Transition Metals (Cr3+) and Lanthanides (Eu3+) in Inorganic Glasses with Extremely Different Glass-Formers B2O3 and GeO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glass Synthesis
2.2. Characterization Techniques
3. Results and Discussion
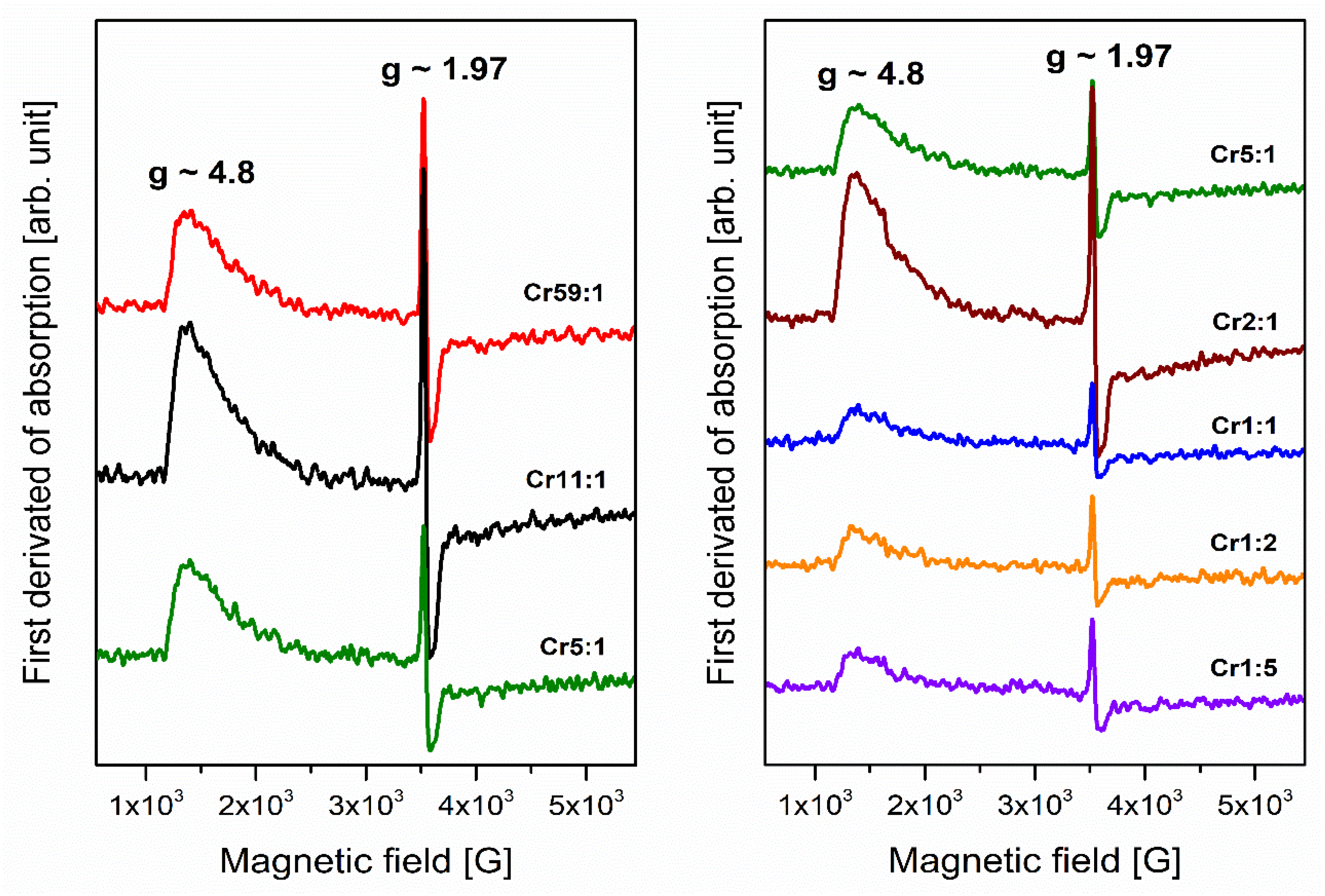
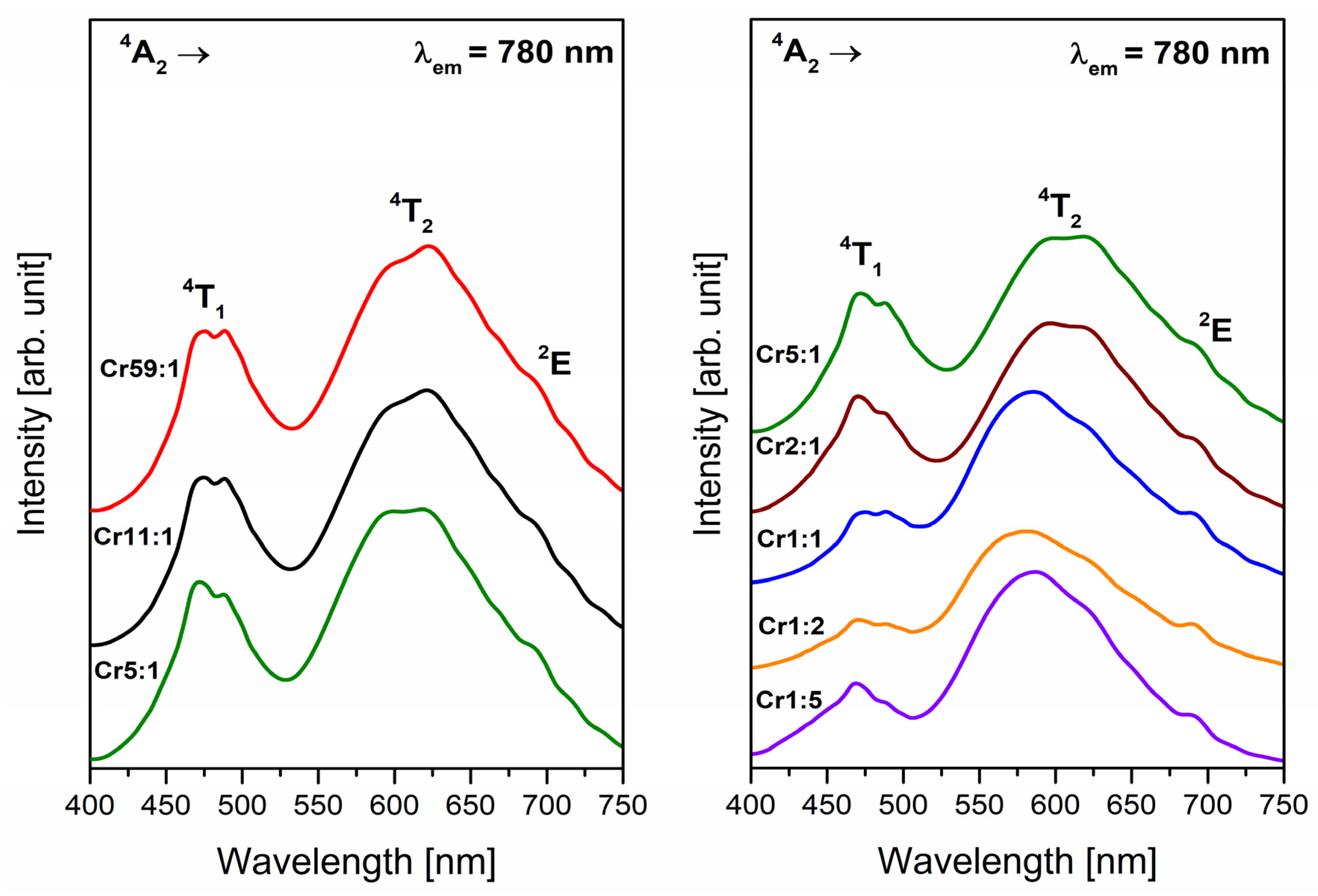
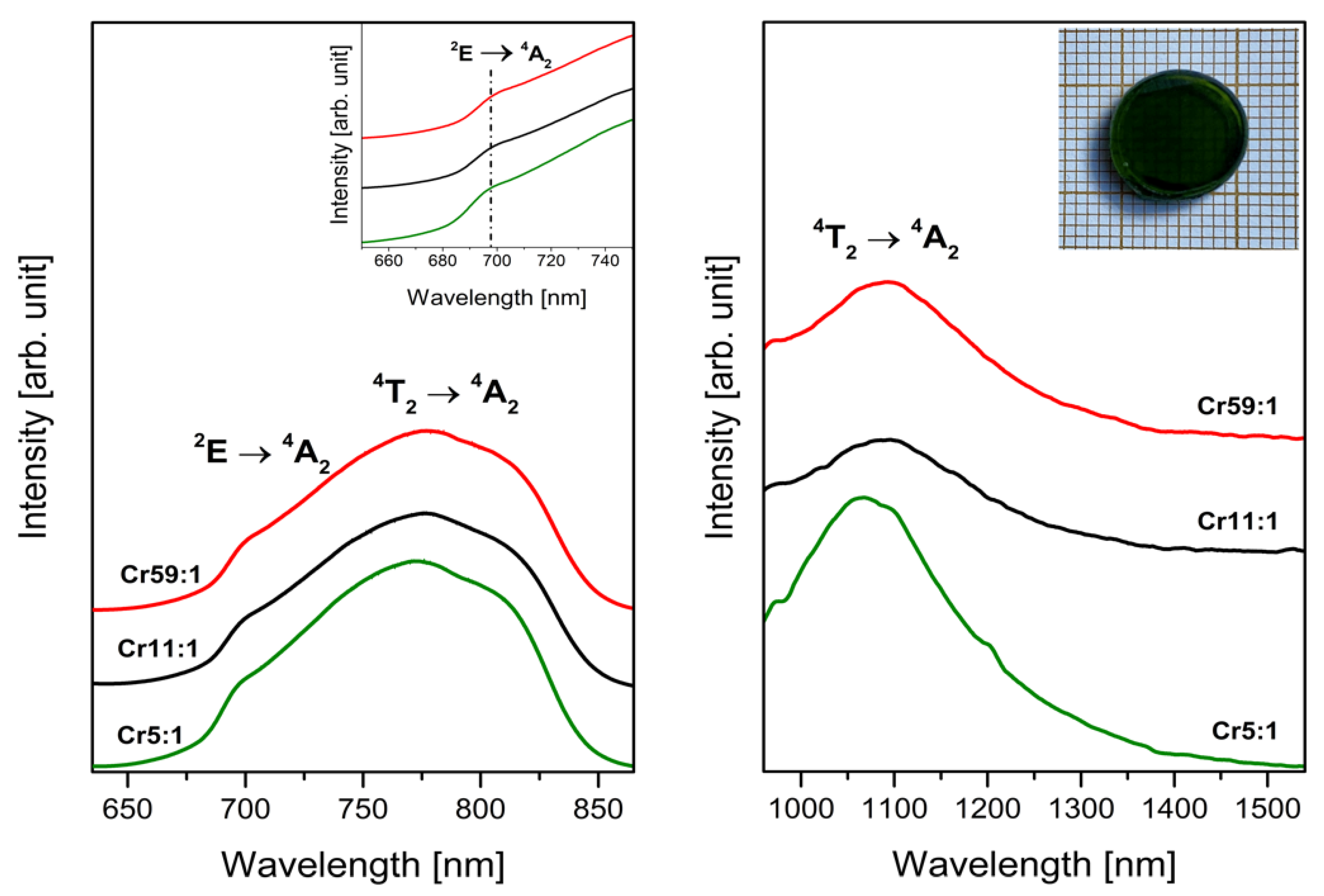
