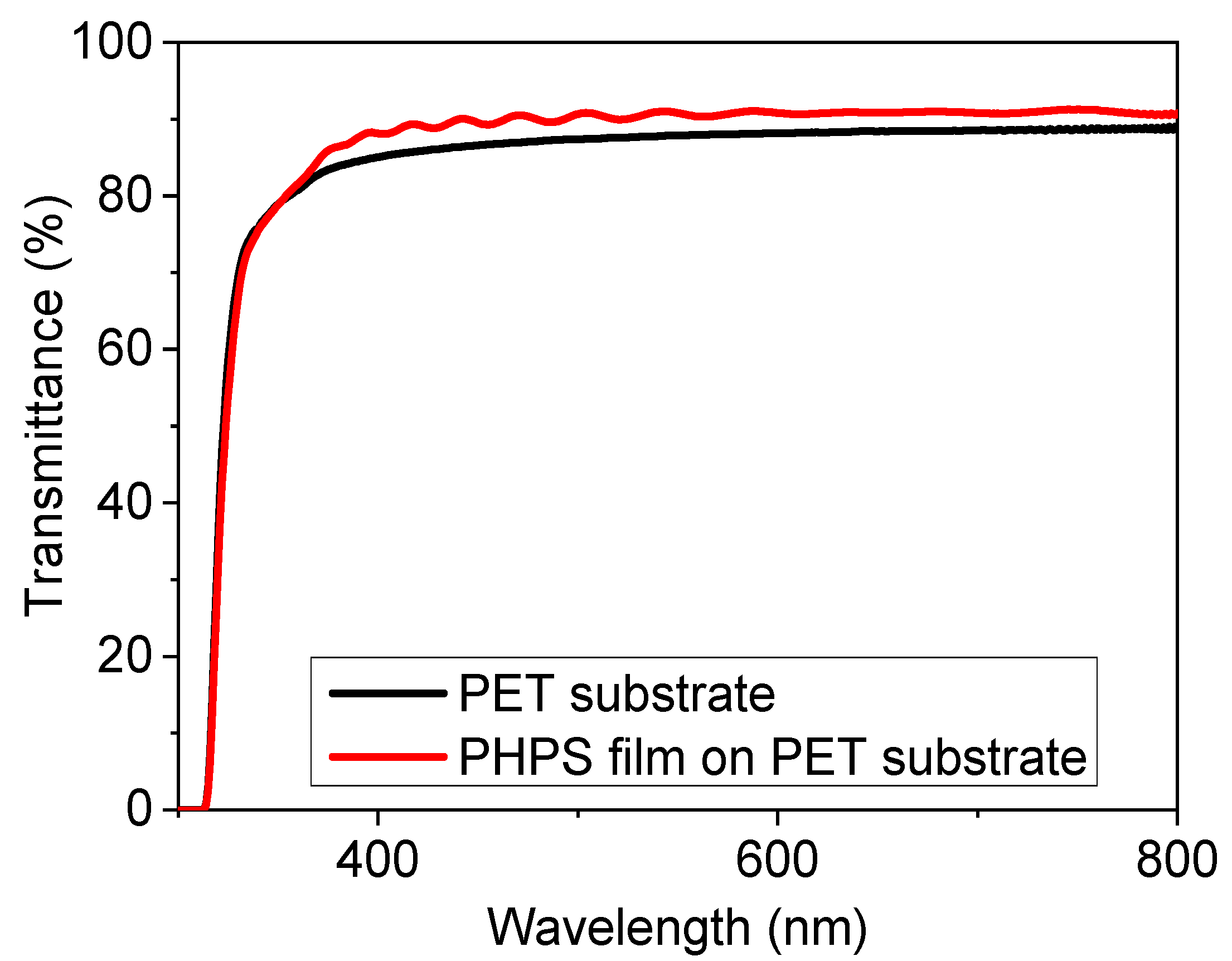
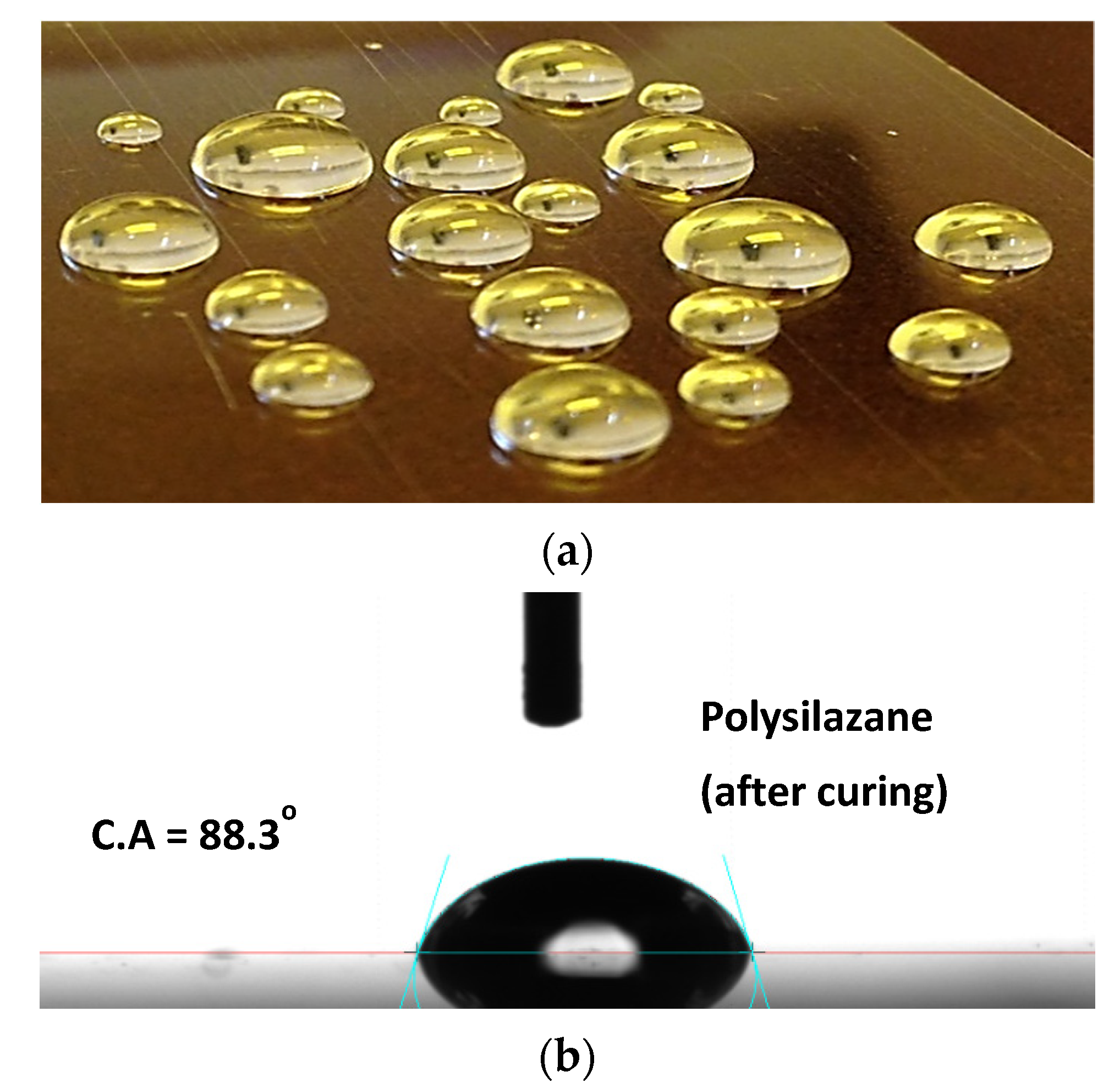
3.2. Transparency and Contact Angle Measurements of PHPS
3.3. Control of PHPS Film Thickness
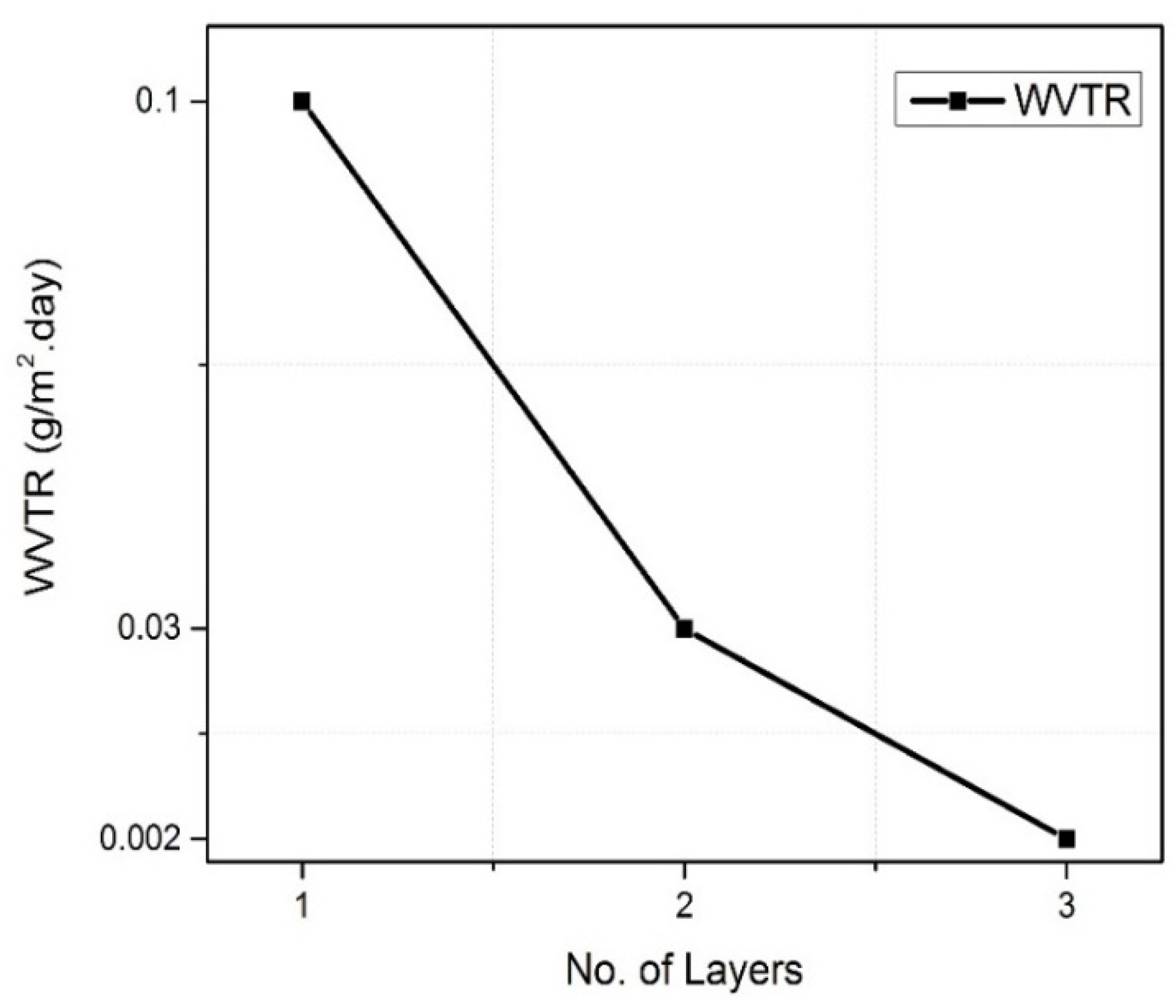
Barrier Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maisch, P.; Lucera, L.; Brabec, C.; Egelhaaf, H.-J. Flexible Carbon-Based Electronics: Flexible Solar Cells; Wiley: Hoboken, NJ, USA, 2018; ISBN 9783527341917. [Google Scholar]
- Brütting, W. Organic LEDs and solar cells united. Nat. Mater. 2019, 18, 432–433. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, K.; Meyer, J.; Riedl, T. Solution processed metal-oxides for organic electronic devices. J. Mater. Chem. C 2013, 1, 4796–4815. [Google Scholar] [CrossRef]
- Kielar, M.; Dhez, O.; Pecastaings, G.; Curutchet, A.; Hirsch, L. Long-Term Stable Organic Photodetectors with Ultra Low Dark Currents for High Detectivity Applications. Sci. Rep. 2016, 6, 39201. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Uddin, A. Progress in Stability of Organic Solar Cells. Adv. Sci. 2020, 7, 1903259. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Egelhaaf, H.; Brabec, C.J. Solution Coated Barriers for Flexible Electronics. In Organic Flexible Electronics, Fundamentals, Devices, and Applications; Cosseddu, P., Caironi, M., Eds.; Woodhead Publishing: Southston, UK, 2020; ISBN 9780128188903. [Google Scholar]
- Channa, I.A. Development of Solution Processed Thin Film Barriers for Encapsulating Thin Film Electronics Entwicklung von lösungsprozessierten Dünnschichtbarrieren für die Verpackung von Dünnschichtelektronik. Ph.D. Thesis, Friedrich Alexander University of Erlangen Nuremberg, Erlangen, Germany, 2019. [Google Scholar]
