Thermal Properties of Calcium Sulphoaluminate Cement as an Alternative to Ordinary Portland Cement
Abstract
1. Introduction
2. Experimental Program
- T(τ), the actual temperature of the cementitious material, °C;
- Tref, the reference temperature usually equal to 20 °C;
- R, the universal gas constant equals 8.314 J mol−1·°C−1;
- , the activation energy, J mol−1;
- , time, h or day.
3. Results and Discussion
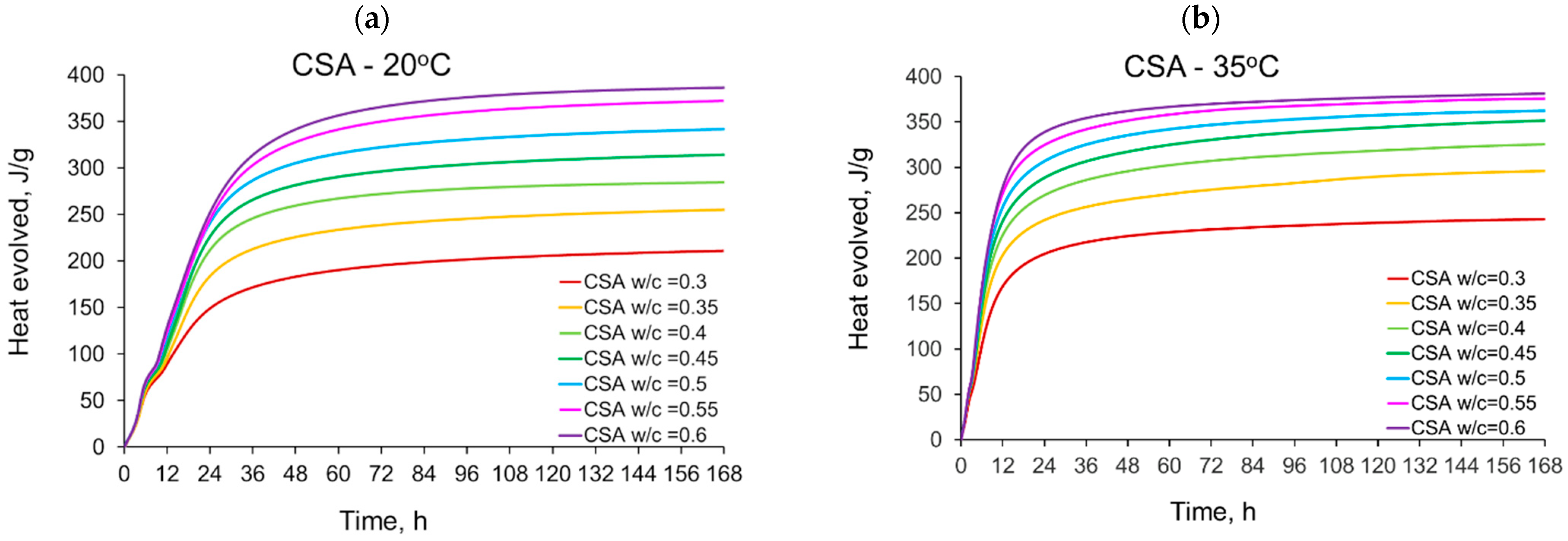
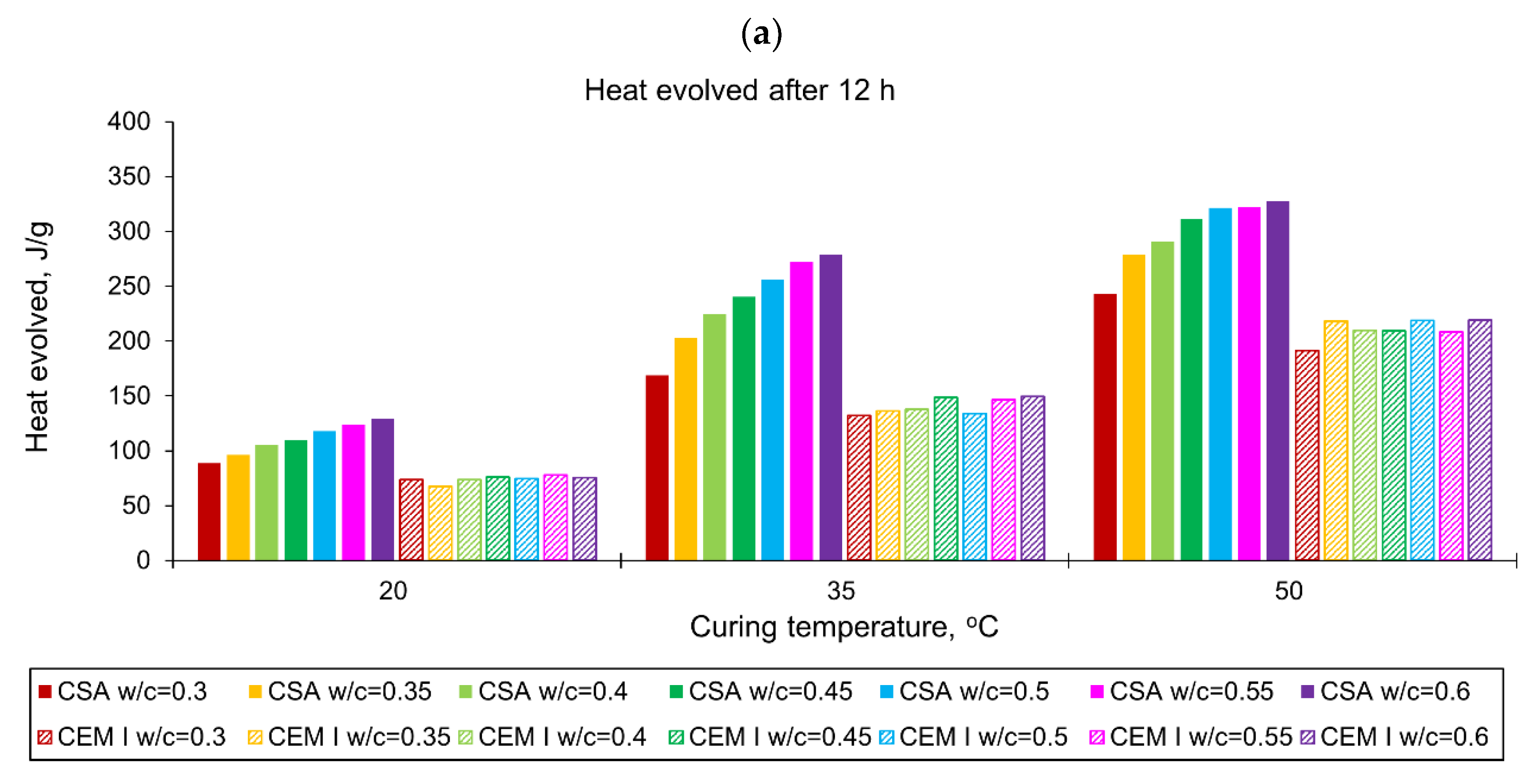
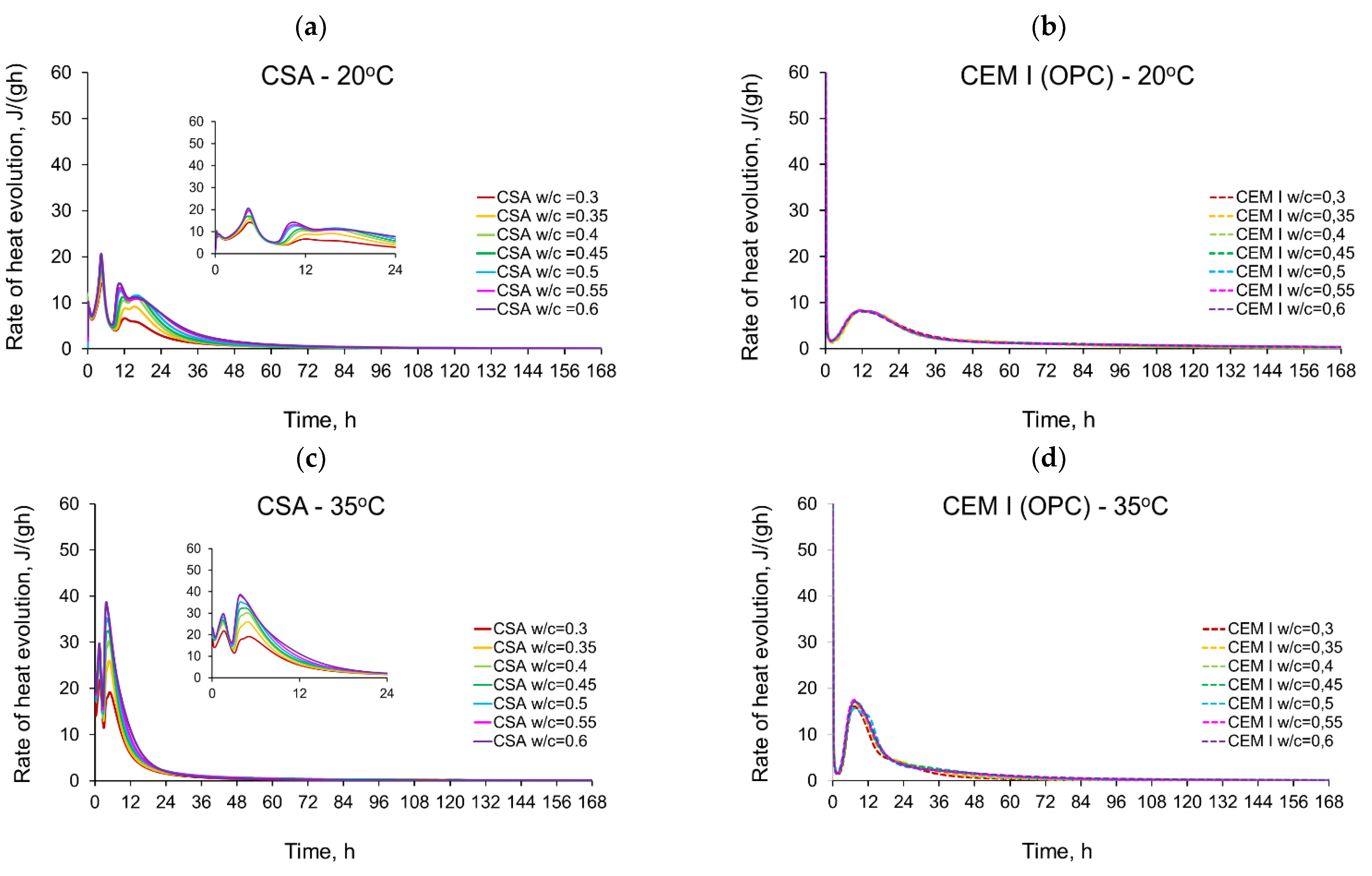
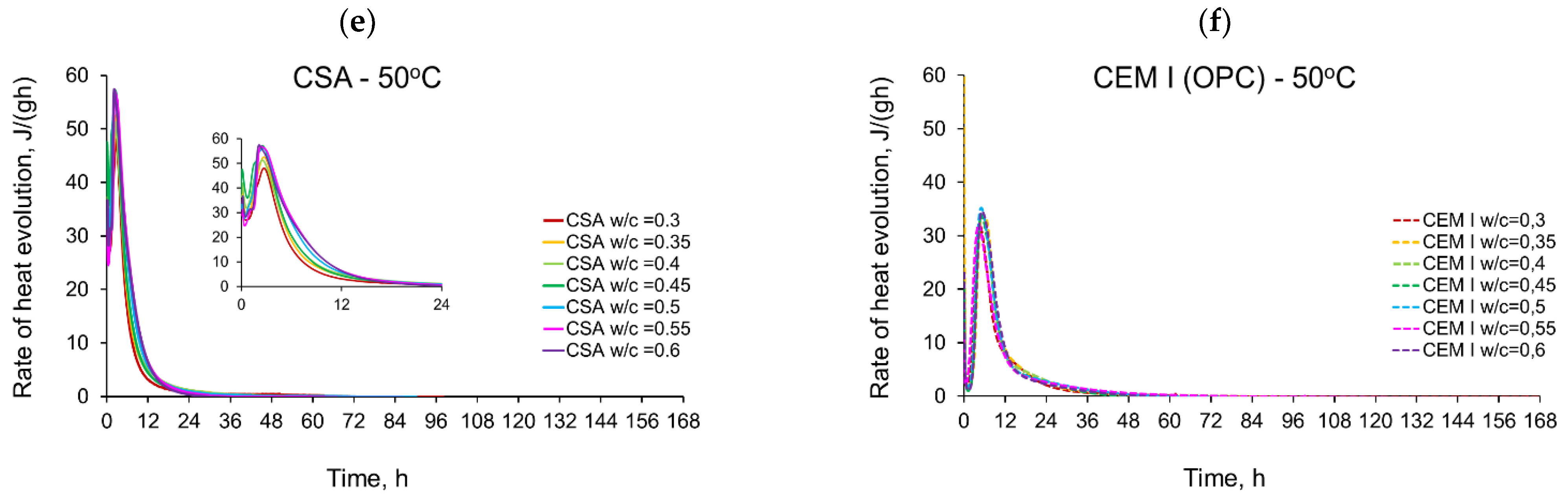