3.1. Transition Metals—Cr3+
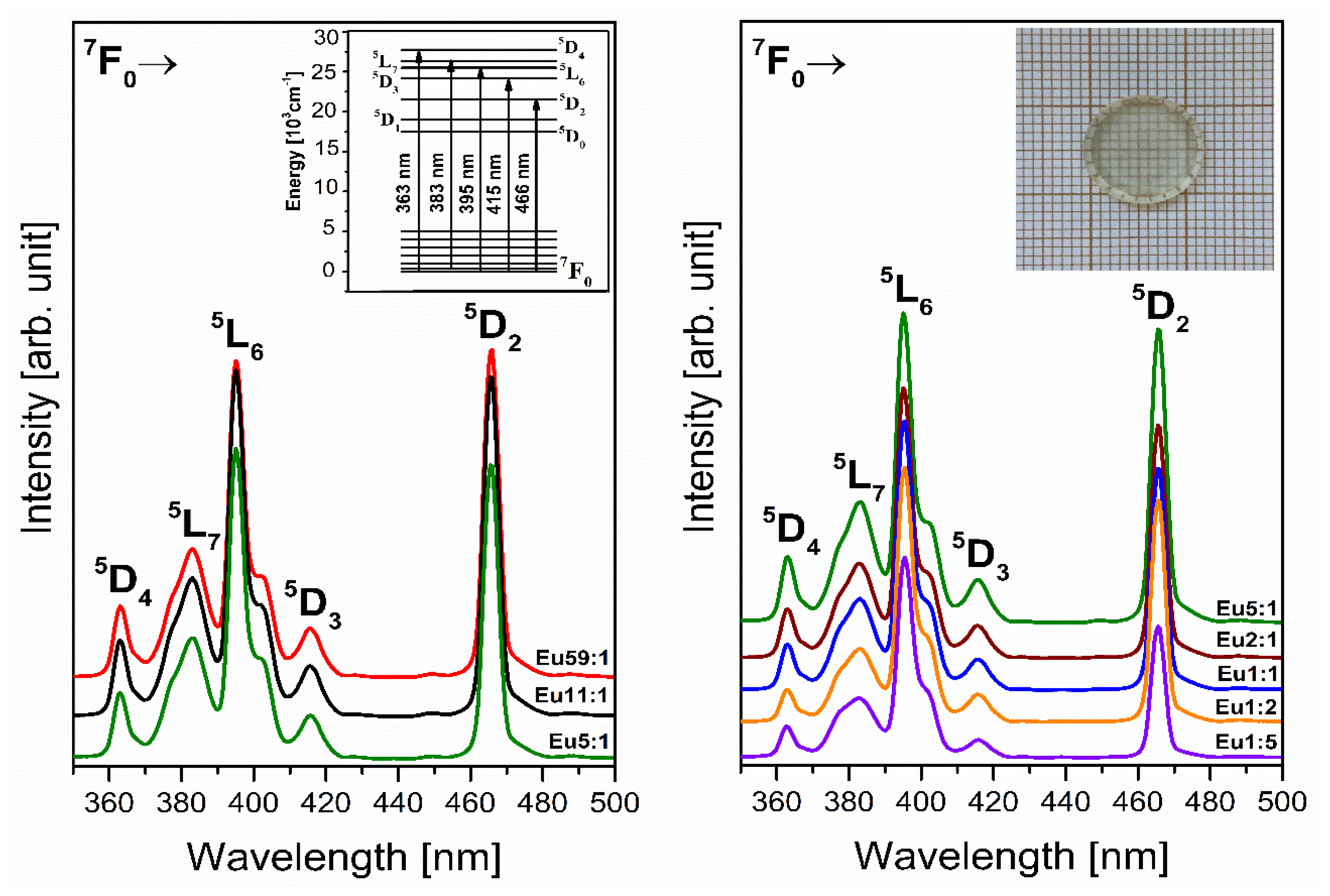
3.2. Lanthanides—Eu3+
4. Conclusions
- Analysis of EPR spectra indicates that the trivalent chromium ions at octahedral sites are present in the glass network. Independently of the molar ratio of different glass-formers, red luminescence was observed for systems doped with Cr3+ ions in octahedral sites. The influence of changing concentration glass-formers on the intensity of R-line attributed to Cr3+ ions in high-field sites was proved. It was stated that the emission bands with maxima at about 780 nm and 1070 nm are related to the same transition (4T2 → 4A2) of trivalent chromium ions in octahedral and tetrahedral sites, respectively.
