Fixed-Bed Modification of Zeolitic Tuffs and Their Application for Cr(VI) Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Used Sorbents and Their Characteristics
- CL:
- 74% clinoptilolite, 11% cristobalite, 6% plagioclase, 4% illite and smectite, 3% tridymite, 1% kaolinite, and 1% quartz;
- CH:
- 54% chabazite, 36% clinoptilolite, 5% quartz, 5% unidentified.
- For CL:
- K+ > Ca2+ > Fe2+ > Mg2+ > Na+ > Ti4+ > Ba2+ > Sr2+,
- For CH:
- Na+ > Fe2+ > Ca2+ > K+ > Mg2+ > Th4+.
2.3. Zeolite Pre-Treatment
2.4. Zeolite Modification
2.5. Chromium Sorption on SMZ
2.5.1. From Artificial Solutions
2.5.2. From Real Wastewaters
3. Results and Discussion
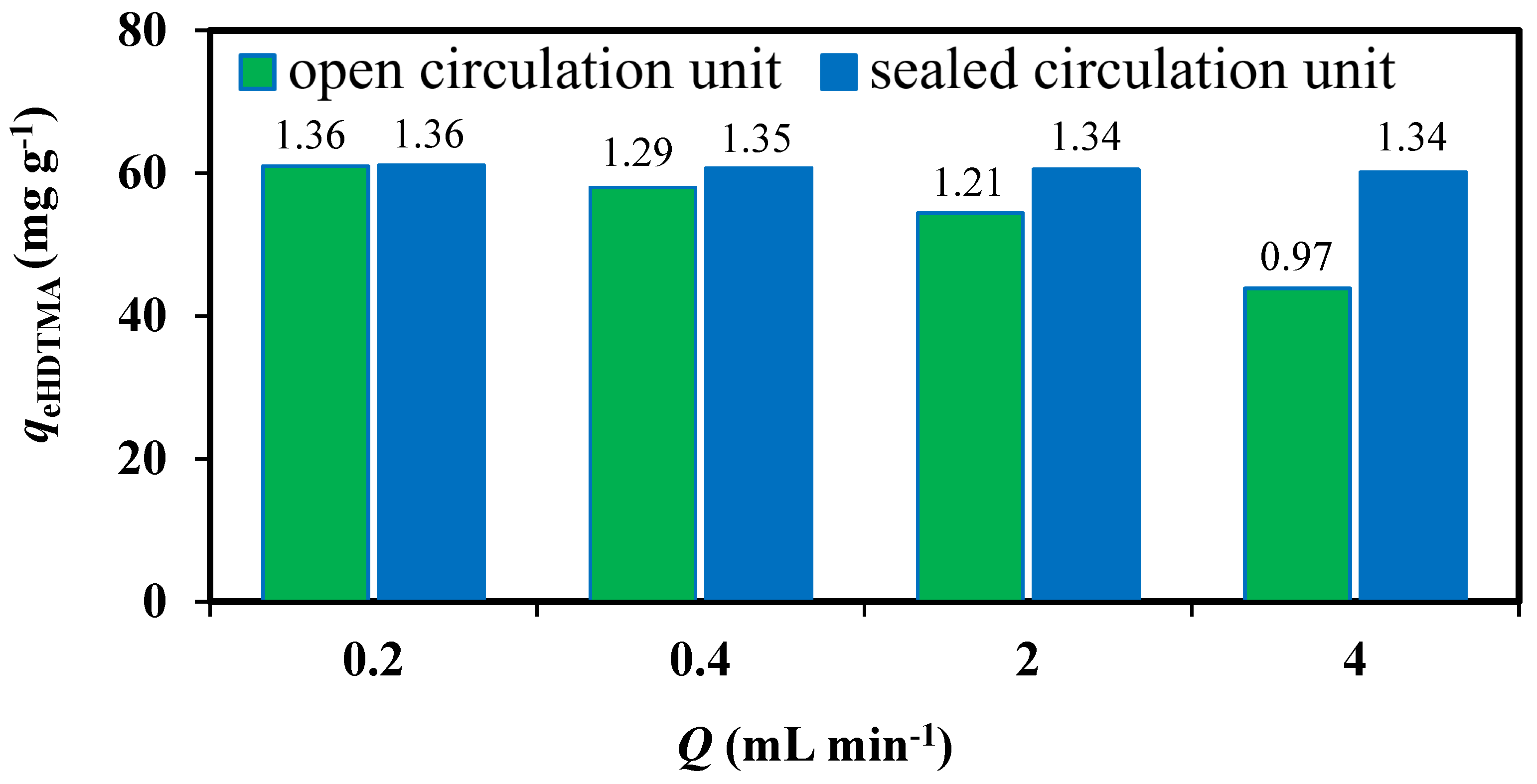
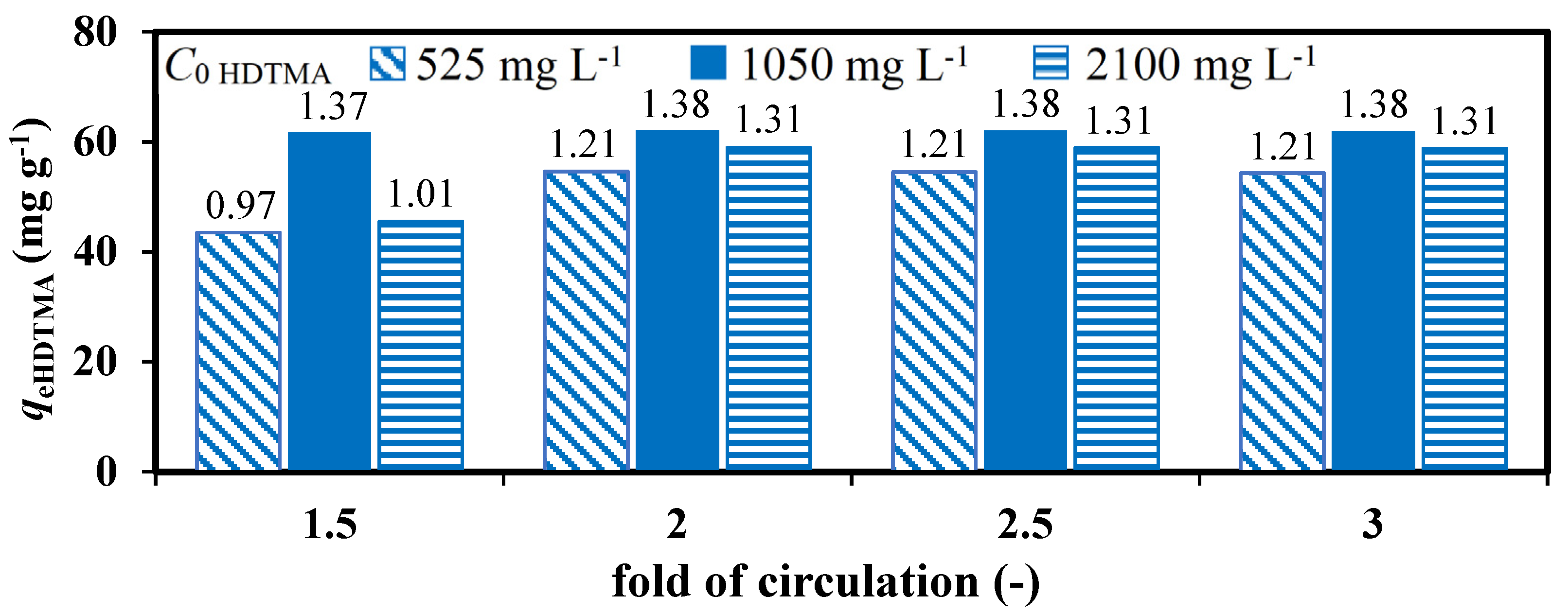
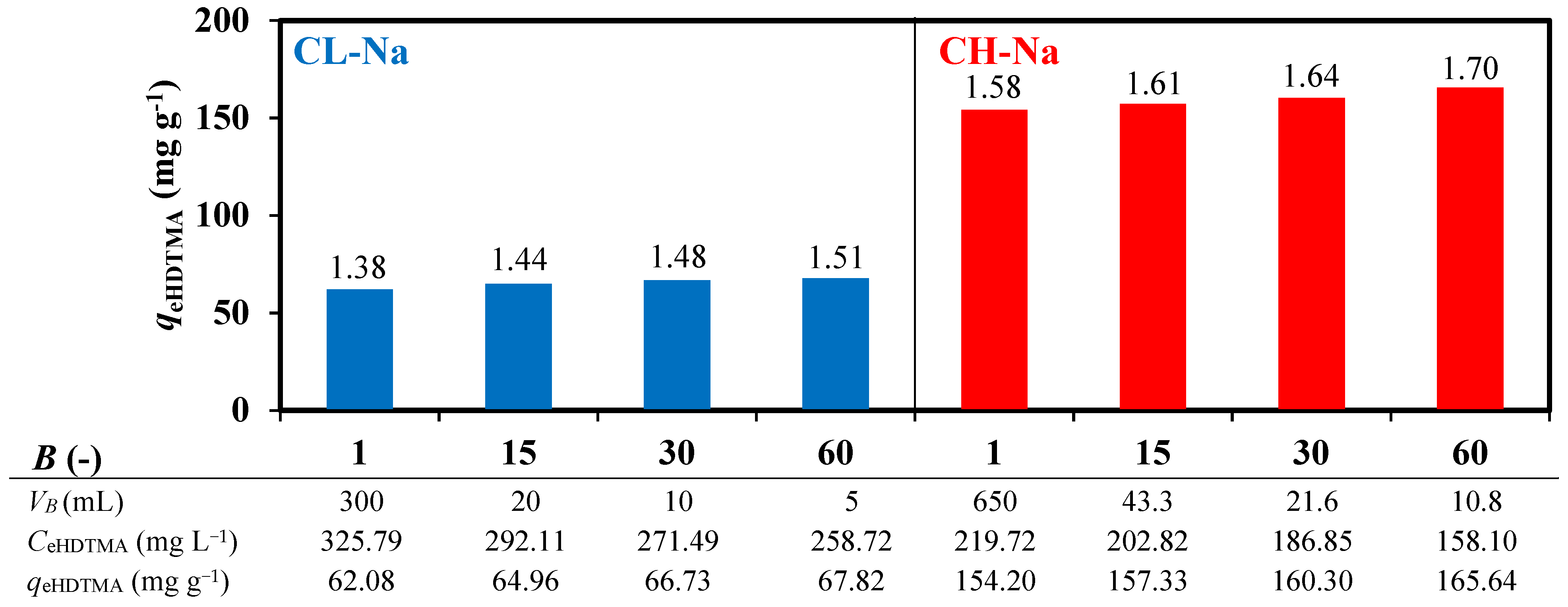
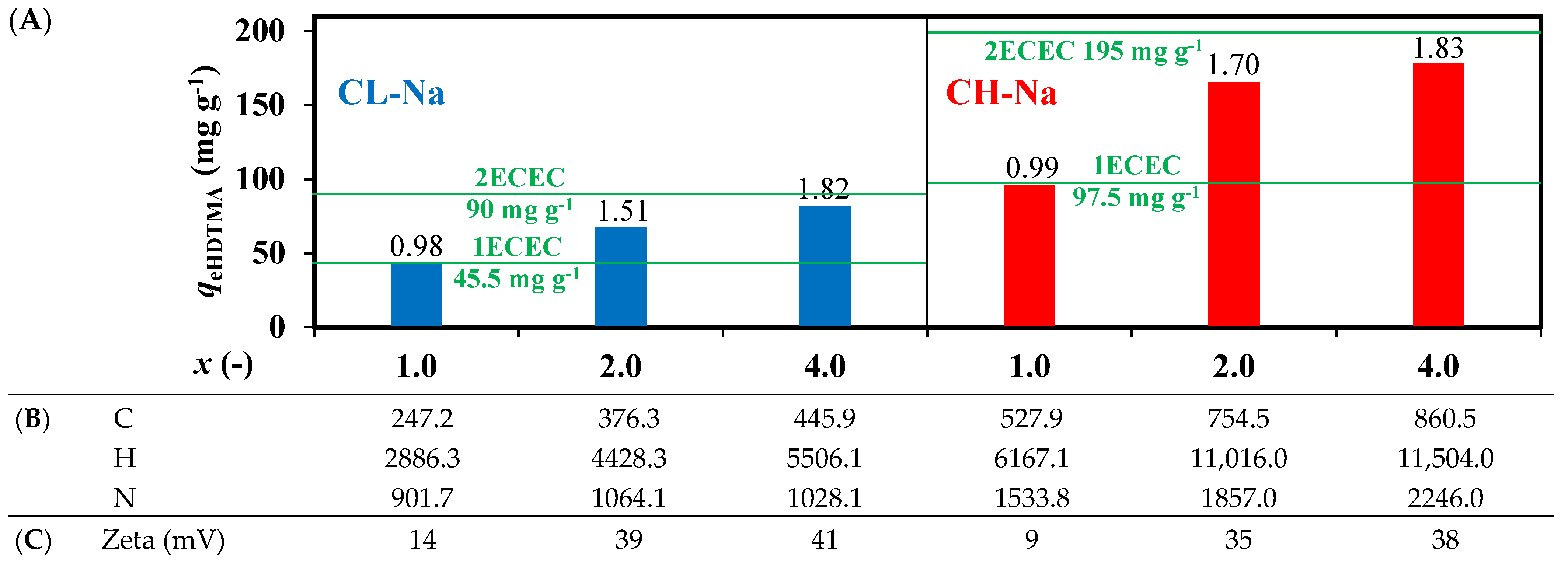
3.1. Zeolites Modification
3.2. Cr(VI) Sorption on SMZ
3.3. Real Wastewater Treatment on SMZ
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water Air Soil Pollut. 2008, 200, 59–77. [Google Scholar] [CrossRef]
- Korngold, E.; Belayev, N.; Aronov, L. Removal of chromates from drinking water by anion exchangers. Sep. Purif. Technol. 2003, 33, 179–187. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr. Activated carbons and low-cost adsorbents for remediation of tri- and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef]
- Thanos, A.; Katsou, E.; Malamis, S.; Psarras, K.; Pavlatou, E.; Haralambous, K. Evaluation of modified mineral performance for chromate sorption from aqueous solutions. Chem. Eng. J. 2012, 211–212, 77–88. [Google Scholar] [CrossRef]