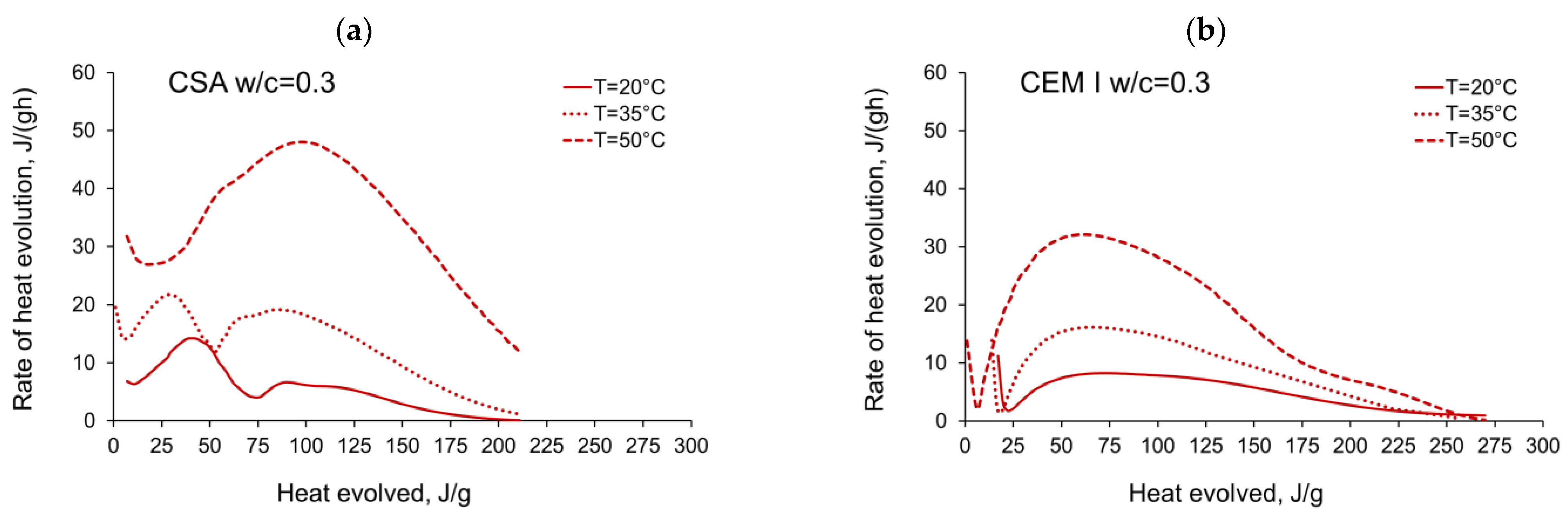
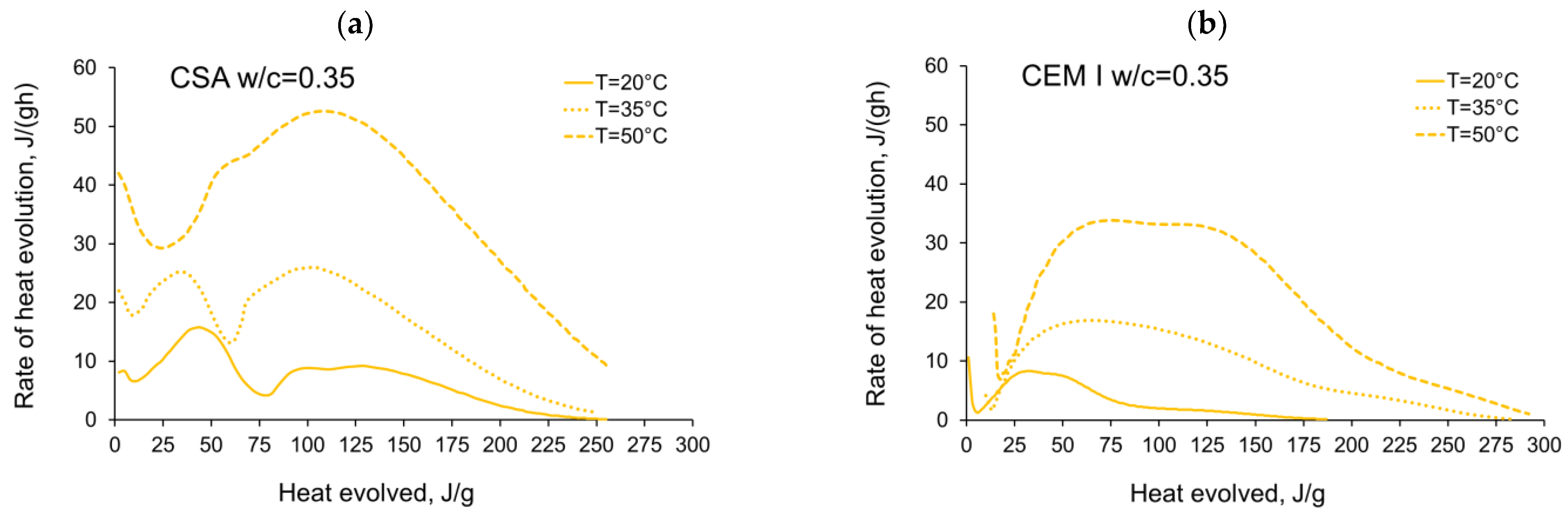
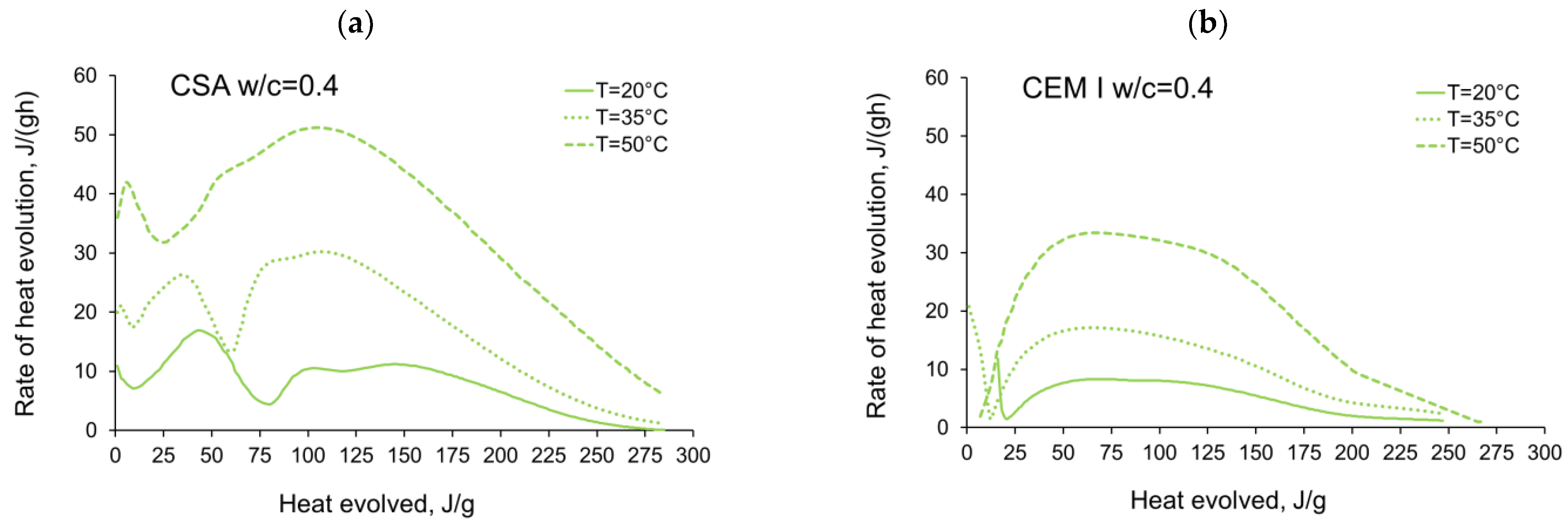
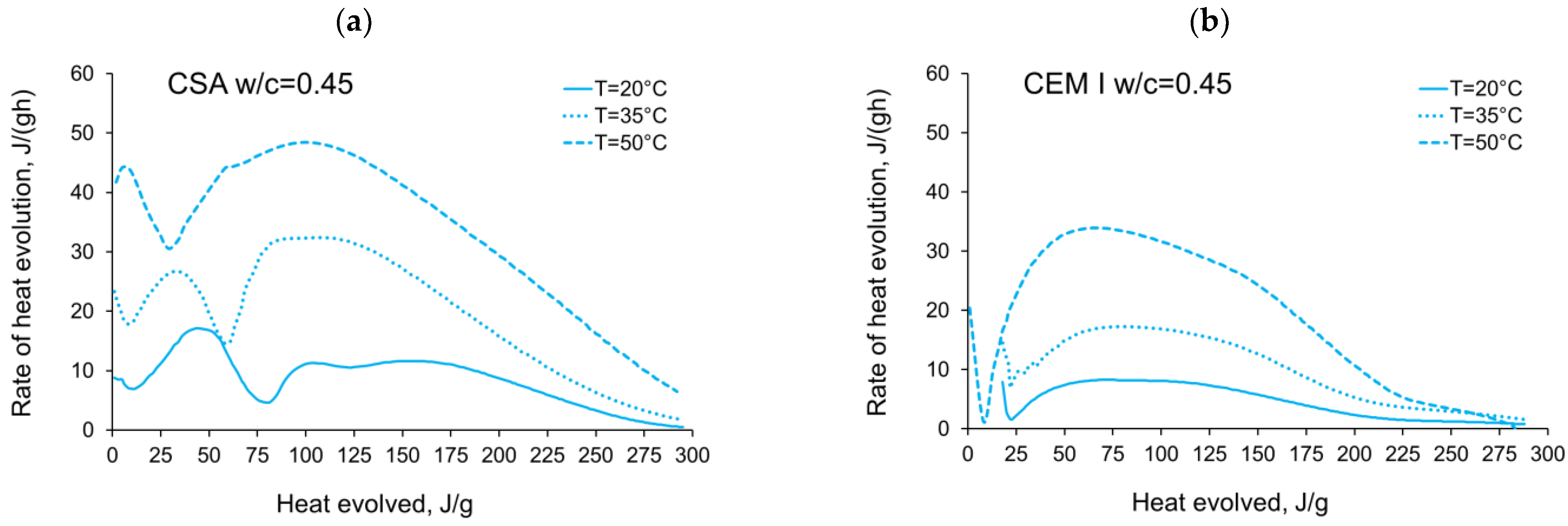
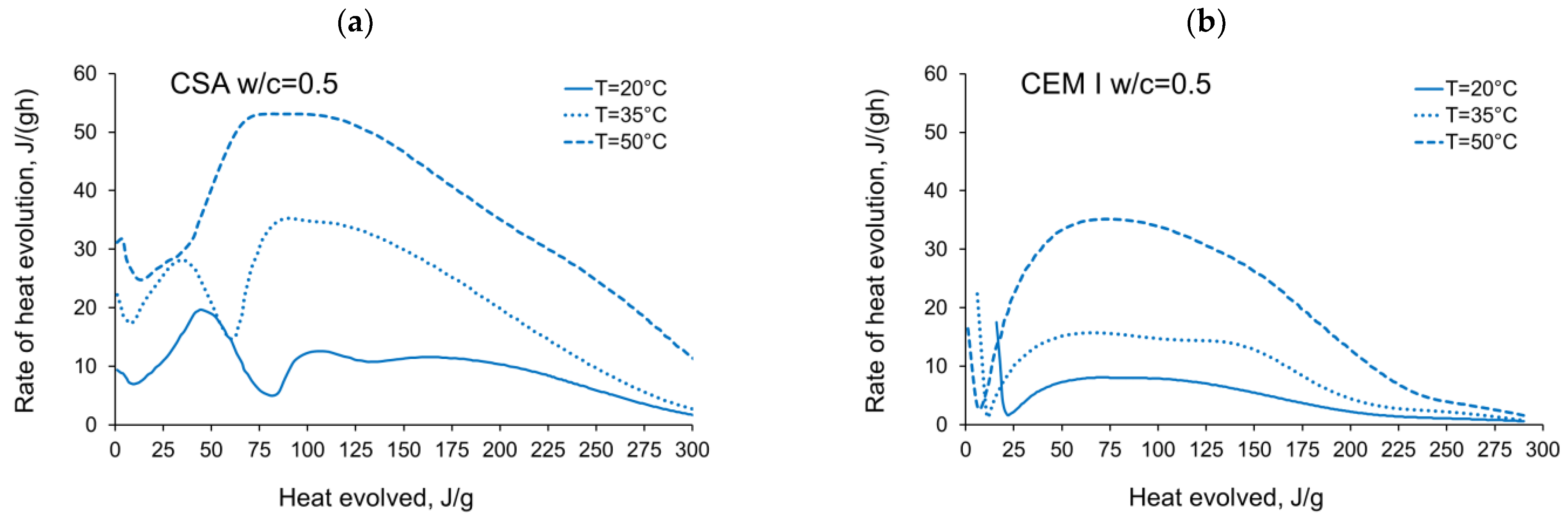
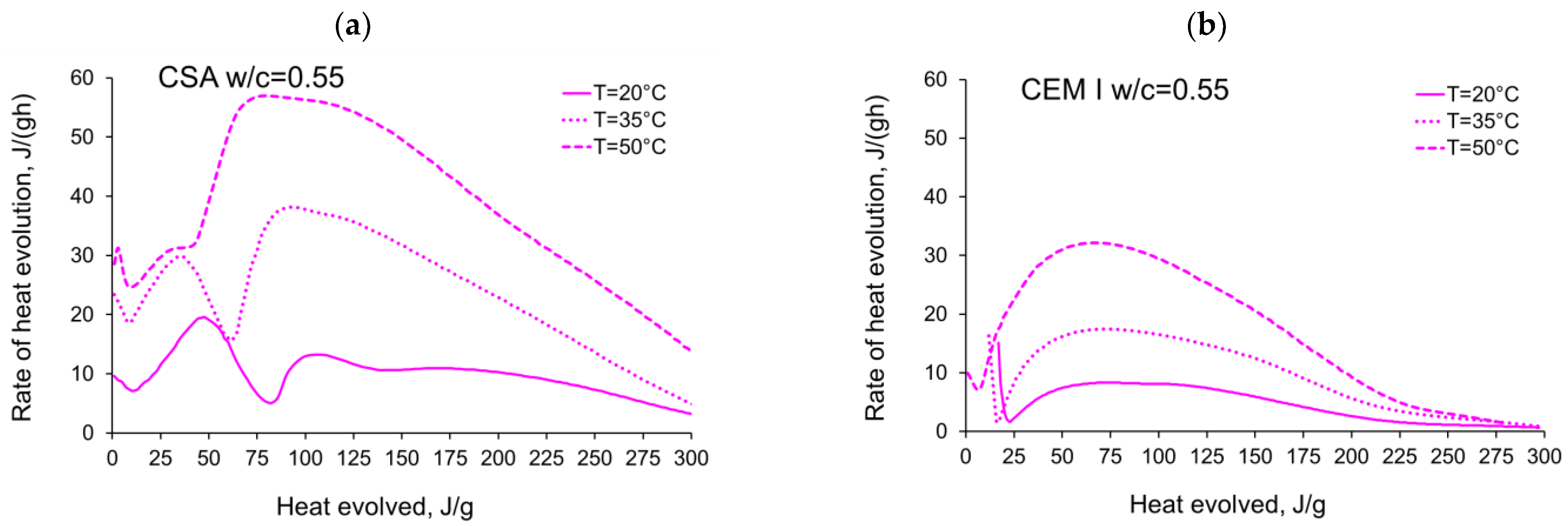
3.1. Evolution and the Rate of Hydration Heat
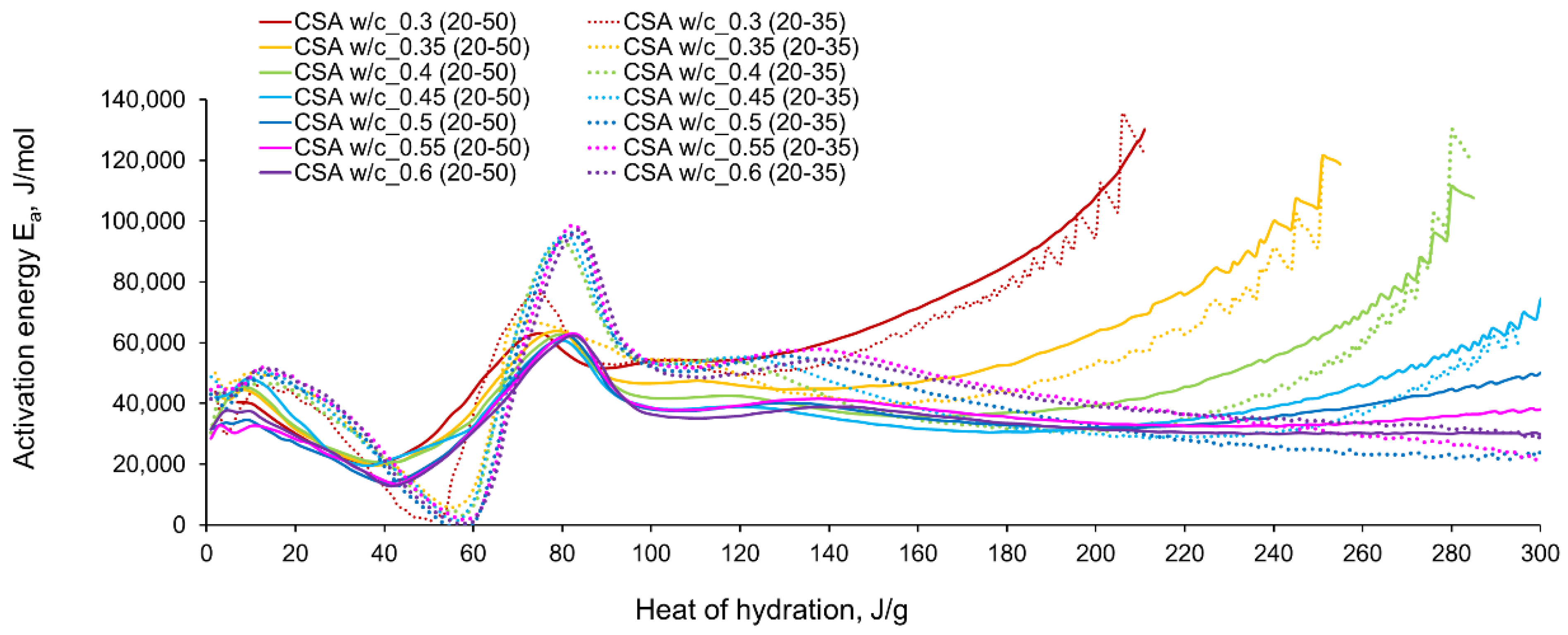
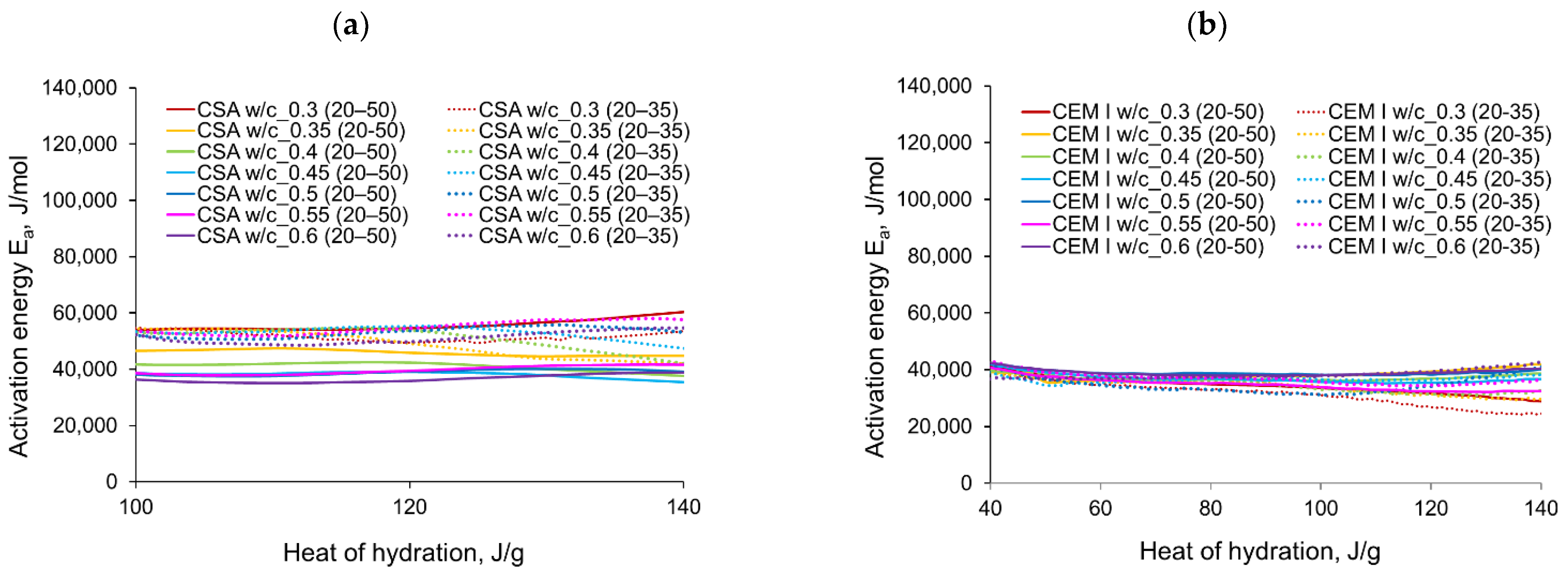
3.2. Apparent Activation Energy
- The variations in apparent activation energy of CSA cement pastes are quite substantial, especially compared to the relatively constant values obtained for reference cement pastes