- Confirmation that the type of glass-formers has a significant contribution to the properties of amorphous materials is presented by the results of spectroscopic studies conducted for glass samples doped which Eu3+ ions. Detailed spectroscopic analysis of the emission spectra showed a gradual quenching of the luminescence as a function of boron oxide concentration. The obtained values of the ratio R indicate a more covalent nature of the bond between the lanthanide ions and the surrounding ligands for samples with a higher concentration of germanium dioxide. On the other hand, the increase in boron oxide concentration results in an increase in the values of luminescence lifetimes of 5D0 level of Eu3+ ions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pascuta, P.; Stefan, R.; Olar, L.E.; Bolundut, L.C.; Culea, E. Effects of Copper Metallic Nanoparticles on Structural and Optical Properties of Antimony Phosphate Glasses Co-Doped with Samarium Ions. Materials 2020, 13, 5040. [Google Scholar] [CrossRef]
- Hongisto, M.; Veber, A.; Petit, Y.; Cardinal, T.; Danto, S.; Jubera, V.; Petit, L. Radiation-Induced Defects and Effects in Germanate and Tellurite Glasses. Materials 2020, 13, 3846. [Google Scholar] [CrossRef]
- Østergaard, M.B.; Bødker, M.S.; Smedskjaer, M.M. Structure Dependence of Poisson’s Ratio in Cesium Silicate and Borate Glasses. Materials 2020, 13, 2837. [Google Scholar] [CrossRef]
- Wang, X.; Sakakura, M.; Liu, Y.; Qiu, J.; Shimotsuma, Y.; Hirao, K.; Miura, K. Modification of long range order in germanate glass by ultra fast laser. Chem. Phys. Lett. 2011, 511, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Zanotto, E.D.; Mauro, J.C. The glassy state of matter: Its definition and ultimate fate. J. Non-Cryst. Solids 2017, 471, 490–495. [Google Scholar] [CrossRef]
- Zachariasen, W.H. The atomic arrangement in glass. J. Am. Chem. Soc. 1932, 54, 3841–3851. [Google Scholar] [CrossRef]
- Laksminarayana, G.; Meza-Rocha, A.N.; Soriano-Romeo, O.; Huerta, E.F.; Caldino, U.; Lira, A.; Lee, D.-E.; Yoon, J.; Park, T. Analysis of fluorescence characteristics of Sm3+-doped B2O3-rich glasses for Orange-light-emitting diodes. J. Alloys Compd. 2021, 884, 161076. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, W.; Lu, F.; Sui, Y.; Wang, J.; Xu, Z. Research of Fluorescent Properties of a New Type of Phosphor with Mn2+-Doped Ca2SiO4. Sensors 2021, 21, 2788. [Google Scholar] [CrossRef]
- Tanabe, S. Rare-earth-doped glasses for fiber amplifiers in broadband telecommunication. Comptes Rendus Chim. 2002, 5, 815–824. [Google Scholar] [CrossRef]
- Zaini, N.A.; Mohamed, S.N.; Mohamed, Z. Effects of Vanadium on the Structural and Optical Properties of Borate Glasses Containing Er3+ and Silver Nanoparticles. Materials 2021, 14, 3710. [Google Scholar] [CrossRef]
- Veselský, K.; Lahti, V.; Petit, L.; Prajzler, V.; Šulc, J.; Jelínková, H. Influence of Y2O3 Content on Structural, Optical, Spectroscopic, and Laser Properties of Er3+, Yb3+ Co-Doped Phosphate Glasses. Materials 2021, 14, 4041. [Google Scholar] [CrossRef] [PubMed]
- Santos Barbosa, J.; Batista, G.; Danto, S.; Fargin, E.; Cardinal, T.; Poirier, G.; Castro Cassanjes, F. Transparent Glasses and Glass-Ceramics in the Ternary System TeO2-Nb2O5-PbF2. Materials 2021, 14, 317. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Shao, C.; Kang, S.; Wang, F.; Wang, X.; Ren, J.; He, D.; Chen, W.; Hu, L. Investigation of luminescence mechanism of Nd3+-doped calcium aluminate glasses: Effect of glass-formers. J. Non-Cryst. Solids 2019, 505, 333–339. [Google Scholar] [CrossRef]
- Kuusela, L.; Veber, A.; Boetti, N.G.; Petit, L. Impact of ZnO Addition on Er3+ Near-Infrared Emission, the Formation of Ag Nanoparticles, and the Crystallization of Sodium Fluorophosphate Glass. Materials 2020, 13, 527. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Chen, M.; Zhu, X.; Zhu, Y.; Zeng, F.; Su, Z. Luminescent properties and structure of Dy3+ doped germanosilicate glass. J. Lumin. 2020, 226, 117378. [Google Scholar] [CrossRef]
