BiFeO3-Based Relaxor Ferroelectrics for Energy Storage: Progress and Prospects
Abstract
:1. Introduction
2. Fundamentals of the Energy Storage Mechanism in Dielectrics
3. Relaxor Ferroelectrics
4. Energy Storage Performance of BiFeO3-Based Relaxor Ferroelectrics
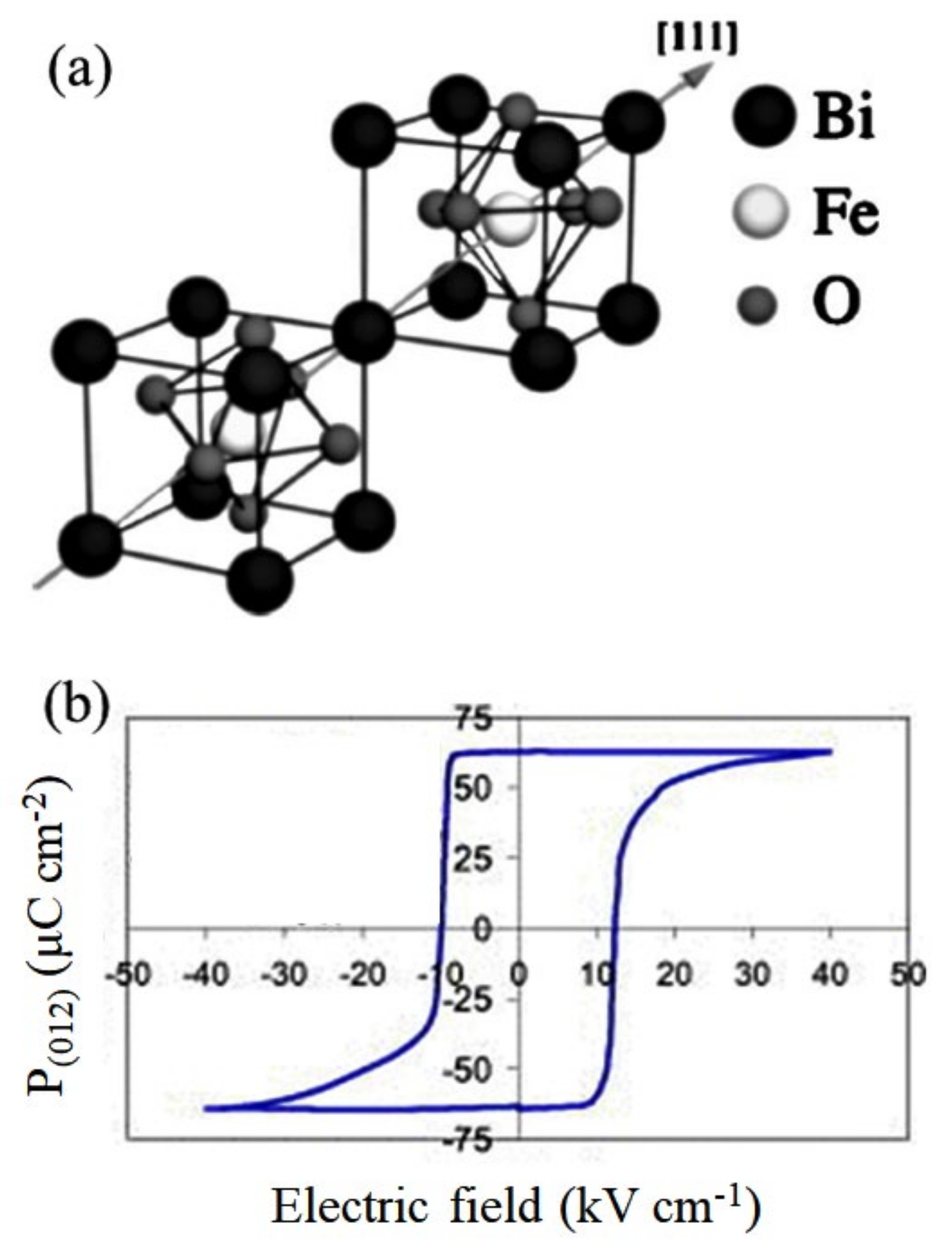
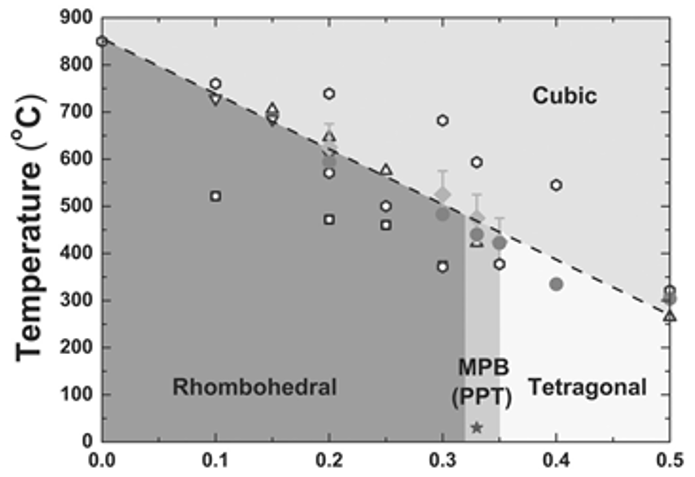
4.1. BiFeO3-Based Binary System
4.2. BiFeO3-Based Ternary System
4.2.1. Bulk Ceramics
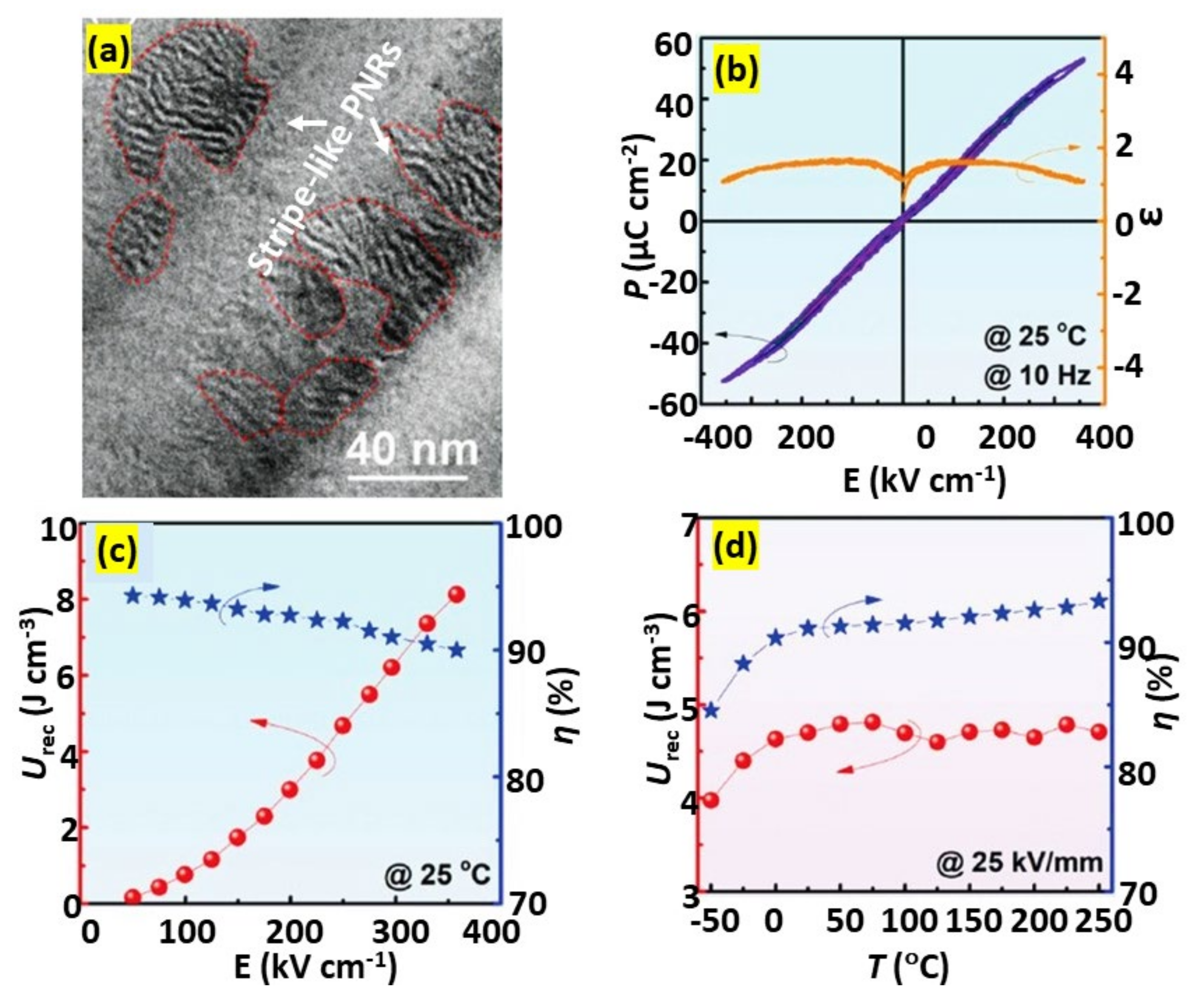
4.2.2. Thin Films
4.3. Multilayered Structure
5. Critical Issues and Strategy
5.1. Leakage Current Control
5.2. Microstructure Engineering
5.3. Band Gap Engineering
5.4. Electromechanical Breakdown
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Goodenough, J.B.; Abruna, H.D.; Buchanan, M.V. Basic Research Needs for Electrical Energy Storage. Report of the Basic Energy Sciences Workshop on Electrical Energy Storage, April 2–4, 2007; U.S. Department of Energy Office of Scientific and Technical Information: Washington, DC, USA, 2007. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Kong, X.; Li, F.; Hao, H.; Cheng, Z.; Liu, H.; Li, J.-F.; Zhang, S. Perovskite lead-free dielectrics for energy storage applications. Prog. Mater. Sc. 2019, 102, 72. [Google Scholar] [CrossRef]
- Kusko, A.; Dedad, J. Stored energy—Short-term and long-term energy storage methods. IEEE Ind. Appl. Mag. 2007, 13, 66. [Google Scholar] [CrossRef]
- Yao, K.; Chen, S.; Rahimabady, M.; Mirshekarloo, M.S.; Yu, S.; Tay, F.E.H.; Sritharan, T.; Lu, L. Nonlinear dielectric thin films for high-power electric storage with energy density comparable with electrochemical supercapacitors. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2011, 58, 1968. [Google Scholar] [PubMed]
- Jow, T.R.; MacDougall, F.W.; Ennis, J.B.; Yang, X.H.; Schneider, M.A.; Scozzie, C.J.; White, J.D.; MacDonald, J.R.; Schalnat, M.C.; Cooper, R.A.; et al. Pulsed power capacitor development and outlook. IEEE 2015, unpublished. [Google Scholar]
- Sarjeant, W.J.; Zirnheld, J.; MacDougall, F.W. Capacitors. IEEE Trans. Plasma Sci. 1998, 26, 1368. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by impedance spectroscopy. Adv. Mater. 1990, 2, 132. [Google Scholar] [CrossRef]