made of ordinary Portland cement, CEM I. Moreover, in the case of CSA cement, both water/cement ratio and curing temperature affect the values and the evolution of apparent activation energy.
- For CSA cement, the variations are qualitatively similar considering the two applied sets of curing temperatures (20 °C/35 °C and 20 °C/50 °C); however, they vary quantitatively. In the case of ordinary Portland cement, CEM I, the differences between the two sets of curing temperatures are not so substantial,
- In the case of CSA, for released heat lower than 100 J/g, particularly rapid changes in the values of activation energy are visible, and no stable period is observed. Moreover, these variations are more distinctive for the activation energy calculated using the isothermal tests at 20 °C/35 °C. In this case, the maximum value of reached nearly 100,000 Jmol−1 for the hydration heat equal to ~80 J/g, while earlier the activation energy almost dropped to 400–1000 Jmol−1 (for heat evolved close to 60 J/g). This effect can already be seen in the graphs presenting the rate of heat released at 20 °C and 35 °C (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10), where the overlap of curves is noticeable in the range of hydration heat 50–60 J/g. For hydration heat greater than 100 J/g, the curves describing the activation energy obtained from two sets of curing temperatures (20 °C/35 °C and 20 °C/50 °C) are more congruous. Simultaneously, the influence of the water/cement ratio is perceivable. In this regard, for w/c ≤ 0.45, the activation energy successively grows up to the value of 120,000 J/mol−1. For w/c equal to 0.5 and 0.55, the increase of the energy value is insignificant, and for w/c = 0.6, a decrease is even visible.