- Jiao, Q.; Li, G.; Zhou, D.; Qiu, J. Effect of the Glass Structure on Emission of Rare-Earth-Doped Borate Glass. J. Am. Ceram. Soc. 2015, 98, 4102–4106. [Google Scholar] [CrossRef]
- Herrmann, A.; Kuhn, S.; Tiegel, M.; Russel, C. Fluorescence properties of Eu3+-doped alumino silicate glasses. Opt. Mater. 2014, 37, 293–297. [Google Scholar] [CrossRef]
- Linganna, K.; Narro-Garcia, R.; Desirens, H.; De la Rosa, E.; Basavapoornima, C.; Venkatramu, V.; Jayasankar, C.K. Effect of P2O5 addition on structural and luminescence properties of Nd3+-doped tellurite glasses. J. Alloys Compd. 2016, 684, 322–327. [Google Scholar] [CrossRef]
- Marzouk, M.A. Optical characterization of some rare earth ions doped bismuth borate glasses and effect of gamma irradiation. J. Mol. Struct. 2012, 1019, 80–90. [Google Scholar] [CrossRef]
- Divina, R.; Evangelin Teresa, P.; Marimuthu, K. Dy3+ ion as optical probe to study the luminescence behavior of Alkali lead bismuth borate glasses for w-LED application. J. Alloys Compd. 2021, 883, 160845. [Google Scholar] [CrossRef]
- Djamal, M.; Yuliantini, L.; Hidayat, R.; Rauf, N.; Horprathum, M.; Rajaramakrishna, R.; Boonin, K.; Yasaka, P.; Kaewkhao, J.; Venkatramu, V.; et al. Spectroscopic study of Nd3+ ion-doped Zn-Al-Ba borate glasses for NIR emitting device applications. Opt. Mater. 2020, 107, 110018. [Google Scholar] [CrossRef]
- Pisarska, J.; Pisarski, W.A.; Lisiecki, R.; Ryba-Romanowski, W. Phonon Sideband Analysis and Near-Infrared Emission in Heavy Metal Oxide Glasses. Materials 2021, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Pipes, R.S.; Shelby, J.E. Formation and properties of soda lime germanate glasses. J. Non-Cryst. Solids 2021, 553, 120506. [Google Scholar] [CrossRef]
- Koroleva, O.N.; Shtenberg, M.V.; Ivanova, T.N. The structure of potassium germanate glasses as revealed by Raman and IR spectroscopy. J. Non-Cryst. Solids 2010, 510, 143–150. [Google Scholar] [CrossRef]
- Żmojda, J.; Kochanowicz, M.; Miluski, P.; Golonko, P.; Baranowska, A.; Ragiń, T.; Dorosz, J.; Kuwik, M.; Pisarski, W.; Pisarska, J.; et al. Luminescent Studies on Germanate Glasses Doped with Europium Ions for Photonics Applications. Materials 2020, 13, 2817. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Kowalska, K.; Kuwik, M.; Polak, J.; Pietrasik, E.; Goryczka, T.; Pisarska, J. Novel Multicomponent Titanate-Germanate Glasses: Synthesis, Structure, Properties, Transition Metal, and Rare Earth Doping. Materials 2020, 13, 4422. [Google Scholar] [CrossRef]
- Tanaka, M.; Kushida, T. Studies of the vibronic interaction between Eu3+ ions and glass-forming units in oxide glasses. Chem. Phys. Lett. 1999, 315, 428–432. [Google Scholar] [CrossRef]
- Rolli, R.; Camagni, P.; Samoggia, G.; Speghini, A.; Wachtler, M.; Bettinelli, M. Fluorescence line narrowing spectroscopy of a lead germanate glass doped with Eu3+. Spectrochim. Acta Part A 1998, 54, 2157–2162. [Google Scholar] [CrossRef]
- Hanyes, W.M. CRC Handbook of Chemistry and Physics, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 4–13. [Google Scholar]
- Binnemans, K. Interpretation of europium (III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, S.; Shibata, Y. Luminescence of Cr3+ ions associated with surpassing the green-emissive defect centers in B-Ga2O3. J. Lumin. 2006, 121, 470–474. [Google Scholar] [CrossRef]
- Rasheed, F.; O’Donnell, K.P.; Henderson, B.; Hollis, D.B. Disorder and the optical spectroscopy of Cr3+-doped glasses: I. Silicate glasses. J. Phys. Condens. Matter 1991, 3, 1915–1930. [Google Scholar] [CrossRef]
- Rossi, F.; Montagna, M.; Ferrari, M.; Capobianco, J.A.; Bettinelli, M. Line width measurements of Cr3+ in a zinc borate glass. J. Non-Cryst. Solids 1998, 240, 232–236. [Google Scholar] [CrossRef]
- Samdani, M.; Ramadevudu, G.; Narasimha Chary, M.; Shareefuddin, M. EPR, optical and physical studies on Cr3+ doped MgO-BaO-B2O3-TeO2 glasses. St. Petersburg Polytech. Univ. J. Phys. Math. 2017, 3, 299–307. [Google Scholar]