- Sarjeant, W.J.; Clelland, I.W.; Price, R.A. Capacitive components for power electronics. Proc. IEEE. 2001, 89, 846. [Google Scholar] [CrossRef]
- Bell., A.J. Ferroelectrics: The role of ceramic science and engineering. J. Eur. Ceram. Soc. 2008, 28, 1307. [Google Scholar] [CrossRef]
- Tan, Q.; Irwin, P.; Cao, Y. Advanced dielectrics for capacitors. IEEJ Trans. Fund. Mater. 2006, 126, 1152. [Google Scholar] [CrossRef] [Green Version]
- Chu, B.J.; Zhou, X.; Ren, K.; Neese, B.; Lin, M.; Wang, Q.; Bauer, F.; Zhang, Q.M. A dielectric polymer with high electric energy density and fast discharge speed. Science 2006, 313, 334. [Google Scholar] [CrossRef]
- Khanchaitit., P.; Han., K.; Gadinski, M.R.; Li, Q.; Wang, Q. Ferroelectric polymer networks with high energy density and improved discharged efficiency for dielectric energy storage. Nat. Commun. 2013, 4, 2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Han, K.; Gadinski, M.R.; Zhang, G.; Wang, Q. High Energy and Power Density Capacitors from Solution-Processed Ternary Ferroelectric Polymer Nanocomposites. Adv. Mater. 2014, 26, 6244. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Yang, T.; Gadinski, M.R.; Zhang, G.; Chen, L.-Q.; Wang, Q. Sandwich-structured polymer nanocomposites with high energy density and great charge–discharge efficiency at elevated temperatures. Proc. Natl. Acad. Sci. USA 2016, 113, 9995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peddigari, M.; Palneedi, H.; Hwang, G.-T.; Ryu, J. Linear and nonlinear dielectric capacitors for high-power energy storage capacitor applications. J. Korean Ceram. Soc. 2019, 56, 1. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, P.; Vidya, R.; Kjekshus, A.; Fjellvåg, H.; Eriksson, O. Theoretical investigation of magnetoelectric behavior in BiFeO3. Phys. Rev. B 2006, 74, 224412. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, X.; Xiong, Z.; Wang, M.; Liu, Q. Environmental Pollution Effect Analysis of Lead Compounds in China Based on Life Cycle. Int. J. Environ. Res. Public Health 2020, 17, 2184. [Google Scholar] [CrossRef] [Green Version]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–164. [Google Scholar]