- for CSA: 51,900 J/mol (tests 20 °C/35 °C) and 42,213 J/mol (tests 20 °C/50 °C),
- for OPC: 35,424 J/mol (tests 20 °C/35 °C) and 37,228 J/mol (tests 20 °C/50 °C).
4. Conclusions
- The evolved heat of CSA cement pastes increases with the increase of the water/cement ratio. The largest, 20% increase in heat emission, was recorded for the water/cement change from 0.3 to 0.35. For higher w/c ratios the average difference between consecutive measured w/c ratios was 10%. In the case of OPC, the heat released at the curing temperature of 20 °C is practically the same for different w/c. At a higher curing temperature of 35 °C, the w/c effect is visible but still less pronounced than for CSA cement.
- The temperature of curing affects the evolved heat of CSA cement the most in the first 12 h. After 168 h, there is no significant difference between the evolved heat at 20 °C and 35 °C, while the evolved heat at 50 °C was significantly lower, which is most likely due to water vaporization. Nevertheless, the hydration of CSA pastes is significantly accelerated at 35 °C and 50 °C. The effect of the elevated temperature on the hydration process is much greater than that of ordinary Portland cement.
- The complex thermal behaviour of CSA pastes with several heat flow maxima has been confirmed in the performed isothermal tests at different curing temperatures. Four heat flow peaks in CSA cement are detected for tests at 20 °C, while at 35 °C and 50 °C three peaks are confirmed. Generally, the strength of the hydration peaks decreased with decreasing water/cement ratio. At 35 °C and 50 °C, the end of the induction period and the occurring time of the main peaks are remarkably shortened. Simultaneously, the exothermic peaks have extremely narrow shapes, and the peak value increases greatly compared to tests at 20 °C.
- The variations in apparent activation energy are substantial, and they cannot be considered as a constant value for the CSA type of cement. Only for the relatively small range of the evolved heat (101–140 J/g) did the curves of energy evolution reveal steady behaviour. In this case, the average activation energy depends both on the water/cement ratio and the curing temperature. The average values in the stable period for CSA cement are much higher than those for ordinary Portland cement.
- The hydration region of CSA with especially rapid variations of activation energy covers the initial period of heat release, up to the value of 100 J/g. Using the isothermal results at 20 °C and 35 °C in the calculation procedure, especially sharp changes in energy are obtained. Moreover, both very small (400–1000 J·mol−1) and extremely high (100,000 Jmol−1) values are observed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Favier, A.; De Wolf, C.; Schrivener, K.; Habert, G. A Sustainable Future for the European Cement and Concrete Industry: Technology Assessment for Full Decarbonisation of the Industry by 2050; ETH Zurich: Zürich, Switzerland, 2018. [Google Scholar] [CrossRef]
- Skjærseth, J.B.; Eikeland, P.O. Corporate Responses to EU Emissions Trading: Resistance, Innovation or Responsibility; Taylor & Francis: London, UK, 2016. [Google Scholar]
- Jain, M. Use and properties of blast furnace slag as a building material—A review. Int. J. Recent Contrib. Eng. Sci. IT 2014, 2, 54. [Google Scholar] [CrossRef][Green Version]
- Lemougna, P.N.; Wang, K.-T.; Tang, Q.; Nzeukou, A.; Billong, N.; Melo, U.C.; Cui, X.-M. Review on the use of volcanic ashes for engineering applications. Resour. Conserv. Recycl. 2018, 137, 177–190. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Wang, D.H.; Shi, C.J.; Farzadnia, N.; Shi, Z.G.; Jia, H.F.; Ou, Z.H. A review on use of limestone powder in cement-based materials: Mechanism, hydration and microstructures. Constr. Build. Mater. 2018, 181, 659–672. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Winnefeld, F. Calcium sulfoaluminate cement: An example of a low CO2—Alternative to portland cement. In Proceedings of the WTA-Colloquium—Effect of Climate Change on Built Heritage, Eindhoven, The Netherlands, 11–12 March 2010; pp. 98–108. [Google Scholar]
- Kubissa, W. Air permeability of air-entrained hybrid concrete containing CSA cement. Buildings 2020, 10, 119. [Google Scholar] [CrossRef]
- Nguyen, K.S.; Nguyen-Ngoc, T.H.; Nguyen-Phung, A.T.; Do, Q.M. Preparation of calcium sulfoaluminate cement from baux-ite/red mud of Tan Rai—Lam Dong. In Proceedings of the 7th International Conference of Asian Concrete Federation ACF 2016, Hanoi, Vietnam, 30 October–2 November 2016; pp. 21–26. [Google Scholar]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11, 074029. [Google Scholar] [CrossRef]
- Chen, I.A.; Hargis, C.W.; Juenger, M.C.G. Understanding expansion in calcium sulfoaluminate–belite cements. Cem. Concr. Res. 2012, 42, 51–60. [Google Scholar] [CrossRef]
- Madan Mohan Reddy, K.; Srimurali, M.; Bhaskar, M.; Mohan Reddy, K. Characterization of calcium sulfoaluminate cement. Int. J. ChemTech Res. 2014, 6, 1696–1698. [Google Scholar]
- Aranda, M.A.G.; De la Torre, A.G. Sulfoaluminate Cement, Eco-Efficient Concrete; Woodhead Publishing: Southston, UK, 2013; pp. 488–522. [Google Scholar]
- Winnefeld, F.; Kaufmann, J. Concrete produced with calcium sulfoaluminate cement—A potential system for energy and heat storage. In Proceedings of the First Middle East Conference on Smart Monitoring, Assessment and Rehabilitation of Civil Structures (SMAR 2011), Dubai, United Arab Emirates, 8–10 February 2011; pp. 1–9. [Google Scholar]
- Ambroise, J.; Pera, J. Immobilization of calcium sulfate contained in demolition waste. J. Hazard. Mater. 2008, 151, 840–846. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J.M. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Martin, L.H.; Winnefeld, F.; Müller, C.J.; Lothenbach, B. Contribution of limestone to the hydration of calcium sulfoaluminate cement. Cem. Concr. Compos. 2015, 62, 204–211. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Berger, S.; Coumes, C.C.D.; Le Bescop, P.; Damidot, D. Influence of a thermal cycle at early age on the hydration of calcium sulphoaluminate cements with variable gypsum contents. Cem. Concr. Res. 2011, 41, 149–160. [Google Scholar] [CrossRef]
- Li, P.; Gao, X.; Wang, K.; Tam, V.W.Y.; Li, W. Hydration mechanism and early frost resistance of calcium sulfoaluminate cement concrete. Constr. Build. Mater. 2020, 239, 117862. [Google Scholar] [CrossRef]
- Batog, M.; Synowiec, K. Cement I Spoiwa Specjalne Zawierające Klinkier Siarczanoglinianowy (Cement and Special Binders Containing Sulphoaluminate Clinker); Budownictwo Technologie Architektura: Kraków, Poland, 2017; Volume 1, pp. 59–64. [Google Scholar]
- Qin, L.; Gao, X.; Zhang, A. Potential application of Portland cement-calcium sulfoaluminate cement blends to avoid early age frost damage. Constr. Build. Mater. 2018, 190, 363–372. [Google Scholar] [CrossRef]
- Ramanathan, S.; Halee, B.; Suraneni, P. Effect of calcium sulfoaluminate cement prehydration on hydration and strength gain of calcium sulfoaluminate cement-ordinary portland cement mixtures. Cem. Concr. Compos. 2020, 112, 103694. [Google Scholar] [CrossRef]
- Li, W. The properties and hydration of portland cement containing calcium sulfoaluminate cement. Ceram. Silik. 2018, 62, 364–373. [Google Scholar] [CrossRef]
- Dovál, M.; Palou, M.; Kovár, V. Heat evolution and mechanism of hydration in CaO-Al2O3-SO3 system. Ceram. Silik. 2005, 49, 104–108. [Google Scholar]
- Jeong, Y.; Hargis, C.W.; Kang, H.; Chun, S.C.; Moon, J. The effect of elevated curing temperatures on high Ye’elimite calcium sulfoaluminate cement mortars. Materials 2019, 12, 1072. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, N.; Xu, L. Hydration evolution and compressive strength of calcium sulphoaluminate cement constantly cured over the temperature range of 0 to 80 °C. Cem. Concr. Res. 2017, 100, 203–213. [Google Scholar] [CrossRef]
- BS EN 197-1:2011 cement. In Composition, Specifications and Conformity Criteria for Common Cements; British Standards Institution: London, UK, 2011.
