An Improved Speciation Method Combining IC with ICPOES and Its Application to Iodide and Iodate Diffusion Behavior in Compacted Bentonite Clay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Bentonite Clay
2.2. Preparation of Iodide (I−) and Iodate (IO3−) Standard Solution
2.3. Qualitative and Quantitative Analyses with IC-ICP-OES to Detect I− and IO3−
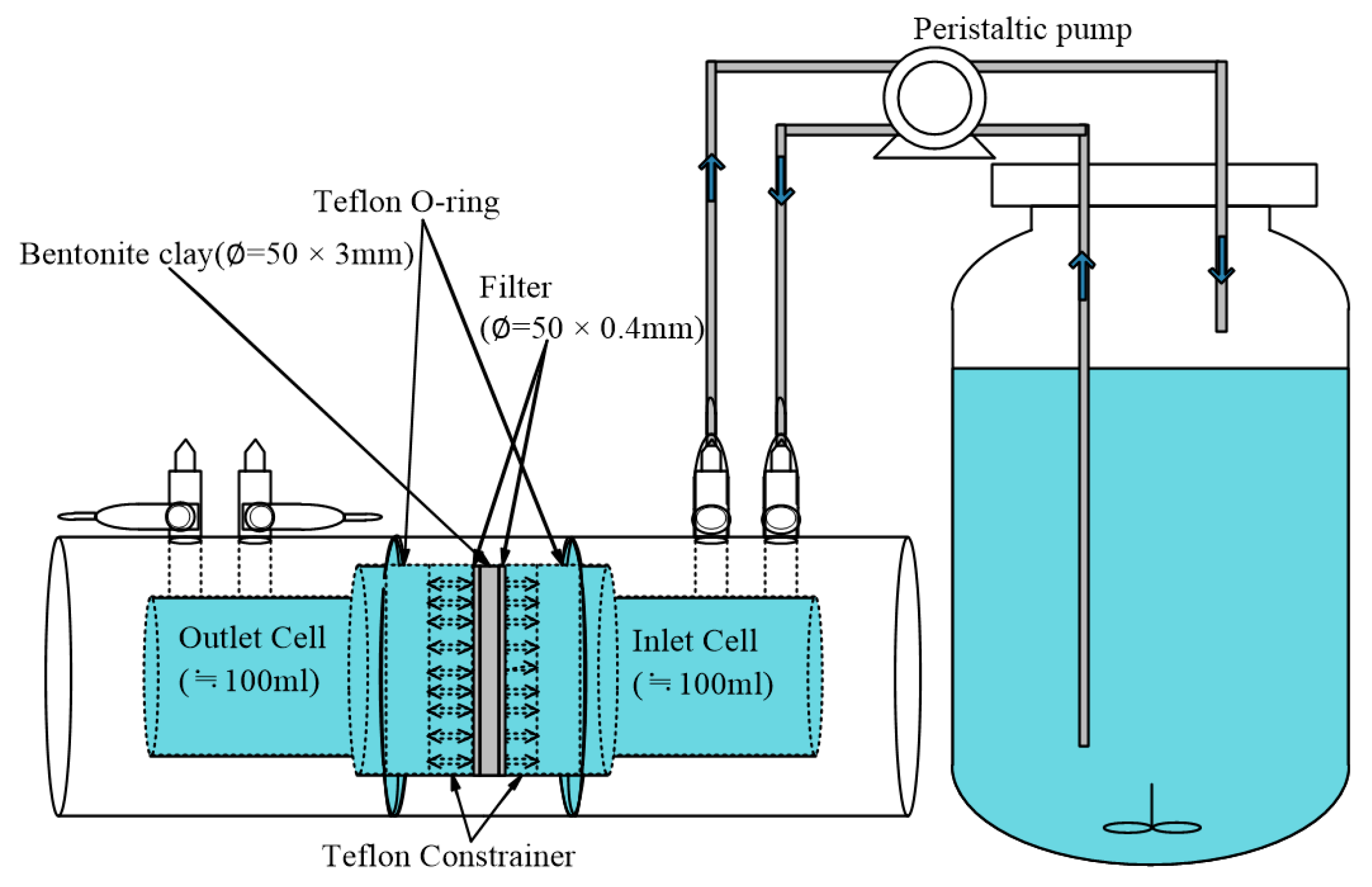
2.4. Through-Diffusion Experiments (TD): Column Tests
2.5. Estimation of Diffusion Coefficient
2.6. Estimation of Distribution Coefficients (Kd)
3. Results
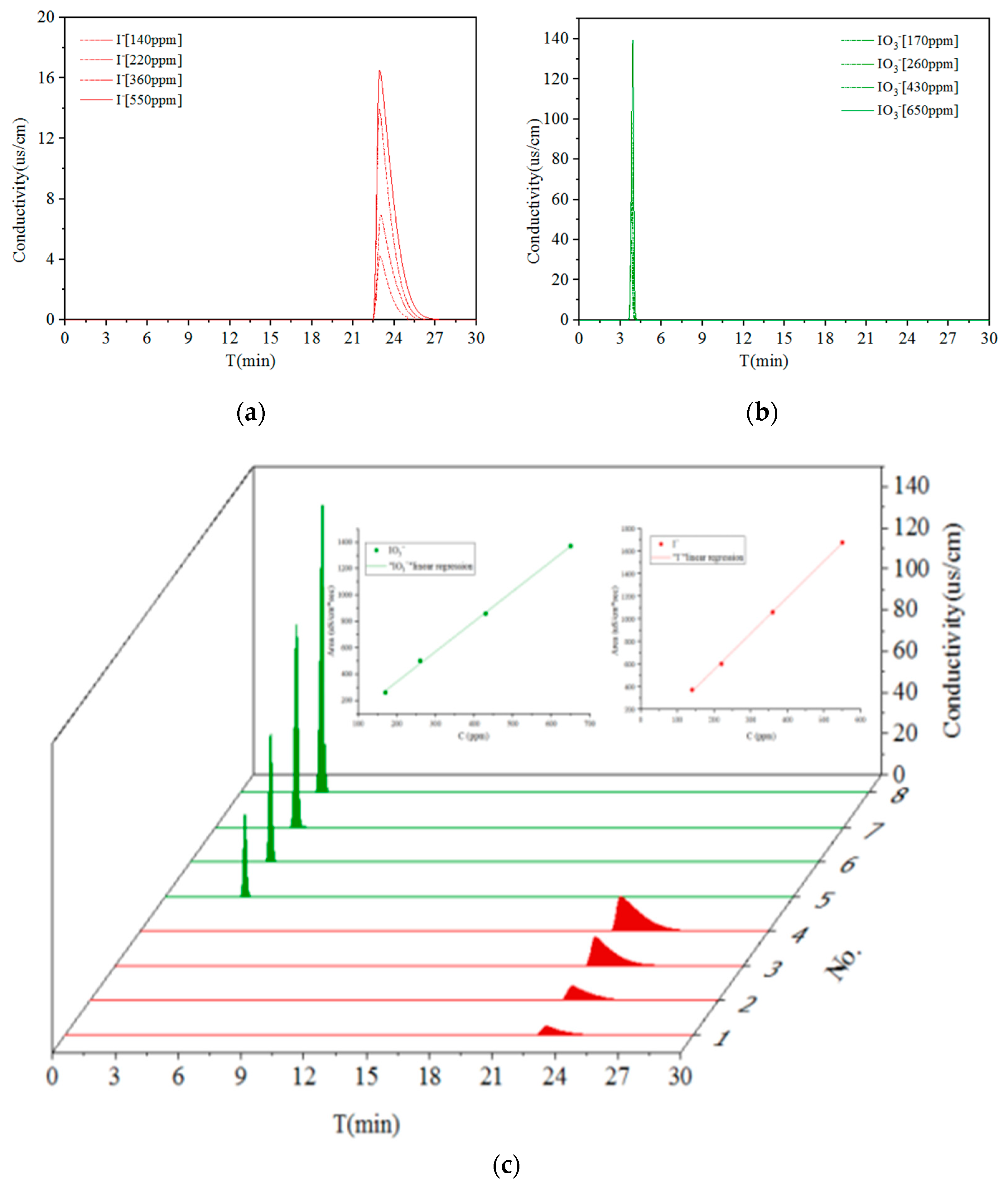
3.1. Identification for Individual I− and IO3−
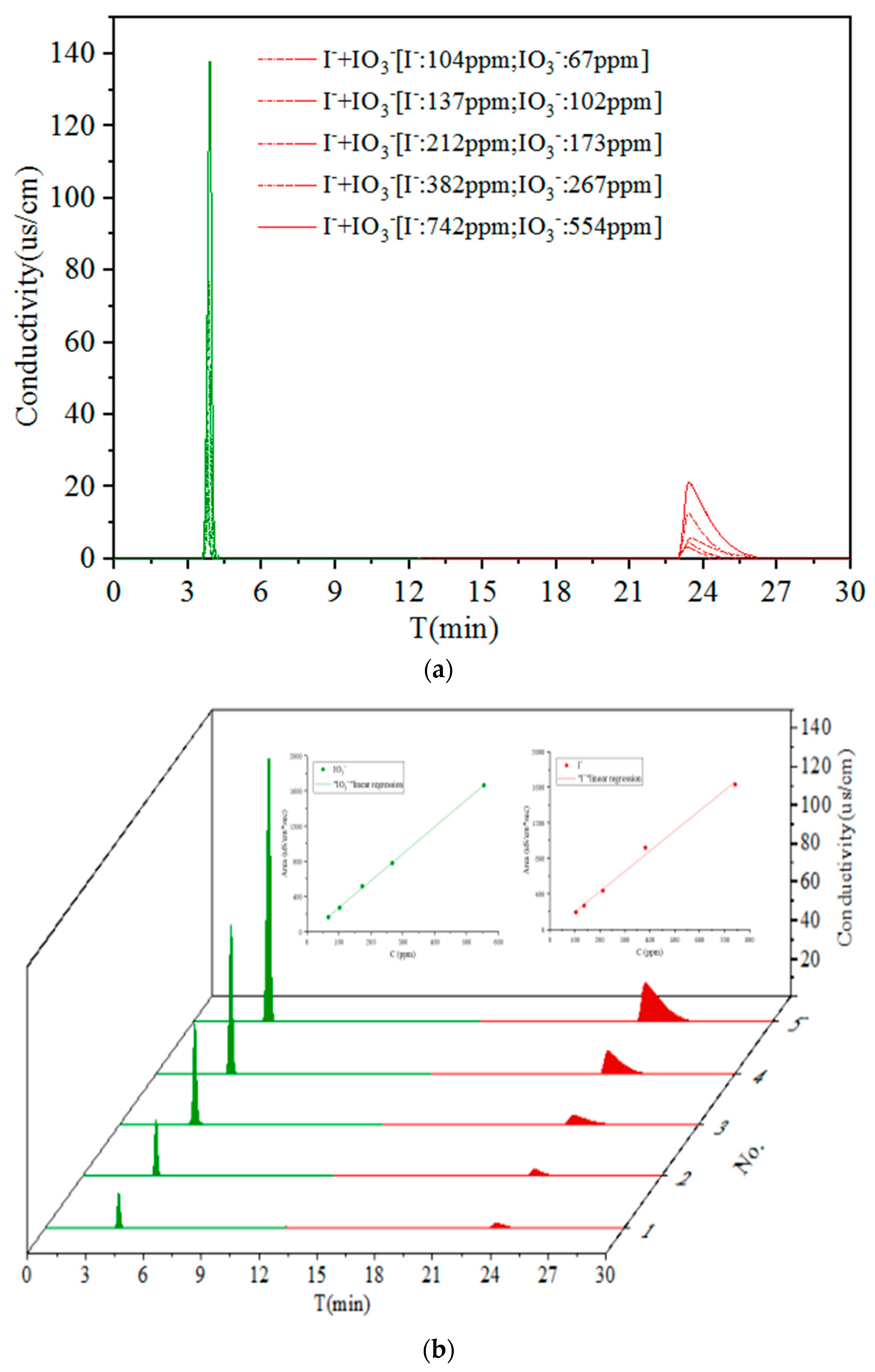
3.2. Performance for Co-Existing I− and IO3−
3.3. Diffusion of I− and IO3−
3.4. Batch Tests of I− and IO3−
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taiwan Power Company. Preliminary Technical Feasibility Study for Final Disposal of Spent Nuclear Fuel—2009; Progress Report (Summary); Taiwan Power Company: Taipei, Taiwan, 2009. [Google Scholar]
- Japan Nuclear Cycle Development Institute. Japan, H12: Project to Establish the Scientific and Technical Basis for HLW Disposal in Japan; Japan Nuclear Cycle Development Institute: Tokyo, Japan, 2000. [Google Scholar]
- Yang, T.; Knutsson, S.; Liu, X. Swelling properties and permeability of expandable clays of potential use for nuclear waste disposal. J. Earth Sci. Geotech. Eng. 2016, 6, 9–61. [Google Scholar]
- Cui, Y.J. On the hydro-mechanical behaviour of MX80 bentonite-based materials. J. Rock Mech. Geotech. Eng. 2017, 9, 565–574. [Google Scholar] [CrossRef]
