Preparation of BiOCl/Bi2WO6 Photocatalyst for Efficient Fixation on Cotton Fabric: Applications in UV Shielding and Self-Cleaning Performances
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of BiOCl/Bi2WO6
2.3. Modification of BiOCl/Bi2WO6
2.4. Pretreatment of Cotton Fabrics
2.5. Finishing of Cotton Fabrics
2.6. Characterization
2.7. Photocatalytic Activity Measurement
2.8. Assessment of Cotton Fabrics
3. Results and Discussion
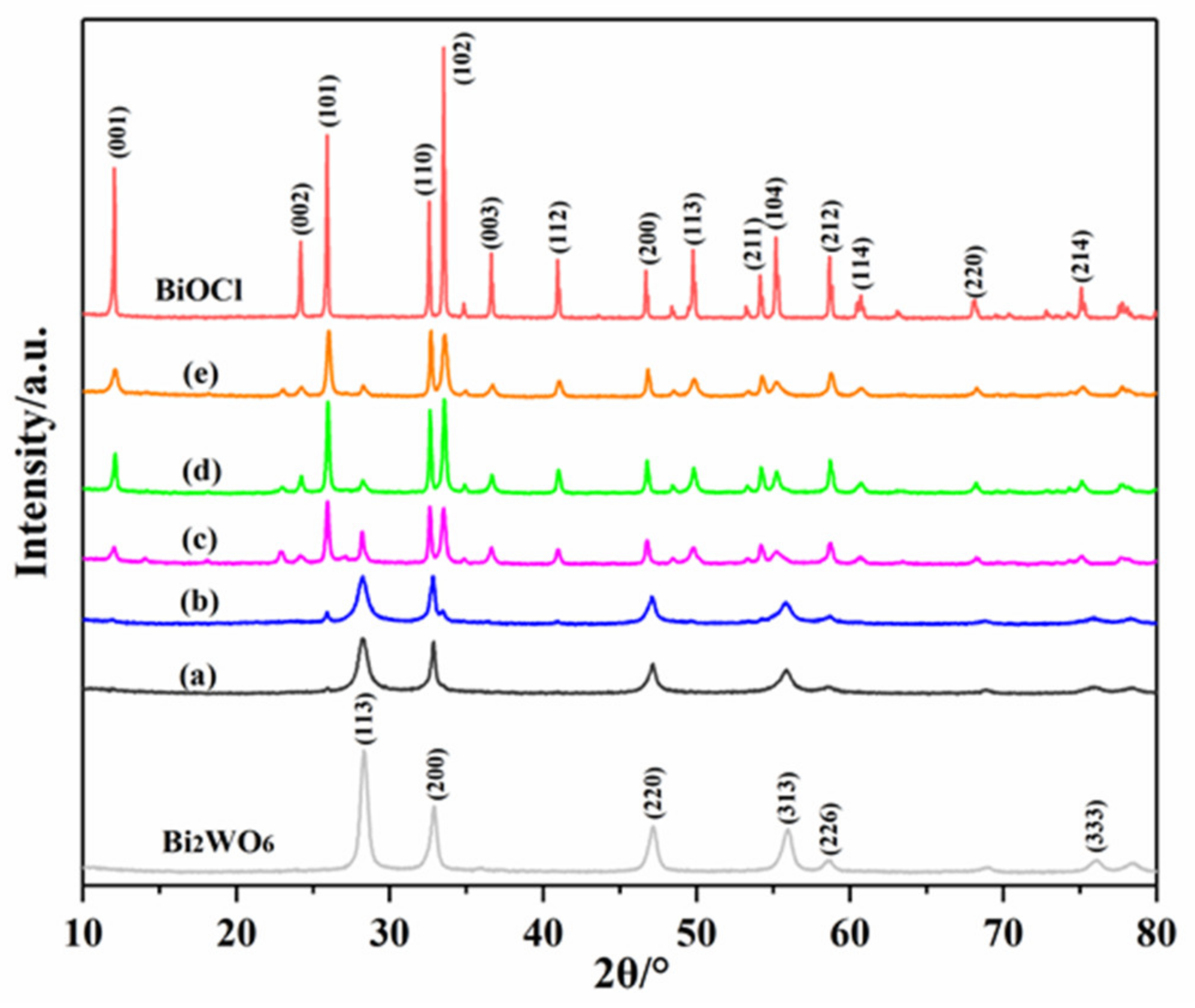
3.1. XRD Analysis of the Photocatalysts
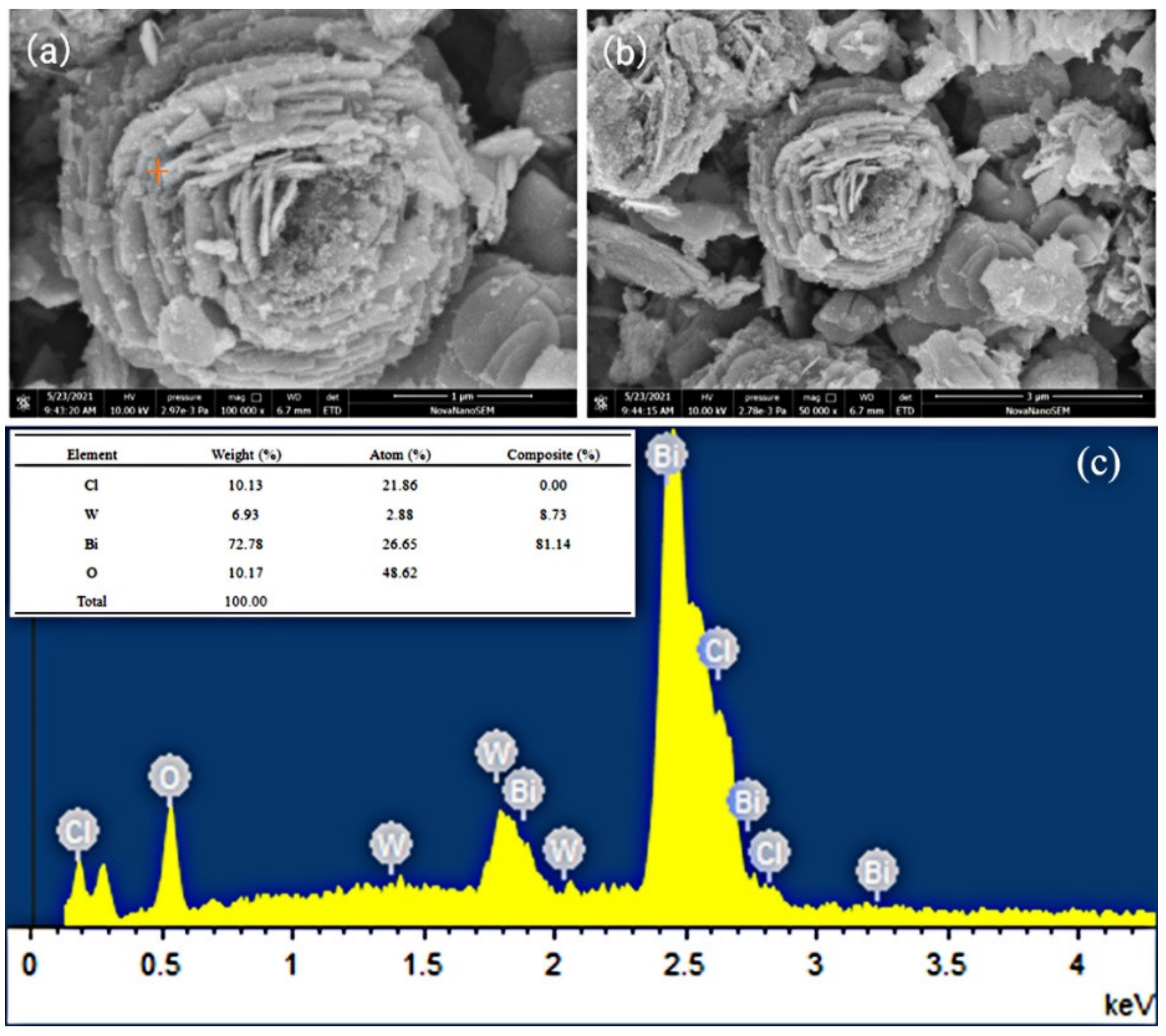
3.2. SEM and EDS Analyses of the Photocatalysts
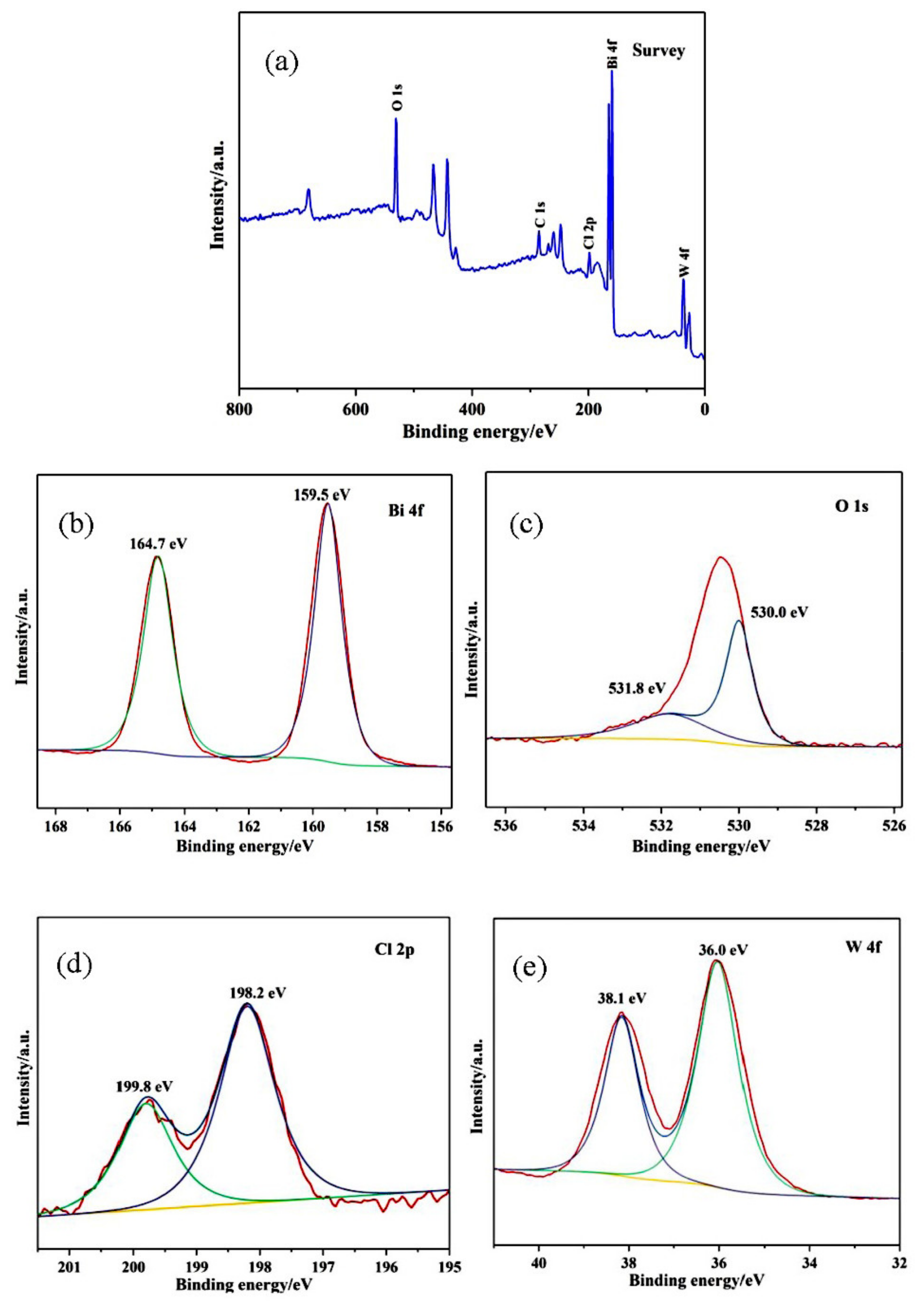
3.3. XPS Analysis
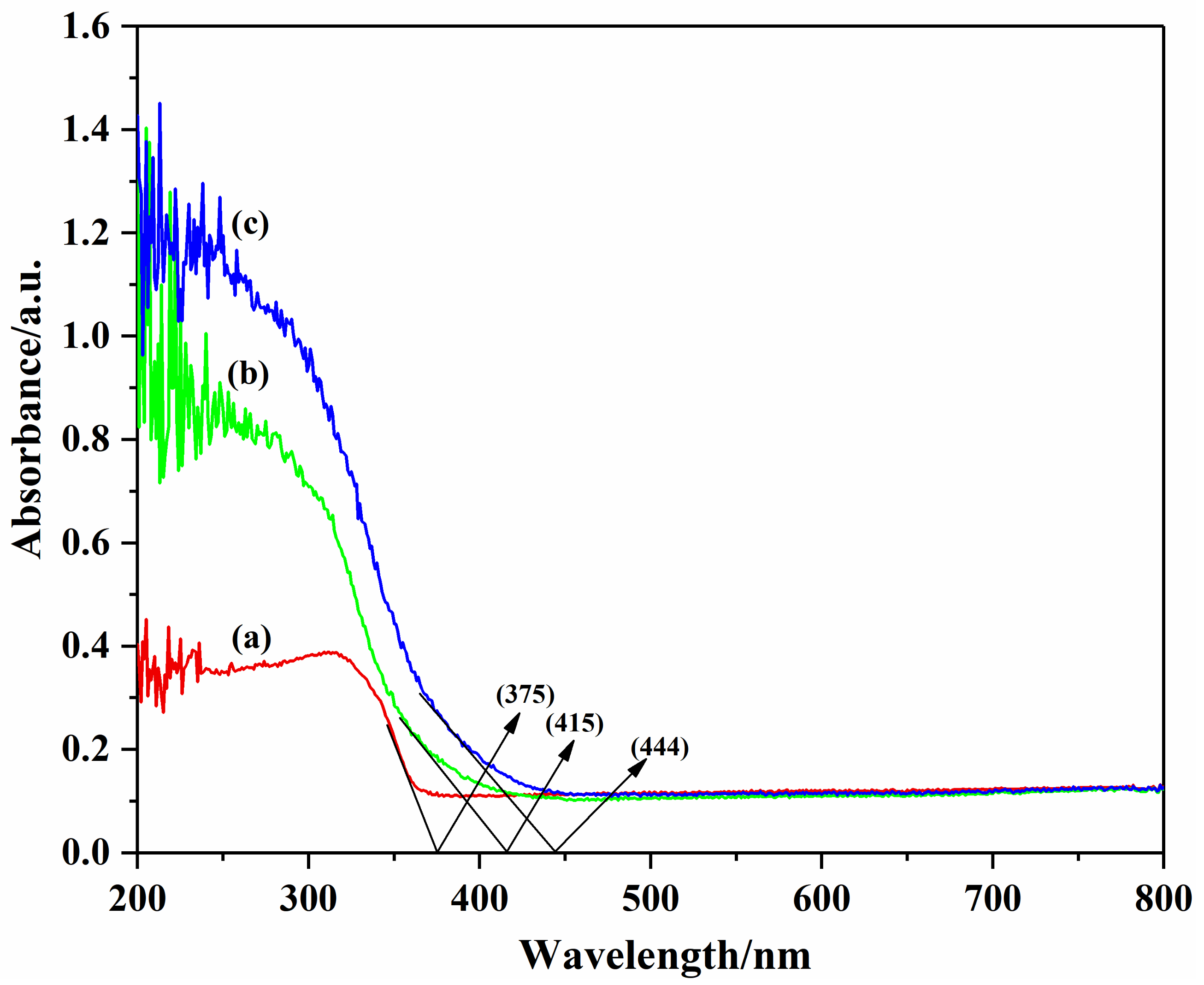
3.4. UV/Vis DRS Analysis of the Photocatalysts
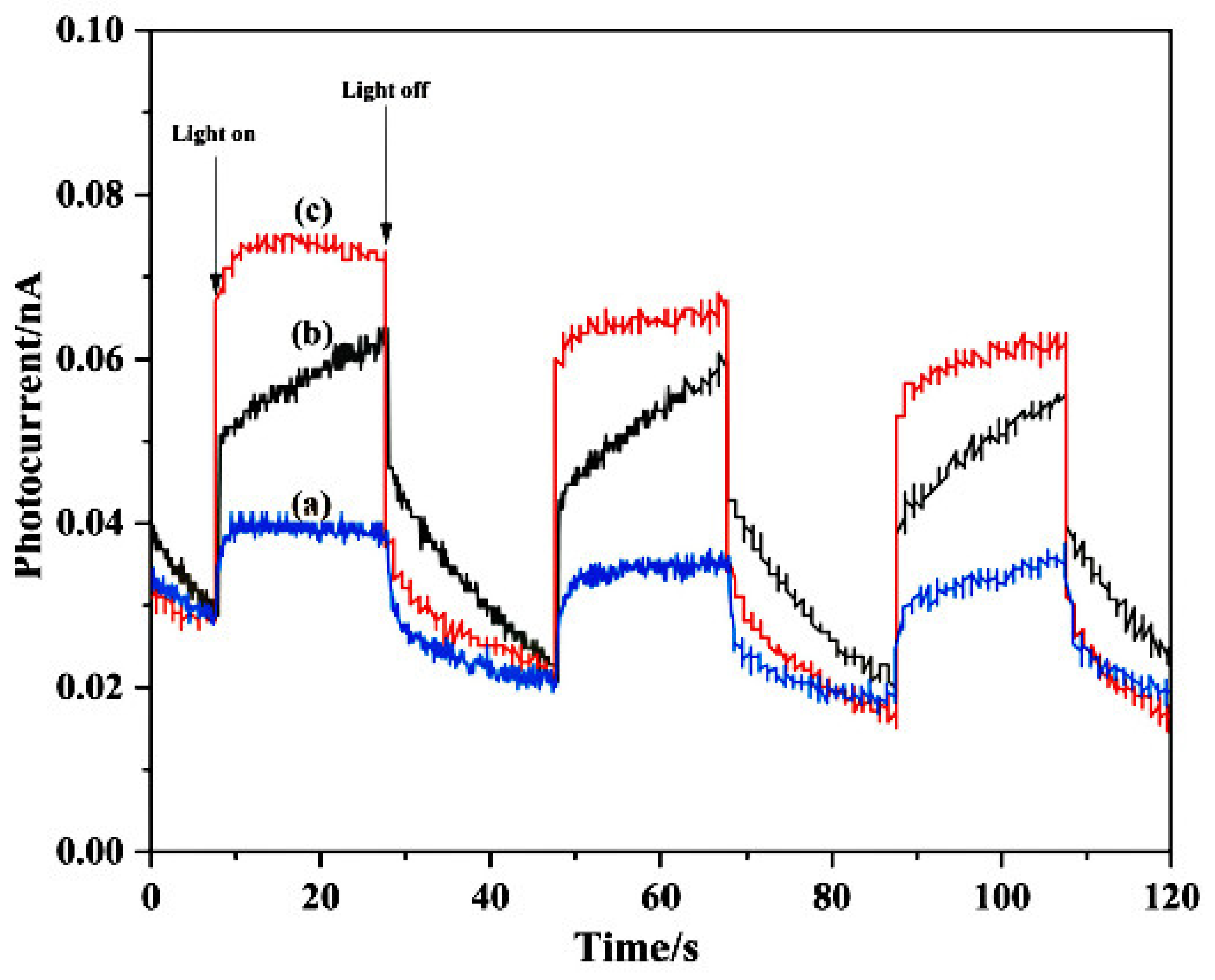
3.5. PC Analysis of the Photocatalysts
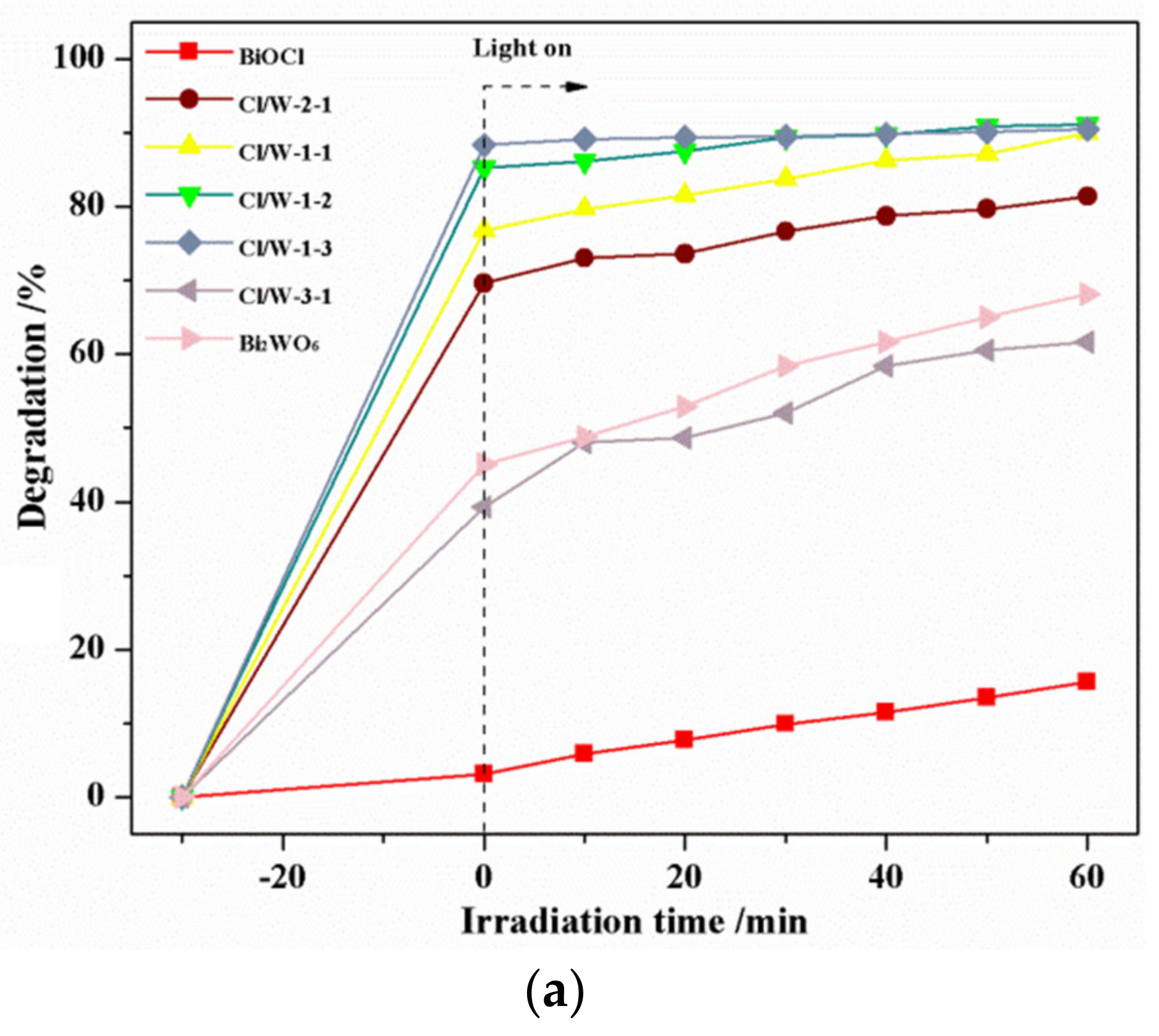
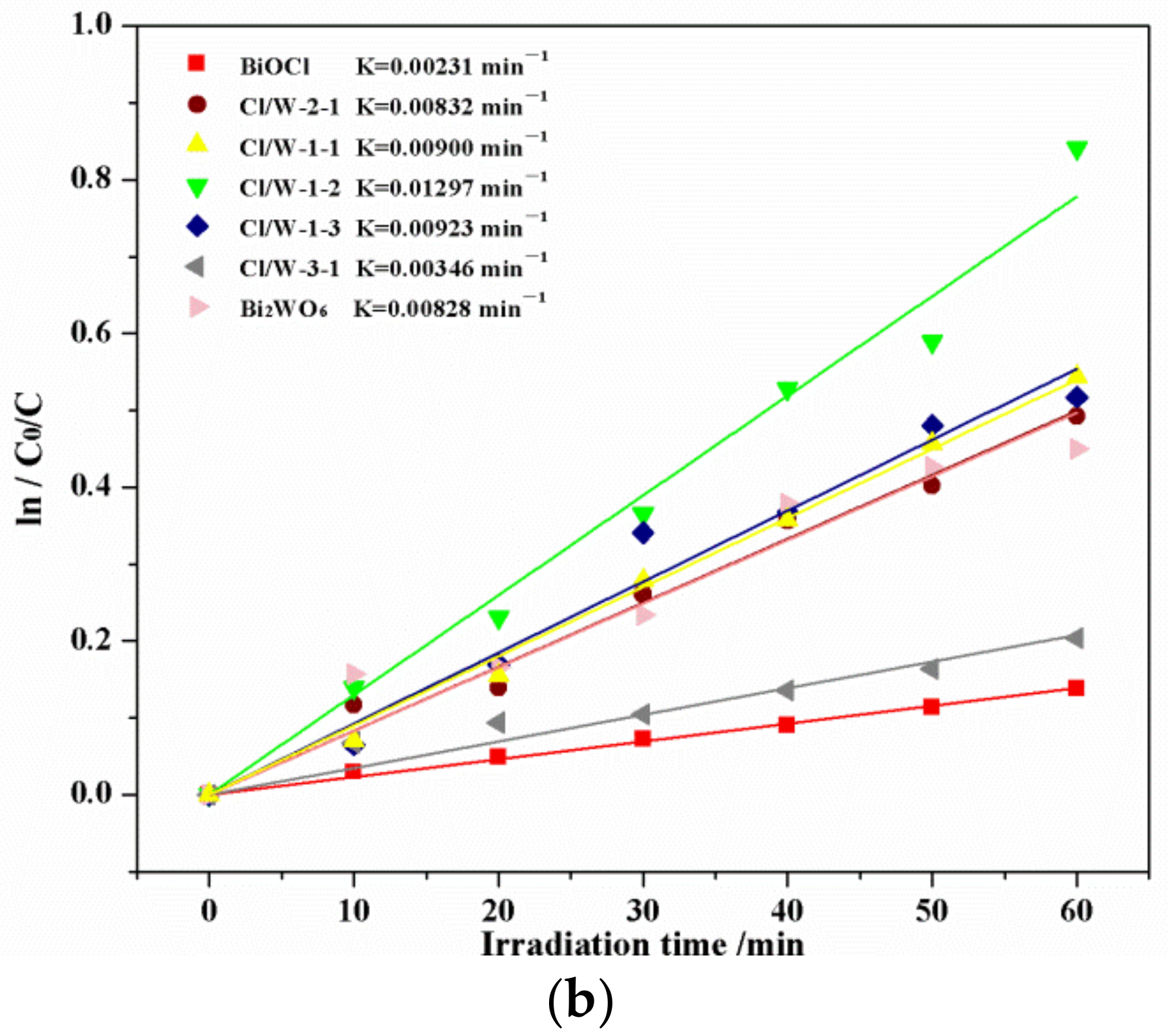
3.6. Photocatalytic Activity of the Photocatalysts
3.7. XRD Analysis of Cotton Fabrics
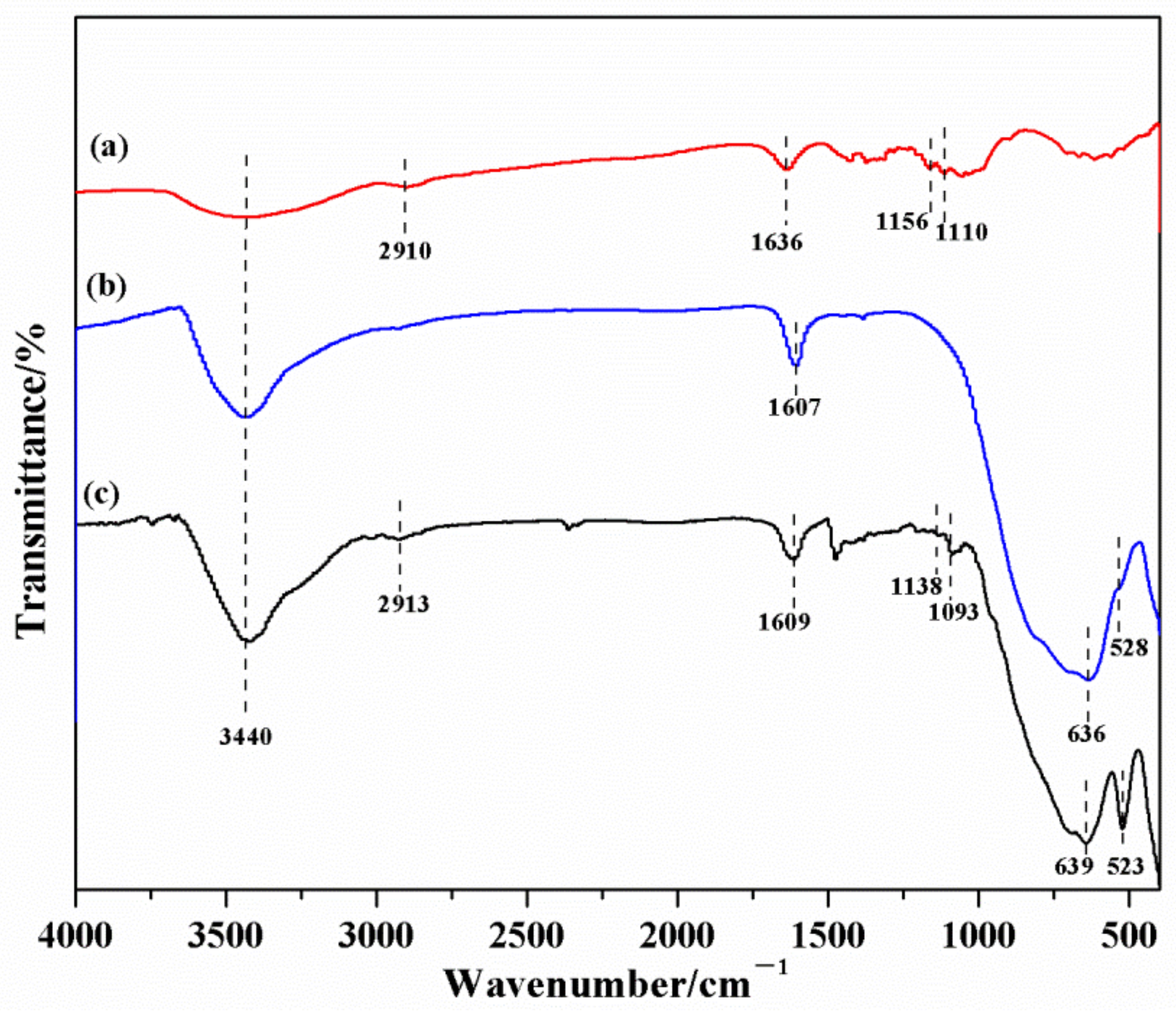
3.8. FT-IR Analysis
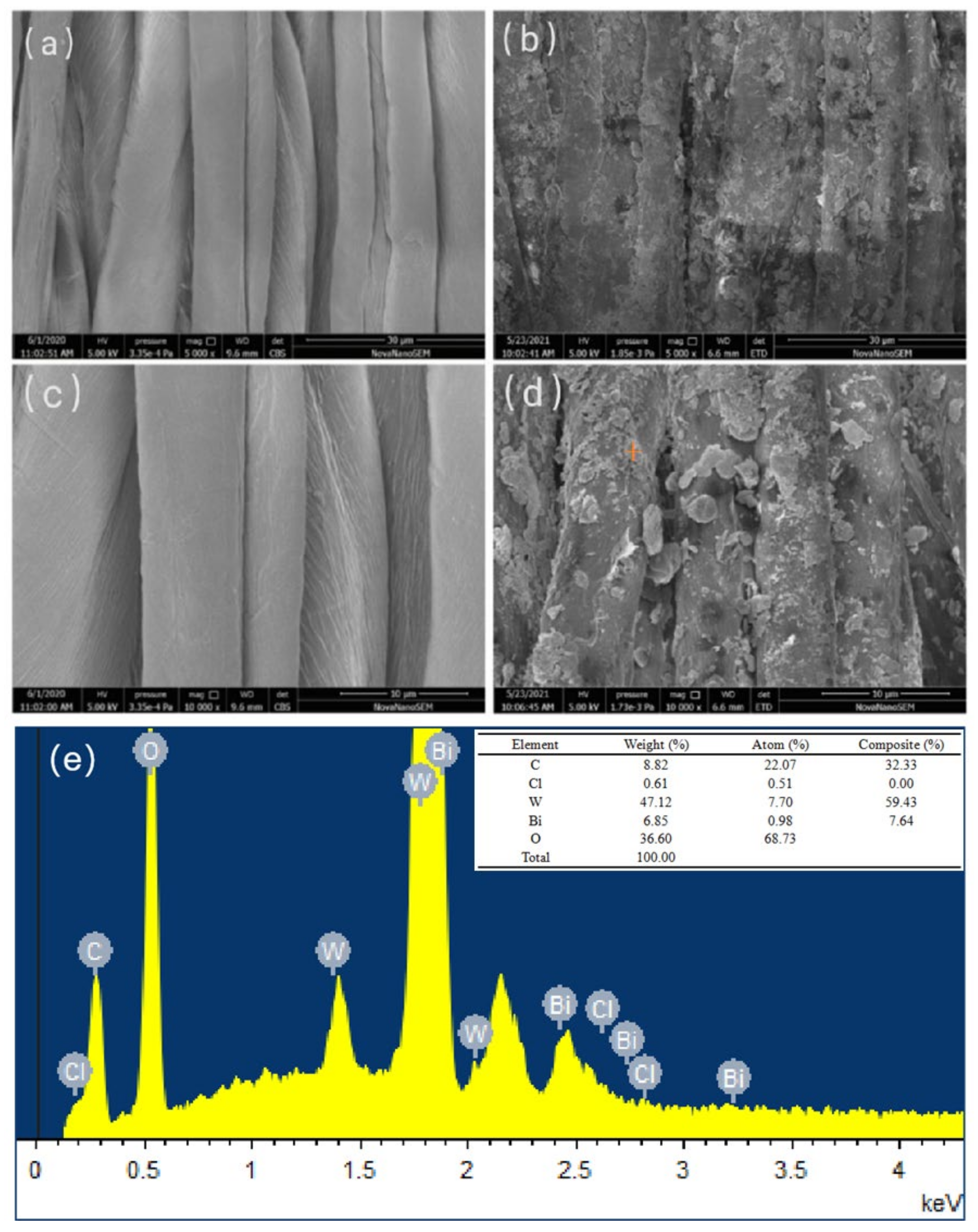
3.9. SEM and EDS Analyses
3.10. Ultraviolet Resistance Evaluation
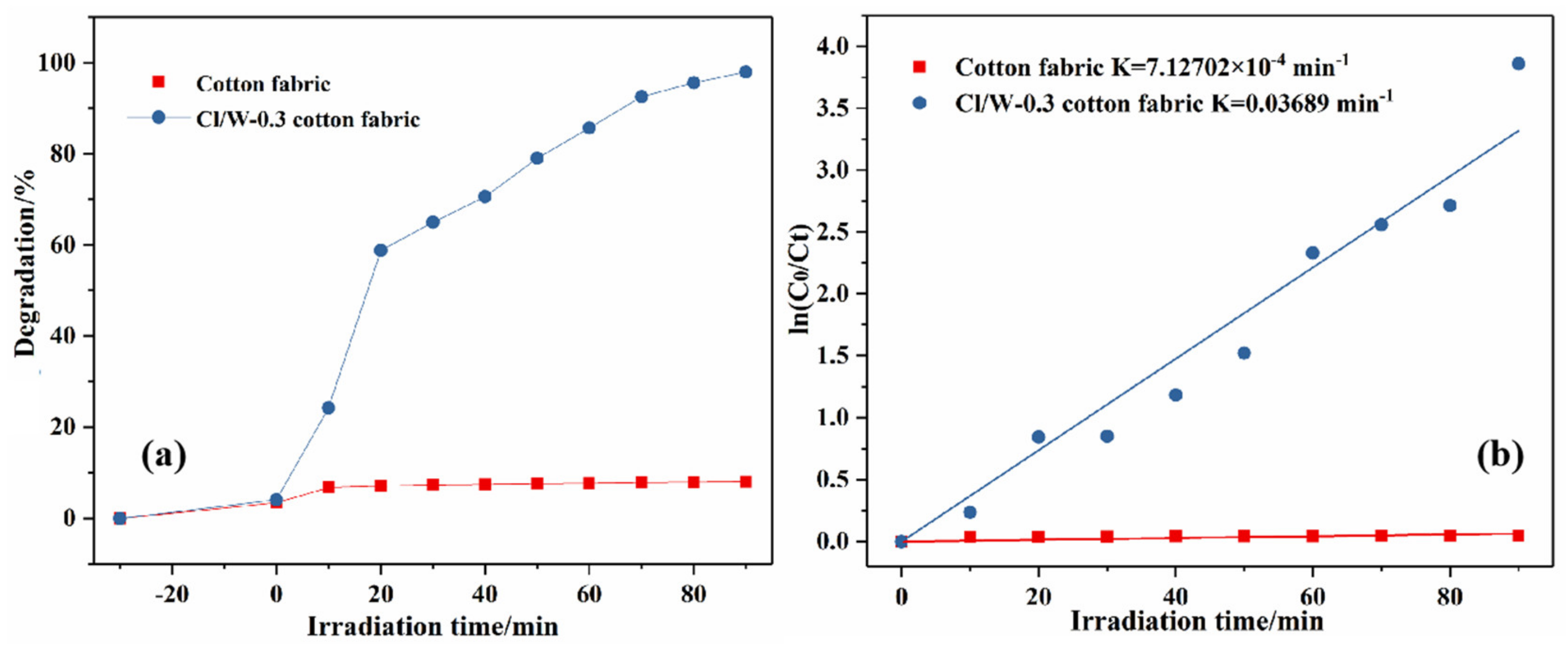
3.11. Photocatalytic Activity of the Cotton Fabrics
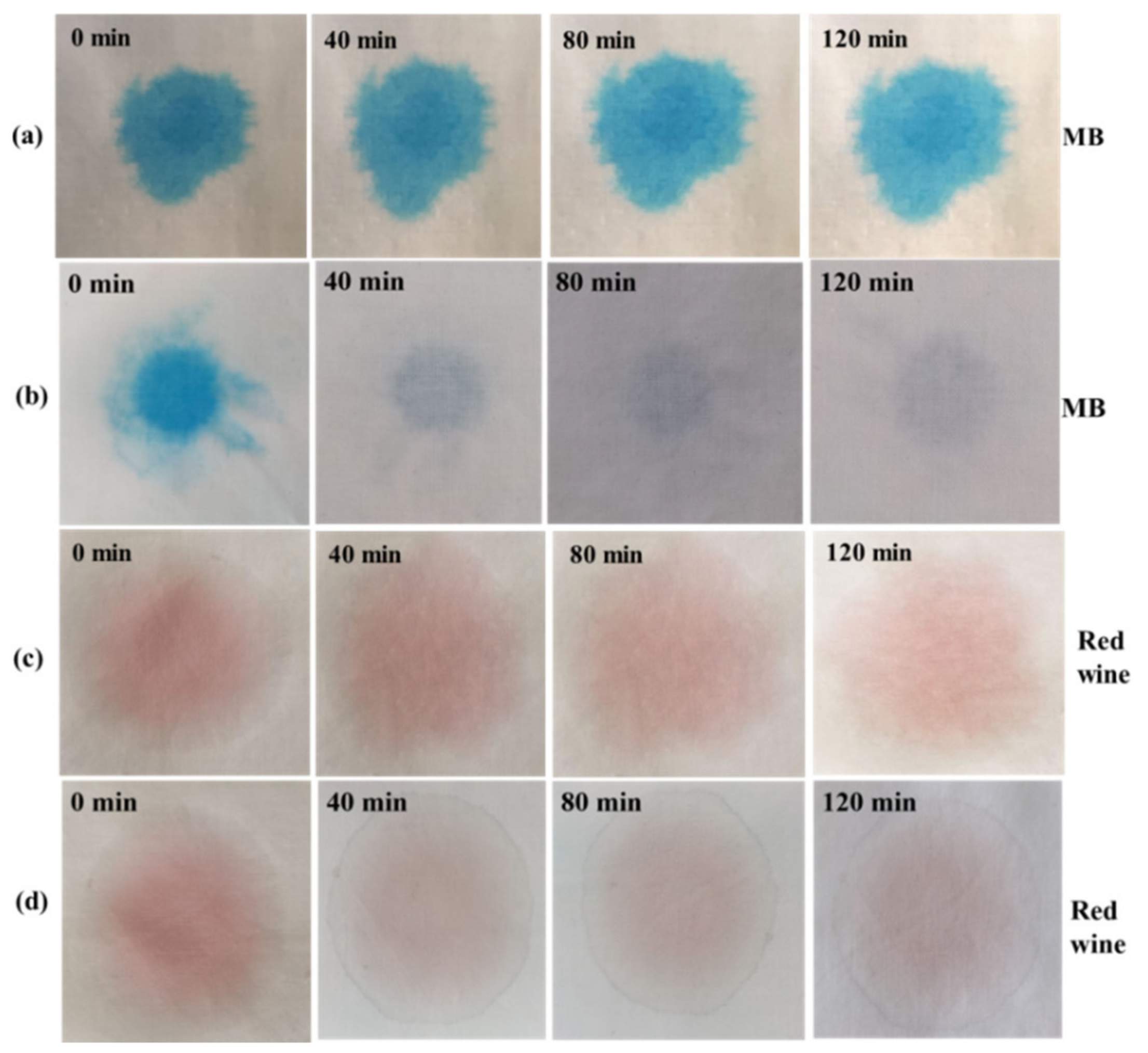
3.12. Self-Cleaning Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, M.; Chen, Z.; Wang, W.; Ma, J.; Shen, J. Hydrothermal synthesis of TiO2 hollow microspheres for the photocatalytic degradation of 4-chloronitrobenzene. J. Hazard. Mater. 2010, 184, 612–619. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Ibañez, P.F.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and Prospects for Wastewater Treatment by UV and Visible-Light-Active Heterogeneous Photocatalysis: A Critical Review. Top. Curr. Chem. 2020, 378, 7. [Google Scholar] [CrossRef]