- Chojnacka, M. Cr(VI) sorption on surface-modified natural zeolites Sorpcja jonów Cr(VI) na powierzchniowo modyfikowanych zeolitach naturalnych. Przem. Chem. 2017, 96, 332–337. [Google Scholar] [CrossRef]
- Jiménez-Castañeda, M.E.; Medina, D.I. Use of Surfactant-Modified Zeolites and Clays for the Removal of Heavy Metals from Water. Water 2017, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Guzel, P.; Aydın, Y.A.; Aksoy, N.D. Removal of chromate from wastewater using amine-based-surfactant-modified clinoptilolite. Int. J. Environ. Sci. Technol. 2016, 13, 1277–1288. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, E. Topological and Thermal Properties of Surfactant-Modified Clinoptilolite Studied by Tapping-Mode™ Atomic Force Microscopy and High-Resolution Thermogravimetric Analysis. Clays Clay Miner. 1997, 45, 42–53. [Google Scholar] [CrossRef]
- Li, Z.; Bowman, R.S. Counterion Effects on the Sorption of Cationic Surfactant and Chromate on Natural Clinoptilolite. Environ. Sci. Technol. 1997, 31, 2407–2412. [Google Scholar] [CrossRef]
- Serri, C.; de Gennaro, B.; Catalanotti, L.; Cappelletti, P.; Langella, A.; Mercurio, M.; Mayol, L.; Biondi, M. Surfactant-modified phillipsite and chabazite as novel excipients for pharmaceutical applications? Microporous Mesoporous Mater. 2016, 224, 143–148. [Google Scholar] [CrossRef]
- Yusof, A.M.; Malek, N.A.N.N. Removal of Cr(VI) and As(V) from aqueous solutions by HDTMA-modified zeolite Y. J. Hazard. Mater. 2009, 162, 1019–1024. [Google Scholar] [CrossRef]