- Zhou, H.Y.; Liu, X.Q.; Zhu, X.L.; Chen, X.M. CaTiO3 Linear Dielectric Ceramics with Greatly Enhanced Dielectric Strength and Energy Storage Density. J. Am. Ceram. Soc. 2017, 101, 1999. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Zhu, X.N.; Ren, G.R.; Chen, X.M. Enhanced Energy Storage Density and Its Variation Tendency in CaZrxTi1−xO3 Ceramics. J. Alloys Compd. 2016, 688, 687. [Google Scholar] [CrossRef]
- Yang, H.; Yan, F.; Lin, Y.; Wang, T. Enhanced Recoverable Energy Storage Density and High Efficiency of SrTiO3-Based Lead-Free Ceramics. Appl. Phys. Lett. 2017, 111, 253903. [Google Scholar] [CrossRef]
- Shay, D.P.; Podraza, N.J.; Randall, C.A. High Energy Density, High Temperature Capacitors Utilizing Mn-Doped 0.8CaTiO3–0.2CaHfO3 Ceramics. J. Am. Ceram. Soc. 2012, 95, 1348. [Google Scholar] [CrossRef]
- Lv, J.; Li, Q.; Li, Y.; Tang, M.; Jin, D.; Yan, Y.; Fan, B.; Jin, L.; Liu, G. Significantly improved energy storage performance of NBT-BT based ceramics through domain control and preparation optimization. Chem. Eng. J. 2021, 420, 129900. [Google Scholar] [CrossRef]
- Gao, F.; Dong, X.; Mao, C.; Cao, F.; Wang, G. c/a Ratio-Dependent Energy-Storage Density in (0.9−x)Bi0.5Na0.5TiO3–xBaTiO3–0.1K0.5Na0.5NbO3 Ceramics. J. Am. Ceram. Soc. 2011, 94, 4162. [Google Scholar] [CrossRef]
- Viola, G.; Ning, H.; Reece, M.J.; Wilson, R.; Correia, T.M.; Weaver, P.; Cain, M.G.; Yan, H. Reversibility in electric field-induced transitions and energy storage properties of bismuth-based perovskite ceramics. J. Phys. D Appl. Phys. 2012, 45, 355302. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, J.; He, Z.; Zhang, L.; Cao, M.; Huang, X.; Lanagan, M.T.; Hao, H.; Yao, Z.; Liu, H. Energy-storage properties of Bi0.5Na0.5TiO3-BaTiO3-KNbO3 ceramics fabricated by wet-chemical method. J. Eur. Ceram. Soc. 2017, 37, 99. [Google Scholar] [CrossRef]
- Puli, V.S.; Pradhan, D.K.; Chrisey, D.B.; Tomozawa, M.; Sharma, G.L.; Scott, J.F.; Katiyar, R.S. Structure, dielectric, ferroelectric, and energy density properties of (1 − x)BZT–xBCT ceramic capacitors for energy storage applications. J. Mater. Sci. 2013, 48, 2151. [Google Scholar] [CrossRef]