- Liu, W.; He, T.; Wang, Y.; Ning, G.; Xu, Z.; Chen, X.; Hu, X.; Wu, Y.; Zhao, Y. Synergistic adsorption-photocatalytic degradation effect and norfloxacin mechanism of ZnO/ZnS@ BC under UV-light irradiation. Sci. Rep. 2020, 10, 11903. [Google Scholar] [CrossRef]
- Ivetić, T.B.; Finčur, N.L.; Abramović, B.F.; Dimitrievska, M.; Štrbac, G.R.; Čajko, K.O.; Miljević, B.B.; Đačanin, L.R.; Lukić-Petrović, S.R. Environmentally friendly photoactive heterojunction zinc tin oxide nanoparticles. Ceram. Int. 2016, 42, 3575–3583. [Google Scholar] [CrossRef]
- Jeon, J.; Kweon, D.H.; Jang, B.J.; Ju, M.J.; Baek, J. Enhancing the Photocatalytic Activity of TiO2 Catalysts. Adv. Sustain. Syst. 2020, 4, 2000197. [Google Scholar] [CrossRef]
- Gu, X.; Li, C.; Yuan, S.; Ma, M.; Qiang, Y.; Zhu, J. ZnO based heterojunctions and their application in environmental photocatalysis. Nanotechnology 2016, 27, 402001. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Yang, H.; Chen, Z.; Qiu, M.; Hu, B.; Wang, X. Bismuth oxychloride-based materials for the removal of organic pollutants in wastewater. Chemosphere 2021, 273, 128576. [Google Scholar] [CrossRef]
- Fazal, T.; Razzaq, A.; Javed, F.; Hafeez, A.; Rashid, N.; Amjad, U.S.; Rehman, M.S.U.; Faisal, A.; Rehman, F. Integrating adsorption and photocatalysis: A cost effective strategy for textile wastewater treatment using hybrid biochar-TiO2 composite. J. Hazard. Mater. 2020, 390, 121623. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhong, Y.; Hu, C.; Luo, J.; Wang, P. Hollow microspheres of BiOCl assembled with nanosheets: Spray drying synthesis and drastically enhanced photocatalytic activity. J. Environ. Chem. Eng. 2018, 6, 6971–6978. [Google Scholar] [CrossRef]