- Channa, I.A.; Distler, A.; Scharfe, B.; Feroze, S.; Forberich, K.; Lipovšek, B.; Brabec, C.J.; Egelhaaf, H.J. Solution processed oxygen and moisture barrier based on glass flakes for encapsulation of organic (opto-) electronic devices. Flex. Print. Electron. 2021, 6, 25006. [Google Scholar] [CrossRef]
- Channa, I.A.; Chandio, A.D.; Rizwan, M.; Shah, A.A.; Bhatti, J.; Shah, A.K.; Hussain, F.; Shar, M.A.; Alhazaa, A. Solution Processed PVB/Mica Flake Coatings for the Encapsulation of Organic Solar Cells. Materials 2021, 14, 2496. [Google Scholar] [CrossRef]
- Chandio, A.D.; Channa, I.A.; Rizwan, M.; Akram, S.; Javed, M.S.; Siyal, S.H.; Saleem, M.; Makhdoom, M.A.; Ashfaq, T.; Khan, S.; et al. Polyvinyl alcohol and nano-clay based solution processed packaging coatings. Coatings 2021, 11, 942. [Google Scholar] [CrossRef]
- Cho, T.Y.; Lee, W.J.; Lee, S.J.; Lee, J.H.; Ryu, J.; Cho, S.K.; Choa, S.H. Moisture barrier and bending properties of silicon nitride films prepared by roll-to-roll plasma enhanced chemical vapor deposition. Thin Solid Films 2018, 660, 101–107. [Google Scholar] [CrossRef]
- Jiang, P.-C.; Chow, Y.-T.; Chien, C.-W.; Chang, C.-H.-T.; Lin, C.-R. Silica Layer Used in Sensor Fabrication from a Low-Temperature Silane-Free Procedure. Chemosens 2021, 9, 32. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, C.; Li, M.; Zou, J.; Tao, H.; Peng, J.; Xu, M. Enhanced moisture barrier performance for ALD-encapsulated OLEDs by introducing an organic protective layer. J. Mater. Chem. C 2017, 5, 4017–4024. [Google Scholar] [CrossRef]
- Lu, Q.; Yang, Z.; Meng, X.; Yue, Y.; Ahmad, M.A.; Zhang, W.; Zhang, S.; Zhang, Y.; Liu, Z.; Chen, W. A Review on Encapsulation Technology from Organic Light Emitting Diodes to Organic and Perovskite Solar Cells. Adv. Funct. Mater. 2021, 31, 2100151. [Google Scholar] [CrossRef]