- Safonov, A.; Popova, N.; Andrushenko, N.; Boldyrev, K.; Yushin, N.; Zinicovscaia, I. Investigation of materials for reactive permeable barrier in removing cadmium and chromium(VI) from aquifer near a solid domestic waste landfill. Environ. Sci. Pollut. Res. 2020, 28, 4645–4659. [Google Scholar] [CrossRef]
- Shang, J.; Li, G.; Singh, R.; Xiao, P.; Liu, J.Z.; Webley, P.A. Potassium Chabazite: A Potential Nanocontainer for Gas Encapsulation. J. Phys. Chem. C 2010, 114, 22025–22031. [Google Scholar] [CrossRef]
- Mansouri, N.; Rikhtegar, N.; Panahi, H.A.; Atabi, F.; Shahraki, B.K. Porosity, characterization and structural properties of natural zeolite-linoptilolite—As a sorbent. Environ. Prot. Eng. 2013, 39, 139–152. [Google Scholar] [CrossRef]
- Elaiopoulos, K.; Perraki, T.; Grigoropoulou, E. Monitoring the effect of hydrothermal treatments on the structure of a natural zeolite through a combined XRD, FTIR, XRF, SEM and N2-porosimetry analysis. Microporous Mesoporous Mater. 2010, 134, 29–43. [Google Scholar] [CrossRef]
- Szala, B.; Bajda, T.; Matusik, J.; Zięba, K.; Kijak, B. BTX sorption on Na-P1 organo-zeolite as a process controlled by the amount of adsorbed HDTMA. Microporous Mesoporous Mater. 2015, 202, 115–123. [Google Scholar] [CrossRef]
- Zeng, Y.; Woo, H.; Lee, G.; Park, J. Removal of chromate from water using surfactant modified Pohang clinoptilolite and Haruna chabazite. Desalination 2010, 257, 102–109. [Google Scholar] [CrossRef]