- Chang, X.; Gondal, M.A.; Al-Saadi, A.A.; Ali, M.A.; Shen, H.; Zhou, Q.; Zhang, J.; Du, M.; Liu, Y.; Ji, G. Photodegradation of Rhodamine B over unexcited semiconductor compounds of BiOCl and BiOBr. J. Colloid Interf. Sci. 2012, 377, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fei, J.; Dai, Y.; Fu, F. Hydrothermal synthesis of series Cu-doped Bi2WO6 and its application in photo-degradative removal of phenol in wastewater with enhanced efficiency. J. Mol. Liq. 2018, 256, 267–276. [Google Scholar] [CrossRef]
- An, W.; Wang, S.; Fu, Y.; Guan, Y.; Li, Z.; Xu, T.; Wang, H. Construction of Bi2WO6 with oxygen vacancies and investigation on mechanisms of significantly enhanced photocatalytic activity. Desalin. Water Treat. 2021, 216, 151–161. [Google Scholar] [CrossRef]
- Guo, M.; Zhou, Z.; Yan, S.; Zhou, P.; Miao, F.; Liang, S.; Wang, J.; Cui, X. Bi2WO6–BiOCl heterostructure with enhanced photocatalytic activity for efficient degradation of oxytetracycline. Sci. Rep. 2020, 10, 18401. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, X.; Yu, C.; Zeng, D.; Chen, F.; Zhang, K.; Huang, W.; Ji, H. Review on heterophase/homophase junctions for efficient photocatalysis: The case of phase transition construction. Chin. J. Catal. 2019, 40, 796–818. [Google Scholar] [CrossRef]
- Ivetic, T.B.; Fincur, N.L.; Merkulov, D.V.S.; Despotovic, V.N.; Cetojevic-Simin, D.D.; Armakovic, S.J.; Uzelac, M.M.; Bognár, S.I.; Zec, N.J.; Lukic-Petrovic, S.R.; et al. Water-Active Titanium/Molybdenum/Mixed-Oxides: Removal Efficiency of Organic Water Pollutants by Adsorption and Photocatalysis and Toxicity Assessment. Catalysts 2021, 11, 1054. [Google Scholar] [CrossRef]