- Ahmad, J.; Bazaka, K.; Anderson, L.J.; White, R.D.; Jacob, M.V. Materials and methods for encapsulation of OPV: A review. Renew. Sustain. Energy Rev. 2013, 27, 104–117. [Google Scholar] [CrossRef]
- Hauch, J.A.; Schilinsky, P.; Choulis, S.A.; Rajoelson, S.; Brabec, C.J. The impact of water vapor transmission rate on the lifetime of flexible polymer solar cells. Appl. Phys. Lett. 2008, 93, 10–13. [Google Scholar] [CrossRef]
- Channa, I.A.; Distler, A.; Zaiser, M.; Brabec, C.J.; Egelhaaf, H. Thin Film Encapsulation of Organic Solar Cells by Direct Deposition of Polysilazanes from Solution. Adv. Energy Mater. 2019, 9, 1900598. [Google Scholar] [CrossRef]
- Prager, L.; Dierdorf, A.; Liebe, H.; Naumov, S.; Stojanović, S.; Heller, R.; Wennrich, L.; Buchmeiser, M.R. Conversion of Perhydropolysilazane into a SiOx Network Triggered by Vacuum Ultraviolet Irradiation: Access to Flexible, Transparent Barrier Coatings. Chem.—A Eur. J. 2007, 13, 8522–8529. [Google Scholar] [CrossRef] [PubMed]
- Prager, L.; Helmstedt, U.; Herrnberger, H.; Kahle, O.; Kita, F.; Münch, M.; Pender, A.; Prager, A.; Gerlach, J.W.; Stasiak, M. Photochemical approach to high-barrier films for the encapsulation of flexible laminary electronic devices. Thin Solid Films 2014, 570, 87–95. [Google Scholar] [CrossRef]
- Lukacs, A. Polysilazane precursors to advanced ceramics. Am Ceram Soc Bull 2007, 86, 9301–9306. [Google Scholar]
- Fedel, M.; Gómez, F.J.R.; Rossi, S.; Deflorian, F. Characterization of polyorganosilazane-derived hybrid coatings for the corrosion protection of mild steel in chloride solution. Coatings 2019, 9, 680. [Google Scholar] [CrossRef]
- Naganuma, T.; Kagawa, Y. Effect of particle size on light transmittance of glass particle dispersed epoxy matrix optical composites. Acta Mater. 1999, 47, 4321–4327. [Google Scholar] [CrossRef]