- Chen, Y.G.; Dong, X.X.; Zhang, X.D.; Ye, W.M.; Cui, Y.J. Cyclic thermal and saline effects on the swelling pressure of densely compacted Gaomiaozi bentonite. Eng. Geol. 2019, 255, 37–47. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, Y.Q.; Pan, K.; Ye, W.M.; Wang, Q. Influence of dry density and water salinity on the swelling pressure and hydraulic conductivity of compacted GMZ01 bentonite–sand mixtures. Acta Geotech. 2021, 1–18. [Google Scholar] [CrossRef]
- Jan, Y.L.; Tsai, S.C.; Wei, Y.Y.; Tung, N.C.; Wei, C.C.; Hsu, C.N. Coupled mechanics, hydraulics and sorption properties of mixtures to evaluate buffer/backfill materials. Phys. Chem. Earth Parts A/B/C 2007, 32, 789–794. [Google Scholar] [CrossRef]
- Jan, Y.L.; Tsai, S.C.; Hsu, C.N. Associating characterization of bentonite-based buffer/backfill materials by distribution ratio (Rd) and Plastic index (PI). J. Mar. Sci. Technol. 2007, 15, 17–23. [Google Scholar] [CrossRef]
- Glaus, M.A.; Müller, W.; Van Loon, L.R. Diffusion of iodide and iodate through Opalinus Clay: Monitoring of the redox state using an anion chromatographic technique. Appl. Geochem. 2008, 23, 3612–3619. [Google Scholar] [CrossRef]
- Van Loon, L.R.; Soler, J.M.; Jakob, A.; Bradbury, M.H. Effect of confining pressure on the diffusion of HTO, 36Cl− and 125I− in a layered argillaceous rock (Opalinus Clay): Diffusion perpendicular to bedding. Appl. Geochem. 2003, 18, 1653–1662. [Google Scholar] [CrossRef]
- Shi, Y.; Lee, C.P.; Yu, H.; Hu, Y.; Liu, H.; Tien, N.C.; Wang, Y.; Liu, W.; Kong, J. Study on Advection-Dispersion Behavior for Simulation of HTO and Se Transport in crushed granite. J. Radioanal. Nucl. Chem. 2021, 328, 1329–1338. [Google Scholar] [CrossRef]