- Catá, G.F.; Rojas, H.C.; Gramatges, A.P.; Zicovich-Wilson, C.M.; Alvarez, L.J.; Searle, C. Initial structure of cetyltrimethylammonium bromide micelles in aqueous solution from molecular dynamics simulations. Soft Matter 2011, 7, 8508–8515. [Google Scholar] [CrossRef]
- Anachkov, S.E.; Danov, K.D.; Basheva, E.S.; Kralchevsky, P.A.; Ananthapadmanabhan, K.P. Determination of the aggregation number and charge of ionic surfactant micelles from the stepwise thinning of foam films. Adv. Colloid Interface Sci. 2012, 183–184, 55–67. [Google Scholar] [CrossRef]
- Spiridonov, A.M.; Aprosimova, E.V.; Zabolotskii, V.I.; Fedoseeva, V.I.; Sokolova, M.D.; Okhlopkova, A.A. Adsorption of Cetyltrimethylammonium Bromide on Zeolite Surface. Russ. J. Phys. Chem. A 2019, 93, 917–923. [Google Scholar] [CrossRef]
- Majdan, M.; Pikus, S.; Rzączyńska, Z.; Iwan, M.; Maryuk, O.; Kwiatkowski, R.; Skrzypek, H. Characteristics of chabazite modified by hexadecyltrimethylammonium bromide and of its affinity toward chromates. J. Mol. Struct. 2006, 791, 53–60. [Google Scholar] [CrossRef]
- Zieliński, R. Surfaktanty: Budowa, Właściwości, Zastosowania; Wydawnictwo UEP: Poznań, Poland, 2017. [Google Scholar]
- Tran, H.; Van Viet, P.; Chao, H.-P. Surfactant modified zeolite as amphiphilic and dual-electronic adsorbent for removal of cationic and oxyanionic metal ions and organic compounds. Ecotoxicol. Environ. Saf. 2018, 147, 55–63. [Google Scholar] [CrossRef]
- Li, Z.; Hong, H. Retardation of chromate through packed columns of surfactant-modified zeolite. J. Hazard. Mater. 2009, 162, 1487–1493. [Google Scholar] [CrossRef]