- Puli, V.S.; Pradhan, D.K.; Riggs, B.C.; Chrisey, D.B.; Katiyar, R.S. Structure, Ferroelectric, Dielectric and Energy Storage Studies of Ba0.70Ca0.30TiO3, Ba(Zr0.20Ti0.80)O3 Ceramic Capacitors. Integr. Ferroelectr. 2014, 157, 139. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhu, H.; Fu, Z.; Zhang, Q. Sintering temperature dependence of dielectric properties and energy-storage properties in (Ba,Zr)TiO3 ceramics. J. Mater. Sci. Mater. Electron. 2017, 28, 514. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Zhao, Q.; Cai, Z.; Chen, Z.; Li, L. Effects of gtain size and temperature on the energy storage and dielectric tunablity of non-reducible BaTiO3-based bulk ceramics. J. Euro. Ceram. Soc. 2019, 39, 1142. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Zhao, Q.; Li, L. Improved energy storage properties of fine crystalline BaTiO3 ceramics by coating powders with Al2O3 and SiO2. J. Am. Ceram. Soc. 2015, 98, 2641. [Google Scholar] [CrossRef]
- Qu, B.; Du, H.; Yang, Z.; Liu, Q. Large recoverable energy storage density and low sintering temperature in potassium-sodium niobate-based ceramics for multilayer pulsed power capacitors. J. Am. Ceram. Soc. 2017, 100, 1517. [Google Scholar] [CrossRef]
- Ciuchi, I.V.; Mitoseriu, L.; Galassi, C. Antiferroelectric to Ferroelectric Crossover and Energy Storage Properties of (Pb1−xLax)(Zr0.90Ti0.10)1−x/4O3 (0.02 ≤ x ≤ 0.04) Ceramics. J. Am. Ceram. Soc. 2016, 99, 2382. [Google Scholar] [CrossRef]
- Dan, Y.; Xu, H.; Zou, K.; Zhang, Q.; Lu, Y.; Chang, G.; Huang, H.; He, Y. Energy storage characteristics of (Pb,La)(Zr,Sn,Ti)O3 antiferroelectric ceramics with high Sn content. Appl. Phys. Lett. 2018, 113, 063902. [Google Scholar] [CrossRef]
- Jo, H.R.; Lynch, C.S. A high energy density relaxor antiferroelectric pulsed capacitor dielectric. J. Appl. Phys. 2016, 119, 024104. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Chen, X.; Yan, S.; Cao, F.; Dong, X.; Wang, G. High charge-discharge performance of Pb0.98La0.02(Zr0.35Sn0.55Ti0.10)0.995O3 antiferroelectric ceramic. J. Appl. Phys. 2016, 120, 074107. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, C.; Xu, J.; Yang, L.; Zhang, X.; Zeng, W.; Yuan, C.; Chen, G.; Rao, G. Tailoring antiferroelectricity with high energy-storage properties in Bi0.5Na0.5TiO3–BaTiO3 ceramics by modulating Bi/Na ratio. J. Mater. Sci. Mater. Electron. 2016, 27, 10810. [Google Scholar]
- Yang, H.; Yan, F.; Lin, Y.; Wang, T. Improvement of dielectric and energy storage properties in SrTiO3-based lead-free ceramics. J. Alloys. Compd. 2017, 728, 780. [Google Scholar] [CrossRef]