- Shih, Y.H.; Lee, I.H.; Ni, C.F.; Tsai, T.L.; Chen, L.C.; Lee, C.P.; Tsai, S.C.; Su, T.Y. Experimental and numerical investigations of 99TcO42− diffusion in compacted SPV 200 bentonite. J. Radioanal. Nucl. Chem. 2018, 316, 1081–1089. [Google Scholar] [CrossRef]
- Fan, Y.; Hou, X.; Fukuda, M.; Zheng, J.; Aono, T.; Chen, N.; Zhang, L.; Zhou, W. 129I in a sediment core offshore Fukushima: Distribution, source and its implication. Chemosphere 2020, 252, 126524. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Zhao, S.W.; Jia, M.L. The study of safety assessment guide of HLW geologic disposal system. J. Nucl. Sci. Eng. 2016, 36, 313–322. [Google Scholar]
- Zhang, S.; Xu, C.; Creely, D.; Ho, Y.F.; Li, H.P.; Grandbois, R.; Schwehr, K.A.; Kaplan, D.I.; Yeager, C.M.; Wellman, D. Iodine-129 and Iodine-127 speciation in groundwater at the Hanford Site, U.S: Iodate Incorporation into Calcite. Environ. Sci. Technol. 2013, 47, 9635–9642. [Google Scholar] [CrossRef] [PubMed]
- Kimmig, S.R.; Thompson, C.; Baum, S.; Brown, C.F. Evaluation of iodine speciation and 129I/127I ratios at low concentrations in environmental samples using IC-ICP-MS. J. Radioanal. Nucl. Chem. 2021, 327, 929–937. [Google Scholar] [CrossRef]
- Bors, J.; Dultz, S.; Riebe, B. Organophilic bentonites as adsorbents for radionuclides: I. Adsorption of ionic fission products. Appl. Clay Sci. 2000, 16, 1–13. [Google Scholar] [CrossRef]
- Riebe, B.; Bors, J.; Dultz, S. Retardation capacity of organophilic bentonite for anionic fission products. J. Contam. Hydrol. 2001, 47, 255–264. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nakayama, S.; Nagao, S.; Kizaki, M. Diffusive transport of neptunium and plutonium through compacted sand-bentonite mixtures under anaerobic conditions. Radiochim. Acta 2007, 95, 115–125. [Google Scholar] [CrossRef]