- Morlier, A.; Cros, S.; Garandet, J.-P.; Alberola, N. Thin gas-barrier silica layers from perhydropolysilazane obtained through low temperature curings: A comparative study. Thin Solid Films 2012, 524, 62–66. [Google Scholar] [CrossRef]
- Morlier, A.; Cros, S.; Garandet, J.-P.; Alberola, N. Structural properties of ultraviolet cured polysilazane gas barrier layers on polymer substrates. Thin Solid Films 2014, 550, 85–89. [Google Scholar] [CrossRef]
- Nakajima, K.; Uchiyama, H.; Kitano, T.; Kozuka, H. Conversion of Solution-Derived Perhydropolysilazane Thin Films into Silica in Basic Humid Atmosphere at Room Temperature. J. Am. Ceram. Soc. 2013, 96, 2806–2816. [Google Scholar] [CrossRef]
- Yamano, A.; Kozuka, H. Perhydropolysilazane-derived silica–polymethylmethacrylate hybrid thin films highly doped with spiropyran: Effects of polymethylmethacrylate on the hardness, chemical durability and photochromic properties. Thin Solid Films 2011, 519, 1772–1779. [Google Scholar] [CrossRef]
- Blankenburg, L.; Schrödner, M. Perhydropolysilazane derived silica for flexible transparent barrier foils using a reel-to-reel wet coating technique: Single- and multilayer structures. Surf. Coat. Technol. 2015, 275, 193–206. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, Z.; Luo, Y.; An, P.; Zhang, M.; Xu, C. Hydrophobic, transparent and hard silicon oxynitride coating from perhydropolysilazane: Silicon oxynitride coating from perhydropolysilazane. Polym. Int. 2015, 64, 971–978. [Google Scholar] [CrossRef]
- Rao, B.G.; Mukherjee, D.; Reddy, B.M. Sol-Gel Novel approaches for preparation of nanoparticles. In Nanostructures for Novel Therapy; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Krüger, C.R.; Rochow, E.G. Polyorganosilazanes. J. Polym. Sci. Part A Polym. Chem. 1964, 2, 3179–3189. [Google Scholar] [CrossRef]