- Cao, W.; Li, W.; Feng, Y.; Bai, T.; Qiao, Y.; Hou, Y.; Zhang, T.; Yu, Y.; Fei, W. Defect dipole induced large recoverable strain and high energy-storage density in lead-free Na0.5Bi0.5TiO3-based systems. Appl. Phys. Lett. 2016, 108, 202902. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Gao, J.; Zhang, S.; Li, J.-F. Lead-Free Antiferroelectric Silver Niobate Tantalate with High Energy Storage Performance. Adv. Mater. 2017, 29, 1701824. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, J.; Liu, Q.; Zhang, S.; Li, J.-F. Silver Niobate Lead-Free Antiferroelectric Ceramics: Enhancing Energy Storage Density by B-Site Doping. ACS Appl. Mater. Interfaces 2018, 10, 819. [Google Scholar] [CrossRef]
- Li, J.; Jin, L.; Tian, Y.; Chen, C.; Lan, Y.; Hu, Q.; Li, C.; Wei, X.; Yan, H. Enhanced energy storage performance under low electric field in Sm3+ doped AgNbO3 ceramics. J. Materiomics 2021, in press. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.; Zhou, C.; Yuan, C.; Li, Q.; Chen, G.; Wang, H.; Yang, L. High energy storage properties and dielectric behavior of (Bi0.5Na0.5)0.94Ba0.06Ti1−x(Al0.5Nb0.5)xO3 lead-free ferroelectric ceramics. Ceram. Int. 2016, 42, 2221. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, T.; Ye, J.; Wang, G.; Dong, X.; Withers, R.; Liu, Y. Antiferroelectrics for energy storage applications: A review. Adv. Mat. Technol. 2018, 3, 1800111. [Google Scholar] [CrossRef]
- Palneedi, H.; Peddigari, M.; Hwang, G.T.; Jeong, D.W.; Ryu, J. High-Performance Dielectric Ceramic Films for Energy Storage Capacitors: Progress and Outlook. Adv. Funct. Mater. 2018, 28, 1803665. [Google Scholar] [CrossRef]
- Marqués, M.I.; Aragó, C. Microscopic model for the formation of nanodomains in relaxor materials. Phys. Rev. B 2010, 81, 064114. [Google Scholar] [CrossRef]
- Li, F.; Zhang, S.; Damjanovic, D.; Chen, L.-Q.; Shrout, T.R. Local Structural Heterogeneity and Electromechanical Responses of Ferroelectrics: Learning from Relaxor Ferroelectrics. Adv. Func. Mater. 2018, 28, 1801504. [Google Scholar] [CrossRef]
- Kirillov, V.V.; Isupov, V.A. Relaxation polarization of PbMg1/3Nb2/3O3 (PMN)-A ferroelectric with a diffused phase transition. Ferroelectrics 1973, 5, 3. [Google Scholar] [CrossRef]