- Kong, J.; Lee, C.P.; Sun, Y.; Hua, R.; Liu, W.; Wang, Z.; Li, Y.; Wang, Y. Anion exclusion and sorption effect for compacted bentonite: The dependency of diffusion coefficients and capacity of HTO and Se (IV). J. Radioanal. Nucl. Chem. 2021, 328, 717–725. [Google Scholar] [CrossRef]
- Lebeau, D.; Leroy, N.; Doizi, D.; Wu, T.D.; Guerquin-Kern, J.L.; Perrin, L.; Ortega, R.; Voiseux, C.; Fournier, J.B.; Potin, P.; et al. Mass spectrometry-based imaging techniques for iodine-127 and iodine-129 detection and localization in the brown alga Laminaria digitata. J. Environ. Radioact. 2021, 231, 106552. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, X.; Zhang, Z.; Zhang, L.; Chen, N.; Fang, M. Rapid Analysis of 129 I in Natural Water Samples Using Accelerator Mass Spectrometry. At. Spectrosc. 2021, 42, 190–196. [Google Scholar] [CrossRef]
- Molera, M.; Eriksen, T. Diffusion of 22Na+, 85Sr2+, 134Cs+ and 57Co2+ in bentonite clay compacted to different densities: Experiments and modeling. Radiochim. Acta 2002, 90, 753–760. [Google Scholar] [CrossRef]
- Wang, T.H.; Payne, T.E.; Harrison, J.J.; Teng, S.P. Interactions involving strontium and various organic acids on the surface of bentonite (MX-80). J. Radioanal. Nucl. Chem. 2015, 304, 95–105. [Google Scholar] [CrossRef]