- Costa, V.C.; Lameiras, F.S.; Pinheiro, M.V.B.; Sousa, D.F.; Nunes, L.A.O.; Shen, Y.R.; Bray, K.L. Laser spectroscopy and electron paramagnetic resonance of Cr3+ doped silicate glasses. J. Non-Cryst. Solids 2000, 273, 209–214. [Google Scholar] [CrossRef]
- Samdani; Ramadevudu, G.; Narasimha Chary, M.; Shareefuddin, M. Physical and spectroscopic studies of Cr3+ doped mixed alkaline earth oxide borate glasses. Mater. Chem. Phys. 2017, 186, 382–389. [Google Scholar] [CrossRef]
- Santhan Kumar, J.; Lakshmi Kumari, J.; Subba Rao, M.; Cole, S. EPR, optical and physical properties of chromium ions in CdO-SrO-B2O3-SiO2 (CdSBSi) glasses. Opt. Mater. 2013, 35, 1320–1326. [Google Scholar] [CrossRef]
- Bala Murali Krishna, S.; Vinaya Teja, P.M.; Krishna Rao, D. Role of chromium ion valence states in ZnO-As2O3-Sb2O3 glass system by means of spectroscopic and dielectric studies. Mater. Res. Bull. 2010, 45, 1783–1791. [Google Scholar] [CrossRef]
- Kesavulu, C.R.; Chakradhar, R.P.S.; Muralidhara, R.S.; Rao, J.L.; Anavekar, R.V. EPR, optical absorption and photoluminescence properties of Cr3+ ions in lithium borophosphate glasses. J. Alloys Compd. 2010, 496, 75–80. [Google Scholar] [CrossRef]
- Babkina, A.; Valiev, D.; Zyryanova, K.; Nuryev, R.; Ignatiev, A.; Kulpina, E.; Kuzmenko, N.; Osipova, A.; Koroleva, A.; Platonova, N. Spectroscopic properties of chromium/antimony co-doped alkali-alumina-borate glass-ceramics. Opt. Mater. 2020, 106, 109983. [Google Scholar] [CrossRef]
- Kiran, N.; Kesavulu, C.R.; Suresh Kumar, A.; Rao, J.L. Spectral studies on Cr3+ ions doped in sodium-lead borophosphate glasses. Phys. B 2011, 406, 1897–1901. [Google Scholar] [CrossRef]
- Wen, H.; Xie, S.; Cui, J.; Mao, S.; Luo, L.; Brik, M.G. Optical properties of 3d transition metal ion-doped aluminophosphate glasses. J. Lumin. 2019, 213, 263–272. [Google Scholar] [CrossRef]
- Saad, N.; Haouari, M.; Bulou, A.; HadiKassiba, A.; Ben Ouada, H. Structural and optical properties of Cr3+ embedded in a P2O5-B2O3-ZnO-BaF2-AlF3 fluoroborophosphate glasses. Mater. Chem. Phys. 2018, 212, 461–470. [Google Scholar] [CrossRef]
- Rodríguez-Mendoza, U.R.; Speghini, A.; Jaque, D.; Zambelli, M.; Bettinelli, M. Optical properties of single doped Cr3+ and co-doped Cr3+-Nd3+ aluminum tantalum tellurite glasses. J. Alloys Compd. 2004, 380, 163–166. [Google Scholar] [CrossRef]
- Narendrudu, T.; Suresh, S.; Chinna Ram, G.; Veeraiah, N.; Krishna Rao, D. Spectroscopic and structural properties of Cr3+ ions in lead niobium germanosilicate glasses. J. Lumin. 2017, 183, 17–25. [Google Scholar] [CrossRef]
- Yang, X.I.; Wang, W.C.; Zhang, Q.Y. BaF2 modified Cr3+/Ho3+ co-doped germanate glass for efficient 2.0 μm fiber lasers. J. Non-Cryst. Solids 2018, 482, 147–153. [Google Scholar] [CrossRef]
- Mikulski, J.; Koepke, C.; Wiśniewski, K.; Padlyak, B.V.; Adamiv, V.T.; Burak, Y.V. Excited state characteristics of the Li2B4O7 and KLiB4O7 glasses active by Cr3+ ions. Opt. Mater. 2014, 38, 24–27. [Google Scholar] [CrossRef]
- Li, Y.; Ye, S.; Zhang, Q. Ultra-broadband near-infrared luminescence of ordered-disordered multi-sited Cr3+ in La3Ga5.5Nb0.5O14:Cr3+. J. Mater. Chem. C 2014, 2, 4636–4641. [Google Scholar] [CrossRef]
- Babu, P.; Jayasankar, C.K. Optical spectroscopy of Eu3+ ions in lithium borate and lithium fluoroborate glasses. Phys. B 2000, 279, 262–281. [Google Scholar] [CrossRef]
- Ramesh, P.; Hegde, V.; Pramod, A.G.; Eraiah, B.; Agarkov, D.A.; Eliseeva, G.M.; Pandey, M.K.; Annapurna, K.; Jagannath, G.; Kokila, M.K. Compositional dependence of red photoluminescence of Eu3+ ions in lead and bismuth containing borate glasses. Solid State Sci. 2020, 107, 106360. [Google Scholar] [CrossRef]
- Fabian, M.; Pinakidou, F.; Tolnai, I.; Czompoly, O.; Osan, J. Lanthanide (Ce, Nd, Eu) environments and leaching behavior in borosilicate glasses. Sci. Rep. 2021, 11, 13272. [Google Scholar] [CrossRef]