- Tahmasebi, N.; Maleki, Z.; Farahnak, P. Enhanced photocatalytic activities of Bi2WO6/BiOCl composite synthesized by one-step hydrothermal method with the assistance of HCl. Mat. Sci. Semicon. Proc. 2019, 89, 32–40. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, C.; Yang, J.; Mo, Q.; Zhang, Y.; Tang, Y. Visible light responsive Bi2WO6/BiOCl heterojunction with enhanced photocatalytic activity for degradation of tetracycline and rohdamine B. Inorg. Chem. Commun. 2018, 93, 136–139. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, S.; Maity, J. Synthesis, characterization and photocatalytic properties of ZnO nanoparticles and cotton fabric modified with ZnO nanoparticles via in-situ hydrothermal coating technique: Dual response. Mater. Technol. 2018, 33, 884–891. [Google Scholar] [CrossRef]
- Wang, C.; Lv, J.; Ren, Y.; Zhou, Q.; Chen, J.; Zhi, T.; Lu, Z.; Gao, D.; Ma, Z.; Jin, L. Cotton fabric with plasma pretreatment and ZnO/Carboxymethyl chitosan composite finishing for durable UV resistance and antibacterial property. Carbohyd. Polym. 2016, 138, 106–113. [Google Scholar]
- Yang, M.; Liu, W.; Jiang, C.; Xie, Y.; Shi, H.; Zhang, F.; Wang, Z. Facile construction of robust superhydrophobic cotton textiles for effective UV protection, self-cleaning and oil-water separation. Colloid. Surf. A 2019, 570, 172–181. [Google Scholar] [CrossRef]
- Hu, J.; Gao, Q.; Xu, L.; Wang, M.; Zhang, M.; Zhang, K.; Liu, W.; Wu, G. Functionalization of cotton fabrics with highly durable polysiloxane–TiO2 hybrid layers: Potential applications for photo-induced water–oil separation, UV shielding, and self-cleaning. J. Mater. Chem. A 2018, 6, 6085–6095. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, X.; Zhang, P.; Wang, Q.; Zheng, K.; Chen, L.; Ding, J.; Tian, X.; Zhang, X. Convenient and recyclable TiO2/g-C3N4 photocatalytic coating: Layer-by-layer self-assembly construction on cotton fabrics leading to improved catalytic activity under visible light. Ind. Eng. Chem. Res. 2019, 58, 3978–3987. [Google Scholar] [CrossRef]
- Cao, C.; Wang, F.; Lu, M. Superhydrophobic CuO coating fabricated on cotton fabric for oil/water separation and photocatalytic degradation. Colloid. Surf. A 2020, 601, 125033. [Google Scholar] [CrossRef]
- Wang, K.; Song, X.; Li, Y.; Tian, J.; Zhang, C.; Qi, Z.; Liu, S.; Gao, D.; Liu, G.; Ren, Y.; et al. In Situ Precipitation of Ag6Si2O7 on the Surface of Cotton Fabric Modified by Plasma Treatment for Highly Efficient and Durable Photocatalytic Activity. Integr. Ferroelectr. 2021, 219, 111–118. [Google Scholar] [CrossRef]
- Tudu, B.K.; Sinhamahapatra, A.; Kumar, A. Surface modification of cotton fabric using TiO2 nanoparticles for self-cleaning, oil–water separation, antistain, anti-water absorption, and antibacterial properties. ACS Omega 2020, 5, 7850–7860. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Feng, K.; Liu, X. Enhanced photocatalytic degradation and UV protection properties of BiOCl nanosheets coating for cotton fabric. Funct. Mater. Lett. 2018, 11, 1850052. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, W.; Tian, Z.; Zhao, Y.; Shen, Z. Facile fabrication of Bi2WO6/BiOCl hierarchical structure as adsorbents for methylene blue dye removal. Mater. Res. Express 2019, 6, 055034. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, K.; Jiang, Y.; Zhu, Y.; Chen, X.; Tang, Q.; Ren, Y.; Zheng, C.; Gao, D.; Wang, C. Preparation and characterization of SnO2−x/GO composite photocatalyst and its visible light photocatalytic activity for self-cleaning cotton fabrics. Cellulose 2019, 26, 8919–8937. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, L.; Guo, H.; Wang, A.; Hu, J.; Zhang, W.; Xu, W.; Wang, Y. Kinetics and thermodynamics studies of cationic dye adsorption onto carboxymethyl cotton fabric. J. Nat. Fibers 2020, 1–12. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Wang, K.; Chen, H.; Jiang, W.; Qi, Z.; Gao, D.; Liu, S.; Song, X.; Liu, G.; et al. Ag6Si2O7/CNTs with highly efficient and stable visible light photocatalytic activity. Integr. Ferroelectr. 2019, 198, 73–79. [Google Scholar] [CrossRef]
- Wang, K.; Zhuo, Y.; Chen, J.; Gao, D.; Ren, Y.; Wang, C.; Qi, Z. Crystalline phase regulation of anatase–rutile TiO2 for the enhancement of photocatalytic activity. RSC Adv. 2020, 10, 43592–43598. [Google Scholar] [CrossRef]
- Gao, D.; Wang, L.; Wang, C.; Chen, T. Photocatalytic self-cleaning cotton fabrics coated by Cu2(OH)PO4 under Vis/NIR irradiation. Materials 2019, 12, 238. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, T.; Ma, R.; Du, J.; Xie, C. Synthesis of flower-like CeO2/BiOCl heterostructures with enhanced ultraviolet light photocatalytic activity. Micro. Nano Lett. 2018, 13, 1394–1398. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, P.; Zhang, W.; Meng, X.; He, H.; Zeng, X.; Shen, X. Pristine Bi2WO6 and hybrid Au-Bi2WO6 hollow microspheres with excellent photocatalytic activities. Appl. Surf. Sci. 2018, 457, 925–932. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Kadirova, Z.C.; Zahedi, E.; Onna, D.; Marchi, M.C.; Zhu, G.; Matsushita, N.; Hasegawa, M.; Bilmes, S.A.; Okada, K. Tuning the morphological structure, light absorption, and photocatalytic activity of Bi2WO6 and Bi2WO6-BiOCl through cerium doping. Arab. J. Chem. 2020, 13, 2844–2857. [Google Scholar] [CrossRef]