- Lee, C.P.; Hu, Y.; Chen, D.; Tien, N.C.; Tsai, S.C.; Shi, Y.; Lee, I.H.; Ni, C.F. A statistical evaluation for comparable analysis for estimating diffusion coefficient of pertechnetate (99TcO4−) in compacted bentonite. Minerals 2021, 11, 1075. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- ASTM. Standard Test Method for Distribution Coefficients of Inorganic Species by the Batch Method. Am. Soc. Test. Mater. 2010, C1733-10, C26, 1–8. [Google Scholar]
- Lee, C.H.; Teng, S.P. A dual period diffusion model for measuring diffusion parameters. Waste Manag. 1993, 13, 15–24. [Google Scholar] [CrossRef]
- Takeda, A.; Tsukada, H.; Takaku, Y.; Satta, N.; Baba, M.; Shinata, T.; Hasegawa, H.; Unno, Y.; Hisamatsu, S. Determination of iodide, iodate and total iodine in natural water samples by HPLC with amperometric and spectrophotometric detection, and off-line UV irradiation. Anal. Sci. 2016, 32, 839–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loon, L.R.; Soler, J.M.; Bradbury, M.H. Diffusion of HTO, 36Cl− and 125I− in Opalinus Clay samples from Mont Terri: Effect of confining pressure. J. Contam. Hydrol. 2003, 61, 73–83. [Google Scholar] [CrossRef]
- Wigger, C.; Van Loon, L.R. Importance of Interlayer Equivalent Pores for Anion Diffusion in Clay-Rich Sedimentary Rocks. Environ. Sci. Technol. 2017, 57, 1998–2006. [Google Scholar] [CrossRef] [Green Version]
- Nightingale, E.R., Jr. Phenomenological theory of ion solvation. Effective radii of hydrated ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Yu, J.W.; Neretnieks, I. Diffusion and Sorption Properties of Radionuclides in Compacted Bentonite; SKB TR 97-12; Svensk Kärnbränslehantering AB: Stockholm, Sweden, 1997. [Google Scholar]

| Element (%) | Wyoming | Taiwan | Inner Mongolia |
|---|---|---|---|
| SiO2 | 53.78 | 50.87 | 63.71 |
| Al2O3 | 17.59 | 15.54 | 13.44 |
| Fe2O3 | 3.24 | 5.93 | 2.01 |