- Hailu, S.L.; Nair, B.U.; Redi-Abshiro, M.; Diaz, I.; Tessema, M. Preparation and characterization of cationic surfactant modified zeolite adsorbent material for adsorption of organic and inorganic industrial pollutants. J. Environ. Chem. Eng. 2017, 5, 3319–3329. [Google Scholar] [CrossRef]
- Alvarez-García, S.; Ramírez-García, J.J.; Granados-Correa, F.; Sánchez-Meza, J.C. Structural and textural influences of surfactant-modified zeolitic materials over the methamidophos adsorption behavior. Sep. Sci. Technol. 2019, 55, 619–634. [Google Scholar] [CrossRef]
- Taffarel, S.R.; Rubio, J. Adsorption of sodium dodecyl benzene sulfonate from aqueous solution using a modified natural zeolite with CTAB. Miner. Eng. 2010, 23, 771–779. [Google Scholar] [CrossRef]
- Castro-Castro, J.D.; Macías-Quiroga, I.F.; Giraldo-Gómez, G.I.; Sanabria-González, N.R. Adsorption of Cr(VI) in Aqueous Solution Using a Surfactant-Modified Bentonite. Sci. World J. 2020, 2020, 3628163. [Google Scholar] [CrossRef]
- He, H.; Zhou, Q.; Frost, R.L.; Wood, B.J.; Duong, L.V.; Kloprogge, J.T. A X-ray photoelectron spectroscopy study of HDTMAB distribution within organoclays. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 66, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Barczyk, K.; Mozgawa, W.; Król, M. Studies of anions sorption on natural zeolites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 876–882. [Google Scholar] [CrossRef]