- Dumrongrojthanath, P.; Saksoong, T.; Patiphatpanya, P.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Microwave-assisted hydrothermal synthesis of BiOCl/Bi2WO6 nanocomposites for the enhancement of photocatalytic efficiency. Res. Chem. Intermed. 2019, 45, 2301–2312. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, Z.; Qu, D.; Shi, J. Synthesis of chemically bonded BiOCl@ Bi2WO6 microspheres with exposed (0 2 0) Bi2WO6 facets and their enhanced photocatalytic activities under visible light irradiation. Appl. Surf. Sci. 2016, 361, 63–71. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, C.; Li, F.; Li, T.; Zhang, M.; Cao, W. Improved photocatalytic Bi2WO6/BiOCl heterojunctions: One-step synthesis via an ionic-liquid assisted ultrasonic method and first-principles calculations. Mol. Catal. 2017, 435, 33–48. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Wang, K.; Chen, J.; Gao, D.; Ren, Y.; Wang, C. Cotton fabric loaded with self-dispersive and reactive biphasic TiO2 for durable self-cleaning activity and ultraviolet protection. New J. Chem. 2021, 45, 11119–11129. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Yang, B.; Wang, B.; Chen, J.; Jing, H. In situ grown heterojunction of Bi2WO6/BiOCl for efficient photoelectrocatalytic CO2 reduction. J. Catal. 2019, 377, 209–217. [Google Scholar] [CrossRef]
- Thi, V.H.T.; Lee, B.-K.J. Development of multifunctional self-cleaning and UV blocking cotton fabric with modification of photoactive ZnO coating via microwave method. Photoch. Photobiol. A 2017, 338, 13–22. [Google Scholar]