- Kim, S.D.; Ko, P.S.; Park, K.S. Perhydropolysilazane spin-on dielectrics for inter-layer-dielectric applications of sub-30 nm silicon technology. Semicond. Sci. Technol. 2013, 28, 035008. [Google Scholar] [CrossRef]
- Seul, H.J.; Kim, H.G.; Park, M.Y.; Jeong, J.K. A solution-processed silicon oxide gate dielectric prepared at a low temperature via ultraviolet irradiation for metal oxide transistors. J. Mater. Chem. C 2016, 4, 10486–10493. [Google Scholar] [CrossRef]
- Ohishi, T.; Yamazaki, Y. Formation and Gas Barrier Characteristics of Polysilazane-Derived Silica Coatings Formed by Excimer Light Irradiation on PET Films with Vacuum Evaporated Silica Coatings. Mater. Sci. Appl. 2017, 08, 1–14. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, Y.; Yokota, H.; Fuchita, Y.; Takahashi, A.; Sugahara, Y. Characterization of gas barrier silica coatings prepared from perhydropolysilazane films by vacuum ultraviolet irradiation. J. Ceram. Soc. Jpn. 2013, 121, 215–218. [Google Scholar] [CrossRef]
- Ohishi, T.; Sone, S.; Yanagida, K. Preparation and Gas Barrier Characteristics of Polysilazane-Derived Silica Thin Films Using Ultraviolet Irradiation. Mater. Sci. Appl. 2014, 5, 105–111. [Google Scholar] [CrossRef]
- Morlier, A.; Cros, S.; Garandet, J.-P.; Alberola, N. Gas barrier properties of solution processed composite multilayer structures for organic solar cells encapsulation. Sol. Energy Mater. Sol. Cells 2013, 115, 93–99. [Google Scholar] [CrossRef]
- Ohishi, T.; Yanagida, K. Preparation and gas barrier characteristics of polysilazane derived multi-layered silica thin films formed on alicyclic polyimide film using ultraviolet irradiation. Front. Nanosci. Nanotechnol. 2016, 2, 173–178. [Google Scholar] [CrossRef]