- Rathnayake, S.I.; Martens, W.N.; Xi, Y.; Frost, R.L.; Ayoko, G.A. Remediation of Cr (VI) by inorganic-organic clay. J. Colloid Interface Sci. 2016, 490, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Fayazi, M.; Ghanbarian, M. One-Pot Hydrothermal Synthesis of Polyethylenimine Functionalized Magnetic Clay for Efficient Removal of Noxious Cr(VI) from Aqueous Solutions. Silicon 2019, 12, 125–134. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Brown, C.; Mycroft, J.R.; Davidson, R.D.; McIntyre, N.S. X-ray photoelectron spectroscopy studies of chromium compounds. Surf. Interface Anal. 2004, 36, 1550–1563. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.-S.; Park, J.M. XAS and XPS studies on chromium-binding groups of biomaterial during Cr(VI) biosorption. J. Colloid Interface Sci. 2007, 317, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Li, S.; Ju, L.; Zhu, N.; Wu, J.; Li, P.; Dang, Z. Mechanism of the reduction of hexavalent chromium by organo-montmorillonite supported iron nanoparticles. J. Hazard. Mater. 2012, 219–220, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Fan, X.; Li, R.; Li, S.; Shen, S.; Hu, D. Efficient removal of Cr(VI) from water by quaternized chitin/branched polyethylenimine biosorbent with hierarchical pore structure. Bioresour. Technol. 2018, 250, 178–184. [Google Scholar] [CrossRef]
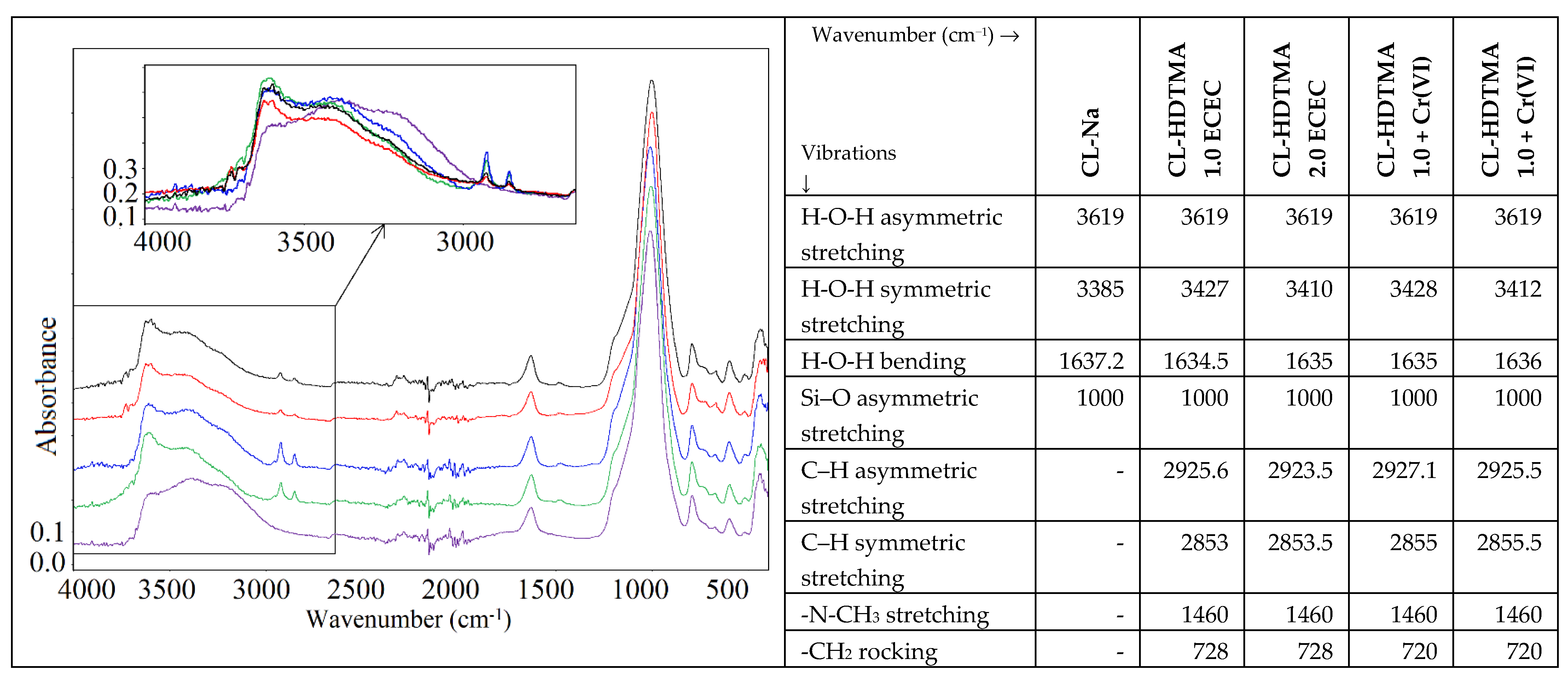
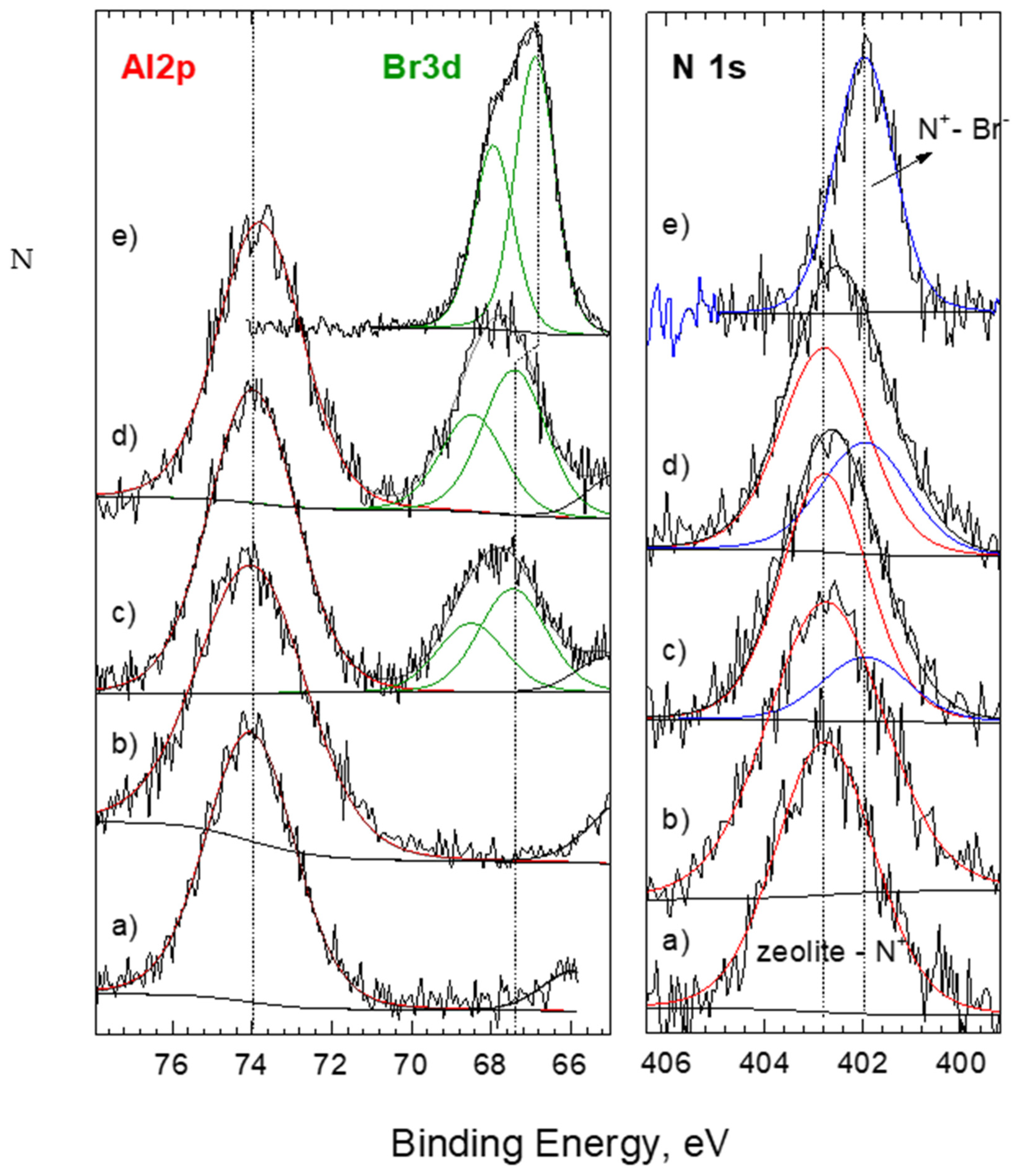

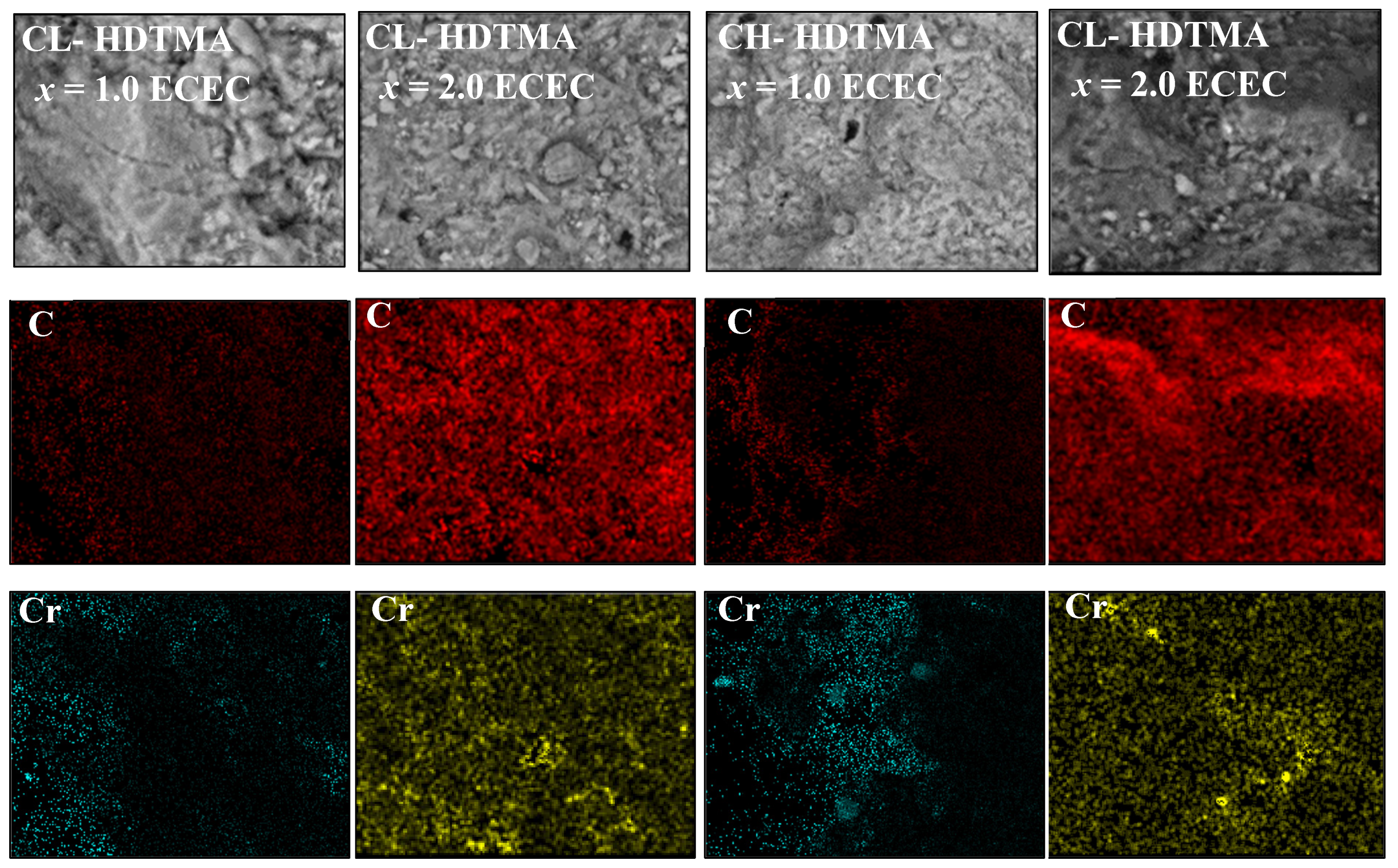

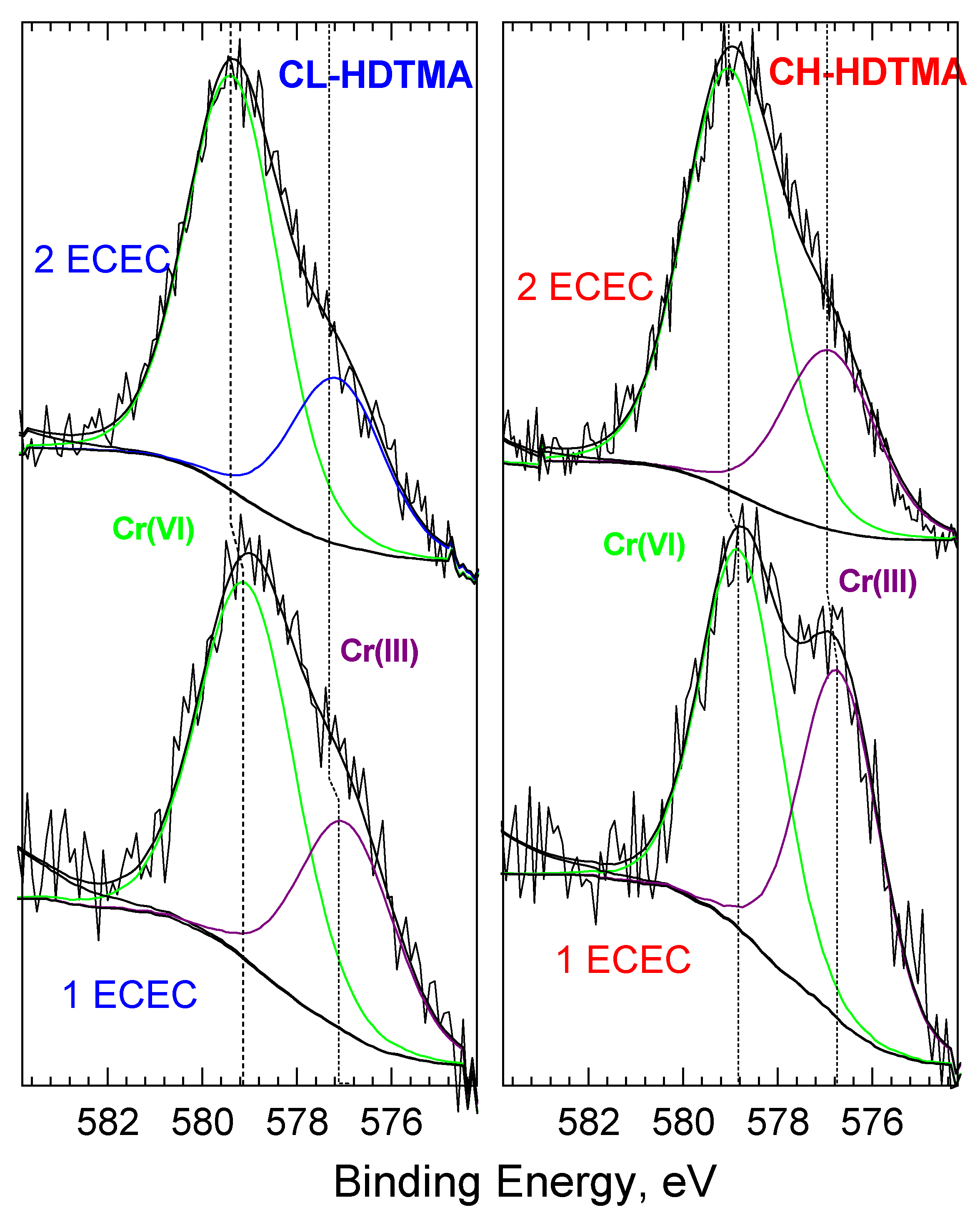

| Sample | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O | Fe2O3 |