- Zheng, C.; Qi, Z.; Shen, W.; Chen, G. Self-cleaning Bombyx mori silk: Room-temperature preparation of anatase nano-TiO2 by the sol-gel method and its application. Color. Technol. 2014, 130, 280–287. [Google Scholar] [CrossRef]


| Sample | UVA (%) | UVB (%) | UPF |
|---|---|---|---|
| Cotton fabric | 20 | 15.45 | 5.95 |
| Cl/W-0.1 cotton fabric | 8.36 | 2.63 | 31.92 |
| Cl/W-0.2 cotton fabric | 5.77 | 3.10 | 37.96 |
| Cl/W-0.3 cotton fabric | 3.51 | 3.45 | 40.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Wang, K.; Tian, J.; Yu, W.; Chen, Y.; Li, N.; Qi, Z.; Wang, C. Preparation of BiOCl/Bi2WO6 Photocatalyst for Efficient Fixation on Cotton Fabric: Applications in UV Shielding and Self-Cleaning Performances. Materials 2021, 14, 7002. https://doi.org/10.3390/ma14227002
Chen J, Wang K, Tian J, Yu W, Chen Y, Li N, Qi Z, Wang C. Preparation of BiOCl/Bi2WO6 Photocatalyst for Efficient Fixation on Cotton Fabric: Applications in UV Shielding and Self-Cleaning Performances. Materials. 2021; 14(22):7002. https://doi.org/10.3390/ma14227002
Chicago/Turabian StyleChen, Jiayi, Kuang Wang, Jialong Tian, Wenhui Yu, Yujie Chen, Na Li, Zhenming Qi, and Chunxia Wang. 2021. "Preparation of BiOCl/Bi2WO6 Photocatalyst for Efficient Fixation on Cotton Fabric: Applications in UV Shielding and Self-Cleaning Performances" Materials 14, no. 22: 7002. https://doi.org/10.3390/ma14227002
APA StyleChen, J., Wang, K., Tian, J., Yu, W., Chen, Y., Li, N., Qi, Z., & Wang, C. (2021). Preparation of BiOCl/Bi2WO6 Photocatalyst for Efficient Fixation on Cotton Fabric: Applications in UV Shielding and Self-Cleaning Performances. Materials, 14(22), 7002. https://doi.org/10.3390/ma14227002