- Klemczak, B.; Batog, M.; Giergiczny, Z.; Żmij, A. Complex effect of concrete composition on the thermo-mechanical behaviour of mass concrete. Materials 2018, 11, 2207. [Google Scholar] [CrossRef] [PubMed]
- Klemczak, B.; Żmij, A. Reliability of standard methods for evaluating the early-age cracking risk of thermal-shrinkage origin in concrete walls. Constr. Build. Mater. 2019, 226, 651–661. [Google Scholar] [CrossRef]
- Hansen, P.F.; Pedersen, E.J. Maturity computer for controlling curing and hardening of concrete. Nord. Betongfoerbundet 1977, 1, 21–25. [Google Scholar]
- D’Aloia, L.; Chanvillard, G. Determining the “apparent” activation energy of concrete: Ea—Numerical simulations of the heat of hydration of cement. Cem. Concr. Res. 2002, 32, 1277–1289. [Google Scholar] [CrossRef]
- Schindler, A.K.; Folliard, K.J. Heat of hydration models for cementitious materials. ACI Mater. J. 2005, 102, 24–33. [Google Scholar]
- Poole, J.L.; Riding, K.A.; Folliard, K.J.; Juenger, M.C.G.; Schindler, A.K. Methods for calculating activation energy for Portland cement. ACI Mater. J. 2007, 104, 202–311. [Google Scholar]
- Wilińska, I.; Pacewska, B. Influence of selected activating methods on hydration processes of mixtures containing high and very high amount of fly ash. J. Therm. Anal. Calorim. 2018, 133, 823–843. [Google Scholar] [CrossRef]
- CEN, EN 196-11:2018 Methods of testing cement. In Heat of Hydration. Isothermal Conduction Calorimetry Method; European Committee for Standardization: Brussels, Belgium, 2018.
- Glasstone, S.; Laidler, K.; Eyring, H. The Theory of Rate Processes; McGraw-Hill Book Company: New York, NY, USA, 1941. [Google Scholar]
- Zákoutský, J.; Tydlitát, V.; Černý, R. Effect of temperature on the early-stage hydration characteristics of Portland cement: A large-volume calorimetric study. Constr. Build. Mater. 2012, 36, 969–976. [Google Scholar] [CrossRef]
- Klemczak, B.; Batog, M. Heat of hydration of low-clinker cements. Pt. 1: Semi-adiabatic and isothermal tests at different temperature. J. Therm. Anal. Calorim. 2016, 123, 1351–1360. [Google Scholar] [CrossRef]
- Han, F.; Zhang, Z.; Wang, D.; Yan, P. Hydration kinetics of composite binder containing slag at different temperatures. J. Therm. Anal. Calorim. 2015, 121, 815–827. [Google Scholar] [CrossRef]
- Wirquin, E.; Broda, M.; Duthoit, B. Determination of the apparent activation energy of one concrete by calorimetric and me-chanical means Influence of a superplasticizer. Cem. Concr. Res. 2002, 32, 1207–1213. [Google Scholar] [CrossRef]
- Kada-Benameur, H.; Wirquin, E.; Duthoit, B. Determination of apparent activation energy of concrete by isothermal calorimetry. Cem. Concr. Res. 2000, 30, 301–305. [Google Scholar] [CrossRef]
- Broda, M.; Wirquin, E.; Duthoit, B. Conception of an isothermal calorimeter for concrete—Determination of the apparent activation energy. Mater. Struct. 2002, 35, 389–394. [Google Scholar] [CrossRef]
- Klemczak, B.; Batog, M. Heat of hydration of low-clinker cements. Pt. 2: Determination of apparent activation energy and validity of the equivalent age approach. J. Therm. Anal. Calorim. 2016, 123, 1361–1369. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, G.; Liu, Y. Early hydration heat of calcium sulfoaluminate cement with influences of supplementary cementitious materials and water to binder ratio. Materials 2021, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Bobrowski, A.; Nocuń-Wczelik, W.; Gawlicki, M.; Łagosz, A.K.; Łój, G. Cement: Metody Badań, Wybrane Kierunki Stosowania. (Cement: Testing Methods, Chosen Uses); Wydawnictwo AGH: Kraków, Poland, 2015. (In Polish) [Google Scholar]
- Neville, A.M. Properties of Concrete; Pearson Education: Harlow, UK, 2012. [Google Scholar]




| Cement Type | Constituent, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LOI | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | Na2Oeq | |
| CSA | 0.46 | 9.2 | 28.1 | 1.52 | 39.2 | 3.5 | 11.4 | 0.08 | 0.35 | - |
| CEM I (OPC) | - | 20.6 | 4.7 | 2.8 | 64.4 | 1.2 | 2.8 | 0.2 | 0.4 | 0.46 |
| Cement Property | Unit | Value | |
|---|---|---|---|
| CSA | CEM I (OPC) | ||
| Initial setting time | min | 50 | 191 |
| The soundness of cement, by Le Chatelier’s method | mm | 1 | 0.4 |
| Compressive strength after 2 days | MPa | 42.6 | 30.1 |
| Compressive strength after 28 days | MPa | 67.7 | 57.4 |
| Specific surface area | cm2/g | 5600 | 4400 |
| Sample, Curing Temperature | The Heat Evolved, Jg−1 | The Peak Value of Heat Evolution Rate, Jg−1·h−1 | |||||
|---|---|---|---|---|---|---|---|
| 12 h | 41 h | 72 h | 168 h | Second Peak | Third Peak | Fourth Peak | |
| CSA w/c = 0.3 | |||||||
| 20 °C | 88.9 | 177.3 | 195.3 | 211.0 | 14.2 | 6.6 | 5.9 |
| 35 °C | 168.7 | 220.8 | 231.7 | 243.0 | 21.8 | 19.2 | - |