|---|---|---|---|---|---|---|---|
| CL | 70.81 | 12.24 | 2.784 | 0.741 | 3.578 | 0.357 | 1.320 |
| CH | 58.62 | 14.55 | 2.519 | 0.828 | 1.253 | 5.082 | 3.246 |
| Sample | Area (m2 g−1) | Pore Volume (mL g−1) | ||
|---|---|---|---|---|
| ABET | Amica | Aext | VT | |
| CL | 29.47 | 8.15 | 21.33 | 0.11 |
| CH | 340.22 | 276.23 | 63.99 | 0.30 |
| Exchange Capacity | CEC (mval g−1) | ECEC (mval g−1) | |
|---|---|---|---|
| Analysis | AAS | TOC | CHN |
| CL | 0.938 | 0.121 | 0.121 |
| CH | 1.657 | 0.262 | 0.262 |
| SMZ | C 1s | O 1s | N 1s | Si 2p | Al 2p | Fe 2p | other | Br 3d |
| CH-1ECEC as rec. | 34.1 | 35.7 | 1.1 | 22.6 | 5.8 | 0.7 | - | |
| +Ar+ 90″ | 26.3 | 40.3 | 1.2 | 24.4 | 6.8 | 1 | - | |
| +Ar+ 120″ | 21.2 | 43.0 | 0.9 | 25.9 | 7.7 | 1.3 | - | |
| CL-1ECEC as rec. | 25.1 | 39.3 | 0.9 | 26.2 | 7.6 | 0.3 | - | |
| +Ar+ 90″ | 21.9 | 41.7 | 0.7 | 27.6 | 7.3 | 0.3 | - | |
| +Ar+ 120″ | 19.2 | 42.5 | 0.4 | 29.3 | 7.7 | 0.3 | - | |
| CH-2ECEC as rec. | 36.1 | 31.7 | 1.4 | 23.7 | 6.4 | 0.1 | - | 0.6 |
| +Ar+ 90″ | 32.6 | 34.5 | 0.9 | 24.8 | 6.7 | 0.1 | - | 0.3 |
| +Ar+ 120″ | 32.7 | 34.0 | 0.9 | 24.8 | 7.2 | 0.2 | - | 0.3 |
| CL-2ECEC as rec. | 45.4 | 29.1 | 1.5 | 18.2 | 4.7 | 0.5 | - | 0.6 |
| +Ar+ 90″ | 38.4 | 33.6 | 1.3 | 20.1 | 5.4 | 0.8 | - | 0.5 |
| +Ar+ 120″ | 39.6 | 32.4 | 1.4 | 20.1 | 5.4 | 0.7 | - | 0.5 |
| HDTMA as rec. | 34.1 | 35.7 | 1.1 | 22.6 | 5.8 | 0.7 | - | |
| 26.3 | 40.3 | 1.2 | 24.4 | 6.8 | 1 | - | ||
| SMZ + Cr(VI) | C 1s | O 1s | N 1s | Si 2p | Al 2p | Fe 2p | other | Br 3d |