| CaO | 1.29 | 2.83 | 0.90 |
| Na2O | 1.92 | 1.21 | 1.67 |
| K2O | 0.47 | 1.41 | 0.67 |
| MnO | 0.01 | 0.10 | 0.04 |
| MgO | 2.04 | 2.11 | 2.67 |
| TiO2 | 0.14 | 0.38 | 0.12 |
| P2O5 | 0.05 | 0.07 | 0.03 |
| * LOI | 16.77 | 12.72 | 12.91 |
| Iodine Speciation | No. | C (ppm) | Area (uS/cm·s) |
|---|---|---|---|
| I− (Retention time:~20 min) | 1 | 140 | 372.2 |
| 2 | 220 | 602.5 | |
| 3 | 360 | 1060.1 | |
| 4 | 550 | 1677.1 | |
| * R2 | 0.99 | ||
| IO3− (Retention time:~4 min) | 5 | 170 | 261.7 |
| 6 | 260 | 503.0 | |
| 7 | 430 | 859.0 | |
| 8 | 650 | 1372.0 | |
| * R2 | 0.99 | ||
| Item | I− (ppm) | Area (uS/cm·s) | IO3− (ppm) | Area (uS/cm·s) | * R | |
|---|---|---|---|---|---|---|
| No. | ||||||
| 1 | 104 | 192.60 | 67 | 166.14 | 79.36 | |
| 2 | 137 | 266.94 | 102 | 273.76 | 68.67 | |
| 3 | 212 | 436.76 | 173 | 518.48 | 52.30 | |
| 4 | 382 | 921.08 | 267 | 781.06 | 71.17 | |
| 5 | 742 | 1631.97 | 554 | 1667.85 | 69.19 | |
| ** R 2 | 0.99 | 0.99 | - | |||
| RN | ρb (g/cm3) | De (m2/s) | αacc | Reference |
|---|---|---|---|---|
| I− | 2.0 | 3.28 × 10−11 | 0.20 | In this work In this work |
| IO3− | 2.0 | 2.43 × 10−11 | 0.25 | |
| 99TcO4− | 2.0 | 4.38 × 10−13 | 0.06 | Lee, C.P. et al. 2021 [25] Lee, C.P. et al. 2021 [25] Lee, C.P. et al. 2021 [25] Lee, C.P. et al. 2021 [25] |
| 1.2 | 8.89 × 10−12 | 0.39 | ||
| HTO | 2.0 | 2.16 × 10−11 | 0.29 | |
| 1.2 | 6.25 × 10−11 | 0.56 | ||
| SeO32− (IV) | 1.2 | 1.50 × 10−11 | 3.52 | Kong, J. et al. 2021 [20] |
| RN | C0 (ppm) | Wyoming | Taiwan | Inner Mongolia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | Eh (mV) | Kd (mL/g) | pH | Eh (mV) | Kd (mL/g) | pH | Eh (mV) | Kd (mL/g) | ||