- Cross, L.E. Relaxor ferroelectrics. Ferroelectrics 1987, 76, 241. [Google Scholar] [CrossRef]
- Viehland, D.; Jang, S.J.; Cross, L.E.; Wuttig, M. Freezing of the polarization fluctuations in lead magnesium niobate relaxors. J. Appl. Phys. 1990, 68, 2916. [Google Scholar] [CrossRef]
- Westphal, V.; Kleemann, W.; Glinchuk, M.D. Diffuse phase transitions and random-field-induced domain states of the relaxor ferroelectric PbMg1/3Nb2/3O3. Phys. Rev. Lett. 1992, 68, 847. [Google Scholar] [CrossRef]
- Akbas, M.A.; Davies, P.K. Domain Growth in Pb(Mg1/3Ta2/3)O3 Perovskite Relaxor Ferroelectric Oxides. J. Am. Ceram. Soc. 1997, 80, 2933. [Google Scholar] [CrossRef]
- Cheng, Z.Y.; Katiyar, R.S.; Yao, X.; Guo, A. Dielectric behavior of lead magnesium niobate relaxors. Phys. Rev. B 1997, 55, 8165. [Google Scholar] [CrossRef]
- Pirc, R.; Blinc, R. Spherical random-bond--random-field model of relaxor ferroelectrics. Phys. Rev. B 1999, 60, 13470. [Google Scholar] [CrossRef]
- Neaton, J.B.; Ederer, C.; Waghmare, U.V.; Spaldin, N.A.; Rabe, K.M. First-principles study of spontaneous polarization in multiferroic BiFeO3. Phys. Rev. B 2005, 71, 014113. [Google Scholar] [CrossRef] [Green Version]
- Moreau, J.M.; Michel, C.; Gerson, R.; James, W.J. Ferroelectric BiFeO3 X-ray and neutron diffraction study. J. Phys. Chem. Solids 1971, 32, 1315. [Google Scholar] [CrossRef]
- Kubel, F.; Schmid, H. Structure of a ferroelectric and ferroelastic monodomain crystal of the perovskite BiFeO3. Acta Cryst. B 1990, 46, 698. [Google Scholar] [CrossRef] [Green Version]
- Fischer, P.; Polomska, M.; Sosnowska, I.; Szymanski, M. Temperature dependence of the crystal and magnetic structures of BiFeO3. J. Phys. C Solid State Phys. 1980, 13, 1931. [Google Scholar] [CrossRef]
- Smith, R.T.; Achenbach, G.D.; Gerson, R.; James, W.J. Dielectric Properties of Solid Solutions of BiFeO3 with Pb(Ti, Zr)O3 at High Temperature and High Frequency. J. Appl. Phys. 1968, 39, 70. [Google Scholar] [CrossRef]
- Ederer, C.; Spaldin, N.A. Effect of Epitaxial Strain on the Spontaneous Polarization of Thin Film Ferroelectrics. Phys. Rev. Lett. 2005, 95, 257601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebeugle, D.; Colson, D.; Forget, A.; Viret, M. Very large spontaneous electric polarization in BiFeO3 single crystals at room temperature and its evolution under cycling fields. Appl. Phys. Lett. 2007, 91, 022907. [Google Scholar] [CrossRef] [Green Version]