| Material (Amount) (70 µL) | Coating Speed (mm/s) | Dilution (ratio) | Final Thickness (nm) | Curing Time (min) |
|---|---|---|---|---|
| PHPS | 1 mm/s | 1:1 | ~450 nm | 10 |
| PHPS | 5 mm/s | 1:1 | ~700 nm | 10 |
| PHPS | 10 mm/s | 1:1 | ~1200 nm | 15 |
| PHPS | 15 mm/s | 1:1 | ~1500 nm | 20 |
| PHPS | 20 mm/s | 1:1 | ~1600 nm | 20 |
| PHPS | 30 mm/s | 1:1 | ~2500 nm | 20 |
| PHPS | 1 mm/s | 1:6 | ~70 nm | 2–3 |
| PHPS | 1 mm/s | 1:5 | ~100 | 2–3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Channa, I.A.; Shah, A.A.; Rizwan, M.; Makhdoom, M.A.; Chandio, A.D.; Shar, M.A.; Mahmood, A. Process Parameter Optimization of a Polymer Derived Ceramic Coatings for Producing Ultra-High Gas Barrier. Materials 2021, 14, 7000. https://doi.org/10.3390/ma14227000
Channa IA, Shah AA, Rizwan M, Makhdoom MA, Chandio AD, Shar MA, Mahmood A. Process Parameter Optimization of a Polymer Derived Ceramic Coatings for Producing Ultra-High Gas Barrier. Materials. 2021; 14(22):7000. https://doi.org/10.3390/ma14227000
Chicago/Turabian StyleChanna, Iftikhar Ahmed, Aqeel Ahmed Shah, Muhammad Rizwan, Muhammad Atif Makhdoom, Ali Dad Chandio, Muhammad Ali Shar, and Asif Mahmood. 2021. "Process Parameter Optimization of a Polymer Derived Ceramic Coatings for Producing Ultra-High Gas Barrier" Materials 14, no. 22: 7000. https://doi.org/10.3390/ma14227000
APA StyleChanna, I. A., Shah, A. A., Rizwan, M., Makhdoom, M. A., Chandio, A. D., Shar, M. A., & Mahmood, A. (2021). Process Parameter Optimization of a Polymer Derived Ceramic Coatings for Producing Ultra-High Gas Barrier. Materials, 14(22), 7000. https://doi.org/10.3390/ma14227000