| 50 °C | 243.1 | 270.0 | 276.9 | 261.1 | 35.5 | 48.0 | - |
| CSA w/c = 0.35 | |||||||
| 20 °C | 96.5 | 219.4 | 238.9 | 255.2 | 15.7 | 9.3 | 9.2 |
| 35 °C | 203.0 | 260.3 | 275.5 | 296.2 | 25.1 | 26.1 | - |
| 50 °C | 278.9 | 312.6 | 311.0 | 287.5 | 40.1 | 51.0 | - |
| CSA w/c = 0.4 | |||||||
| 20 °C | 105.6 | 253.0 | 272.3 | 284.6 | 16.8 | 10.5 | 11.2 |
| 35 °C | 225.1 | 290.6 | 307.2 | 325.4 | 26.5 | 30.2 | - |
| 50 °C | 291.0 | 331.7 | 330.6 | 305.0 | 41.0 | 51.2 | - |
| CSA w/c = 0.45 | |||||||
| 20 °C | 109.5 | 273.9 | 296.5 | 314.4 | 17.1 | 11.3 | 11.6 |
| 35 °C | 240.4 | 311.6 | 330.5 | 351.6 | 26.8 | 32.4 | - |
| 50 °C | 311.5 | 337.2 | 326.0 | 281.6 | 47.1 | 57.2 | - |
| CSA w/c = 0.5 | |||||||
| 20 °C | 118.1 | 296.0 | 322.4 | 341.8 | 19.2 | 12.6 | 11.7 |
| 35 °C | 255.8 | 330.0 | 346.8 | 362.5 | 28.1 | 35.3 | - |
| 50 °C | 321.4 | 347.6 | 336.3 | 291.8 | 33.9 | 56.3 | - |
| CSA w/c = 0.55 | |||||||
| 20 °C | 124.3 | 315.7 | 350.1 | 372.3 | 19.5 | 13.2 | 10.9 |
| 35 °C | 271.8 | 346.6 | 362.7 | 375.8 | 29.4 | 38.5 | - |
| 50 °C | 322.0 | 347.9 | 337.8 | 299.5 | 31.2 | 56.8 | - |
| CSA w/c = 0.6 | |||||||
| 20 °C | 129.2 | 327.9 | 365.8 | 386.4 | 20.7 | 14.3 | 11.2 |
| 35 °C | 278.5 | 358.1 | 369.9 | 388.3 | 29.7 | 38.7 | - |
| 50 °C | 327.4 | 351.1 | 340.3 | 297.6 | 36.7 | 57.4 | - |
| Sample, Curing Temperature | The Heat Evolved, Jg−1 | The Peak Value of Heat Evolution Rate, Jg−1·h−1 | |||||
|---|---|---|---|---|---|---|---|
| 12 h | 41 h | 72 h | 168 h | Second Peak | Third Peak | Fourth Peak | |
| CEM I w/c = 0.3 | |||||||
| 20 °C | 73.8 | 213.6 | 257.0 | 320.8 | 8.2 | - | - |
| 35 °C | 132.1 | 235.2 | 250.9 | 250.2 | 16.1 | - | - |
| 50 °C | 191.3 | 263.8 | 270.4 | 258.0 | 32.1 | - | - |
| CEM I w/c = 0.35 | |||||||
| 20 °C | 67.5 | 204.7 | 252.4 | 291.6 | 8.3 | - | - |
| 35 °C | 136.5 | 254.2 | 275.3 | 282.4 | 16.9 | - | - |
| 50 °C | 218.4 | 297.2 | 299.9 | 279.1 | 33.9 | - | - |
| CEM I w/c = 0.4 | |||||||
| 20 °C | 73.9 | 206.1 | 251.3 | 295.4 | 8.3 | - | - |
| 35 °C | 137.6 | 258.9 | 282.1 | 282.3 | 17.1 | - | - |
| 50 °C | 209.6 | 290.4 | 291.2 | 257.7 | 33.5 | - | - |
| CEM I w/c = 0.45 | |||||||
| 20 °C | 76.1 | 211.3 | 254.3 | 317.8 | 8.3 | - | - |
| 35 °C | 148.5 | 273.3 | 311.4 | 338.6 | 17.2 | - | - |
| 50 °C | 209.5 | 282.3 | 272.8 | 199.8 | 33.9 | - | - |
| CEM I w/c = 0.5 | |||||||
| 20 °C | 74.8 | 208.2 | 249.7 | 311.5 | 8.1 | - | - |
| 35 °C | 133.9 | 257.7 | 294.0 | 306.9 | 15.7 | - | - |
| 50 °C | 218.7 | 299.5 | 304.7 | 271.1 | 35.2 | - | - |
| CEM I w/c = 0.55 | |||||||
| 20 °C | 78.0 | 214.3 | 255.4 | 319.3 | 8.3 | - | - |
| 35 °C | 146.6 | 266.6 | 302.1 | 315.1 | 17.5 | - | - |
| 50 °C | 208.4 | 285.4 | 297.0 | 278.7 | 32.1 | - | - |
| CEM I w/c = 0.6 | |||||||
| 20 °C | 75.7 | 209.7 | 251.2 | 313.5 | 8.1 | - | - |
| 35 °C | 149.7 | 271.2 | 311.2 | 342.6 | 17.1 | - | - |
| 50 °C | 219.2 | 290.9 | 290.4 | 233.4 | 34.6 | - | - |
| Value | Apparent Activation Energy, J mol−1 | ||||||
|---|---|---|---|---|---|---|---|
| w/c = 0.3 | w/c = 0.35 | w/c = 0.4 | w/c = 0.45 | w/c = 0.5 | w/c = 0.55 | w/c = 0.6 | |
| Average values calculated from tests 20 °C/35 °C | |||||||
| CSA—period | in range of evolved heat: 101–136 Jg−1 | ||||||
| CSA—value | 50,917 | 49,131 | 51,623 | 53,477 | 53,230 | 53,906 | 51,016 |
| CEM I (OPC)—period | in range of evolved heat: 40–140 Jg−1 | ||||||
| CEM I (OPC)—value | 31,211 | 33,737 | 34,638 | 36,667 | 36,615 | 36,820 | 38,282 |
| Average values calculated from tests 20 °C/50 °C | |||||||
| CSA—period | in range of evolved heat: 101–136 Jg−1 | ||||||
| CSA—value | 54,978 | 45,968 | 41,158 | 38,169 | 38,915 | 39,712 | 36,592 |
| CEM I (OPC)—period | in range of evolved heat: 40–140 Jg−1 | ||||||
| CEM I (OPC)—value | 33,922 | 38,208 | 37,085 | 36,805 | 39,010 | 36,601 | 38,967 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołaszewska, M.; Klemczak, B.; Gołaszewski, J. Thermal Properties of Calcium Sulphoaluminate Cement as an Alternative to Ordinary Portland Cement. Materials 2021, 14, 7011. https://doi.org/10.3390/ma14227011
Gołaszewska M, Klemczak B, Gołaszewski J. Thermal Properties of Calcium Sulphoaluminate Cement as an Alternative to Ordinary Portland Cement. Materials. 2021; 14(22):7011. https://doi.org/10.3390/ma14227011
Chicago/Turabian StyleGołaszewska, Małgorzata, Barbara Klemczak, and Jacek Gołaszewski. 2021. "Thermal Properties of Calcium Sulphoaluminate Cement as an Alternative to Ordinary Portland Cement" Materials 14, no. 22: 7011. https://doi.org/10.3390/ma14227011
APA StyleGołaszewska, M., Klemczak, B., & Gołaszewski, J. (2021). Thermal Properties of Calcium Sulphoaluminate Cement as an Alternative to Ordinary Portland Cement. Materials, 14(22), 7011. https://doi.org/10.3390/ma14227011