- Wang, B.; Li, D.S.; Shen, L.F.; Pun, E.Y.B.; Lin, H. Eu3+ doped high-brightness fluorophosphate laser-driven glass phosphors. Opt. Mater. Exp. 2019, 9, 1749–1762. [Google Scholar] [CrossRef]
- Mahato, K.K.; Rai, S.B.; Rai, A. Optical studies of Eu3+ doped oxyfluoroborate glass. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 979–985. [Google Scholar] [CrossRef]
- Rodriguez, V.D.; Lavin, V.; Rodriquez-Mendoza, U.R.; Martin, I.R. Spectroscopy of rare earth ions in fluoride glasses for laser applications. Opt. Mater. 1999, 13, 1–7. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Q.; Song, J.; Xiao, Z.; Qiang, Y.; Ye, X.; You, W.; Lu, A. A novel Eu3+-doped phosphate glass for reddish orange emission: Preparation, structure and fluorescence properties. J. Lumin. 2020, 221, 117041. [Google Scholar] [CrossRef]
- Aryal, P.; Kim, H.J.; Khan, A.; Saha, S.; Kang, S.J.; Kothan, S.; Yamsuk, Y.; Kaewkhao, J. Development of Eu3+-doped phosphate glass. For luminescent solid-state optical devices. J. Lumin. 2020, 227, 117564. [Google Scholar] [CrossRef]
- Chen, M.; Liu, R.; Wang, D.; Zhou, Y.; Zeng, F.; Su, Z. Luminescent properties of Eu3+ doped germanosilicate red glass. J. Non-Cryst. Solids 2021, 566, 120895. [Google Scholar] [CrossRef]
- Balda, R.; Fernandez, J.; Duhamel, N.; Adam, J.L.; Imbusch, G.F. Site-selection spectroscopy and energy transfer studies of Eu3+ ions in a new fluorophosphate glass. J. Lumin. 1995, 66–67, 290–293. [Google Scholar] [CrossRef]
- Cascales, C.; Balda, R.; Gercia-Revilla, S.; Lezama, L.; Barredo-Zuriarrain, M.; Fernandez, J. Site symmetry and host-sensitization-dependence of Eu3+ real time luminescence in tin dioxide nanoparticles. Opt. Exp. 2018, 26, 16155–16170. [Google Scholar] [CrossRef] [Green Version]
- Wen, H.; Jia, G.; Duan, C.K.; Tanner, P.A. Understanding Eu3+ emission spectra in glass. Phys. Chem. Chem. Phys. 2010, 12, 9933–9937. [Google Scholar] [CrossRef]
- De, M.; Jana, S. Optical characterization of Eu3+ doped titanium barium lead phosphate glass. Optik 2020, 215, 164718. [Google Scholar] [CrossRef]
- Mariselvam, K.; Arun Kumar, R.; Karthik, S. Optical and luminescence characteristics of europium doped barium lithium fluoroborate glasses. Chem. Phys. 2019, 525, 110379. [Google Scholar] [CrossRef]
- Oomen, E.W.J.L.; van Dongen, A.M.A. Europium (III) in oxide glasses: Dependence of the emission spectrum upon glass composition. J. Non-Cryst. Solids 1989, 111, 205–213. [Google Scholar] [CrossRef]
- Reisfeld, R.; Boehm, L.; Ish-Shalom, M.; Fischer, R. 4f yields 4f transitions and charge transfer spectra of Eu3+, 4f yields 5d spectra of Tb3+, and 1S0 yields 3P1 spectra of Pb2+ in alkaline earth metaphosphate glasses. Phys. Chem. Glasses 1974, 15, 76. [Google Scholar]
- Gokce, M.; Senturk, U.; Uslu, D.K.; Burgaz, G.; Sahin, Y.; Gokce, A.G. Investigation of europium concentration dependence on the luminescent dependence on the luminescent properties of borogermanate glasses. J. Lumin. 2017, 192, 263–268. [Google Scholar] [CrossRef]
- Zmoja, J.; Kochanowicz, M.; Miluski, P.; Baranowska, A.; Pisarski, W.A.; Pisarska, J.; Jadach, R.; Sitarz, M.; Dorosz, D. Structural and optical properties of antimony-germanate-borate glass and glass fiber co-doped Eu3+ and Ag nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 201, 1–7. [Google Scholar] [CrossRef]
- Sołtys, M.; Janek, J.; Zur, L.; Pisarska, J.; Pisarski, W.A. Compositional-dependent europium-doped lead phosphate glasses and their spectroscopic properties. Opt. Mater. 2015, 40, 91–96. [Google Scholar] [CrossRef]
- Kesavulu, C.R.; Kumar, K.K.; Vijaya, N.; Lim, K.S.; Jayasankar, C.K. Thermal vibration and optical properties of Eu3+-doped lead fluorophosphate glasses for red laser applications. Mater. Chem. Phys. 2013, 141, 903–911. [Google Scholar] [CrossRef]
- Deopa, N.; Kaur, S.; Prasad, A.; Joshi, B.; Rao, A.S. Spectral studies of Eu3+ doped lithium lead alumino borate glasses for visible photonic applications. Opt. Laser Technol. 2018, 108, 434–440. [Google Scholar] [CrossRef]