| CH-1ECEC + Cr as rec. | 36.3 | 34.1 | 1.3 | 20.7 | 5.2 | 0.6 | 1.5 | 0.4 |
| +Ar+ 90″ | 26.6 | 40.0 | 0.9 | 23.6 | 6.4 | 0.9 | 1.3 | 0.4 |
| +Ar+ 120″ | 26.6 | 39.6 | 0.9 | 23.7 | 6.8 | 0.9 | 1.0 | 0.5 |
| CL-1ECEC + Cr as rec. | 46.6 | 27.8 | 1.6 | 17.3 | 4.3 | 0.3 | 1.3 | 0.7 |
| +Ar+ 90″ | 37.1 | 34.5 | 1.2 | 19.7 | 5.1 | 0.6 | 1.0 | 0.8 |
| +Ar+ 120″ | 36.6 | 33.5 | 1.2 | 20.5 | 5.8 | 0.7 | 0.8 | 0.9 |
| CH-2ECEC + Cr as rec. | 30.5 | 36.2 | 1 | 24.3 | 5.9 | 0.1 | 1.6 | 0.4 |
| +Ar+ 90″ | 20.8 | 42.1 | 0.4 | 26.9 | 7.4 | 0.2 | 1.34 | 0.5 |
| +Ar+ 120″ | 19.1 | 42 | 0.7 | 28.9 | 7.5 | 0.2 | 1.23 | 0.5 |
| CL-2ECEC + Cr as rec. | 38.8 | 30.8 | 1.4 | 21.4 | 5.9 | 0.03 | 1.1 | 0.7 |
| +Ar+ 90″ | 31.4 | 35.9 | 1.1 | 23.9 | 6.2 | 0.07 | 0.8 | 0.6 |
| +Ar+ 120″ | 32.5 | 35.1 | 0.8 | 24.0 | 6.2 | 0.10 | 0.6 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warchoł, J.K.; Sobolewska, P.; Tylus, W.; Petrus, R. Fixed-Bed Modification of Zeolitic Tuffs and Their Application for Cr(VI) Removal. Materials 2021, 14, 7061. https://doi.org/10.3390/ma14227061
Warchoł JK, Sobolewska P, Tylus W, Petrus R. Fixed-Bed Modification of Zeolitic Tuffs and Their Application for Cr(VI) Removal. Materials. 2021; 14(22):7061. https://doi.org/10.3390/ma14227061
Chicago/Turabian StyleWarchoł, Jolanta Karolina, Paulina Sobolewska, Włodzimierz Tylus, and Roman Petrus. 2021. "Fixed-Bed Modification of Zeolitic Tuffs and Their Application for Cr(VI) Removal" Materials 14, no. 22: 7061. https://doi.org/10.3390/ma14227061