| I− | 5 | 9.46 ± 0.13 | 251 ± 6 | 0.01 ± 0.00 | 7.23 ± 0.08 | 170 ± 1 | ~0 | 9.22 ± 0.03 | 221 ± 3 | 0.01 ± 0.00 |
| 10 | 9.55 ± 0.07 | 116 ± 5 | ~0 | 7.94 ± 0.45 | 282 ± 7 | 0.03 ± 0.00 | 9.05 ± 0.27 | 186 ± 15 | ~0 | |
| 20 | 9.57 ± 0.06 | 241 ± 2 | ~0 | 8.53 ± 0.04 | 306 ± 4 | ~0 | 9.11 ± 0.16 | 211 ± 8 | ~0 | |
| IO3− | 5 | 9.53 ± 0.04 | 243 ± 9 | ~0 | 8.24 ± 0.01 | 321 ± 9 | 0.02 ± 0.00 | 9.13 ± 0.14 | 273 ± 8 | 0.01 ± 0.00 |
| 10 | 9.26 ± 0.33 | 243 ± 8 | 0.01 ± 0.00 | 8.05 ± 0.02 | 247 ± 17 | 0.01 ± 0.00 | 9.36 ± 0.03 | 233 ± 12 | 0.01 ± 0.00 | |
| 20 | 9.38 ± 0.02 | 187 ± 9 | ~0 | 8.07 ± 0.11 | 225 ± 3 | ~0 | 9.28 ± 0.22 | 197 ± 4 | ~0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-P.; Hu, Y.; Chen, D.; Wu, E.; Wang, Z.; Wen, Z.; Tien, N.-C.; Yang, F.; Tsai, S.-C.; Shi, Y.; et al. An Improved Speciation Method Combining IC with ICPOES and Its Application to Iodide and Iodate Diffusion Behavior in Compacted Bentonite Clay. Materials 2021, 14, 7056. https://doi.org/10.3390/ma14227056
Lee C-P, Hu Y, Chen D, Wu E, Wang Z, Wen Z, Tien N-C, Yang F, Tsai S-C, Shi Y, et al. An Improved Speciation Method Combining IC with ICPOES and Its Application to Iodide and Iodate Diffusion Behavior in Compacted Bentonite Clay. Materials. 2021; 14(22):7056. https://doi.org/10.3390/ma14227056
Chicago/Turabian StyleLee, Chuan-Pin, Yanqin Hu, Dongyang Chen, Enhui Wu, Ziteng Wang, Zijin Wen, Neng-Chuan Tien, Fan Yang, Shih-Chin Tsai, Yunfeng Shi, and et al. 2021. "An Improved Speciation Method Combining IC with ICPOES and Its Application to Iodide and Iodate Diffusion Behavior in Compacted Bentonite Clay" Materials 14, no. 22: 7056. https://doi.org/10.3390/ma14227056