- Szal, R.; Zmoja, J.; Kochanowicz, M.; Miluski, P.; Dorosz, J.; Lesniak, M.; Jeleń, P.; Starzyk, B.; Sitarz, M.; Kuwik, M.; et al. Spectroscopic properties of antimony modified germanate glass doped with Eu3+ ions. Ceram. Int. 2019, 45, 24811–24817. [Google Scholar] [CrossRef]
- Venkatramu, V.; Babu, P.; Jayasankar, C.K. Fluorescence properties of Eu3+ ions doped borate and fluoroborate glasses containing lithium, zinc and lead. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 63, 276–281. [Google Scholar] [CrossRef]
- Shojiya, M.; Kawamoto, Y. Judd-Ofelt parameters and multiphonon relaxation of Ho3+ ions in ZnCl2-based glass. J. Appl. Phys. 2001, 89, 4944–4950. [Google Scholar] [CrossRef]
- Wang, X.; Huang, L.; Zhao, S.; Xu, S. Eu3+ doped heavy germanate scintillating glasses. J. Lumin. 2018, 196, 256–258. [Google Scholar] [CrossRef]
- Zur, L.; Janek, J.; Pietrasik, E.; Sołtys, M.; Pisarska, J.; Pisarski, W.A. Influence of Mo/MF2 modifiers (M = Ca, Sr, Ba) on spectroscopic properties of Eu3+ ions in germanate and borate glasses. Opt. Mater. 2016, 61, 59–63. [Google Scholar] [CrossRef]
- Vijayalakshmi, L.; Kumar, K.N.; Hwang, P. Dazzling red luminescence dynamics of Eu3+ doped lithium borate glasses for photonic applications. Optic 2019, 193, 163019. [Google Scholar] [CrossRef]
- Yao, L.; Chen, G.; Cui, S.; Zhong, H.; Wen, C. Fluorescence and optical properties of Eu3+-doped borate glasses. J. Non-Cryst. Solids 2016, 444, 38–42. [Google Scholar] [CrossRef]



| Sample Code | GeO2 | B2O3 | BaO | Ga2O3 | Cr2O3 |
|---|---|---|---|---|---|
| Cr59:1 | 59 | 1 | 30 | 9.75 | 0.25 |
| Cr11:1 | 55 | 5 | 30 | 9.75 | 0.25 |
| Cr5:1 | 50 | 10 | 30 | 9.75 | 0.25 |
| Cr2:1 | 40 | 20 | 30 | 9.75 | 0.25 |
| Cr1:1 | 30 | 30 | 30 | 9.75 | 0.25 |
| Cr1:2 | 20 | 40 | 30 | 9.75 | 0.25 |
| Cr1:5 | 10 | 50 | 30 | 9.75 | 0.25 |
| Sample Code | GeO2 | B2O3 | BaO | Ga2O3 | Eu2O3 |
|---|---|---|---|---|---|
| Eu59:1 | 59 | 1 | 30 | 9.75 | 0.25 |
| Eu11:1 | 55 | 5 | 30 | 9.75 | 0.25 |
| Eu5:1 | 50 | 10 | 30 | 9.75 | 0.25 |
| Eu2:1 | 40 | 20 | 30 | 9.75 | 0.25 |
| Eu1:1 | 30 | 30 | 30 | 9.75 | 0.25 |
| Eu1:2 | 20 | 40 | 30 | 9.75 | 0.25 |
| Eu1:5 | 10 | 50 | 30 | 9.75 | 0.25 |
| Glass Composition [mol%] | R/O | References |
|---|---|---|
| 10GeO2-50B2O3-30BaO-9.75Ga2O3-0.25Eu2O3 | 3.08 | Present work |
| 59GeO2-1B2O3-30BaO-9.75Ga2O3-0.25Eu2O3 | 3.63 | Present work |
| 89.5B2O3-10Li2O-0.5Eu2O3 | 2.41 | [63] |
| 0.5GeO2-63.5SiO2-16K2O-16BaO-4Eu2O3 | 3.46 | [63] |
| 84.5GeO2-12.5K2O-3Eu2O3 | 4.60 | [63] |
| 49.5BaO-49.5P2O5-1Eu2O3 | 5.28 | [64] |
| 30B2O3-40GeO2-29Gd2O3-1Eu2O3 | 3.54 | [65] |
| 25Sb2O3-25GeO2-29.2B2O3-5Al2O3-10Na2O-0.6AgNO3-0.2Eu2O3 | 2.75 | [66] |
| 45P2O5-45PbO-9Ga2O3-1Eu2O3 | 1.70 | [67] |
| 44P2O5-17K2O-9Al2O3-23PbF2-6Na2O-1Eu2O3 | 2.36 | [68] |
| 10Li2O-10PbO-7Al2O3-70B2O3-3Eu2O3 | 2.02 | [69] |
| 59.8GeO2-15Ga2O3-25BaO-0.2Eu2O3 | 3.72 | [70] |
| Sample Code | GeO2:B2O3 [mol%] | R/O (Eu3+) | τm [ms] |
|---|---|---|---|
| Eu59:1 | 59:1 | 3.63 | 1.25 |
| Eu11:1 | 55:5 | 3.43 | 1.30 |
| Eu5:1 | 50:10 | 3.38 | 1.36 |
| Eu2:1 | 40:20 | 3.30 | 1.43 |
| Eu1:1 | 30:30 | 3.26 | 1.50 |
| Eu1:2 | 20:40 | 3.17 | 1.60 |
| Eu1:5 | 10:50 | 3.08 | 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, K.; Kuwik, M.; Polak, J.; Pisarska, J.; Pisarski, W.A. Transition Metals (Cr3+) and Lanthanides (Eu3+) in Inorganic Glasses with Extremely Different Glass-Formers B2O3 and GeO2. Materials 2021, 14, 7156. https://doi.org/10.3390/ma14237156
Kowalska K, Kuwik M, Polak J, Pisarska J, Pisarski WA. Transition Metals (Cr3+) and Lanthanides (Eu3+) in Inorganic Glasses with Extremely Different Glass-Formers B2O3 and GeO2. Materials. 2021; 14(23):7156. https://doi.org/10.3390/ma14237156
Chicago/Turabian StyleKowalska, Karolina, Marta Kuwik, Justyna Polak, Joanna Pisarska, and Wojciech A. Pisarski. 2021. "Transition Metals (Cr3+) and Lanthanides (Eu3+) in Inorganic Glasses with Extremely Different Glass-Formers B2O3 and GeO2" Materials 14, no. 23: 7156. https://doi.org/10.3390/ma14237156