- Teague, J.R.; Gerson, R.; James, W.J. Dielectric hysteresis in single crystal BiFeO3. Solid State Commun. 1970, 8, 1073. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, L.; Zhang, M.F.; Chen, X.Y.; Liu, J.-M.; Liu, Z.G. Room-temperature saturated ferroelectric polarization in BiFeO3 ceramics synthesized by rapid liquid phase sintering. Appl. Phys. Lett. 2004, 84, 1731. [Google Scholar] [CrossRef]
- Ueda, K.; Tabata, H.; Kawai, T. Coexistence of ferroelectricity and ferromagnetism in BiFeO3–BaTiO3 thin films at room temperature. Appl. Phys. Lett. 1999, 75, 555. [Google Scholar] [CrossRef]
- Palkar, V.R.; John, J.; Pinto, R. Observation of saturated polarization and dielectric anomaly in magnetoelectric BiFeO3 thin films. Appl. Phys. Lett. 2002, 80, 1628. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures. Science 2003, 299, 1719. [Google Scholar] [CrossRef]
- Yun, K.Y.; Noda, M.; Okuyama, M. Prominent ferroelectricity of BiFeO3 thin films prepared by pulsed-laser deposition. Appl. Phys. Lett. 2003, 83, 3981. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, D.J.; Park, J.S.; Kim, S.W.; Song, T.K.; Kim, M.-H.; Kim, W.-J.; Do, D.; Jeong, I.-K. High-Performance Lead-Free Piezoceramics with High Curie Temperatures. Adv. Mater. 2015, 27, 6976. [Google Scholar] [CrossRef]
- Kumar, M.M.; Srinivas, A.; Suryanarayana, S.V. Structure property relations in BiFeO3/BaTiO3 solid solutions. J. Appl. Phys. 2000, 87, 855. [Google Scholar] [CrossRef]
- Zhaludkevich, D.V.; Latushka, S.I.; Latushka, T.V.; Sysa, A.V.; Shaman, Y.P.; Dronova, D.A.; Chobot, A.N.; Chobot, G.M.; Nekludov, K.N.; Silibin, M.V.; et al. Crystal structure and magnetic properties of (1-x) BiFeO3-xBaTiO3 ceramics across the phase boundary. Nanomaterials Sci. Eng. 2020, 2, 93. [Google Scholar]
- Wei, Y.; Wang, X.; Jia, J.; Wang, X. Multiferroic and piezoelectric properties of 0.65BiFeO3–0.35BaTiO3 ceramic with pseudo-cubic symmetry. Ceram. Int. 2012, 38, 3499. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, H.; Zhou, Q.; Cen, Z.; Li, W.; Yuan, C.; Wang, H. Dielectric, ferroelectric and piezoelectric properties of La-substituted BiFeO3–BaTiO3 ceramics. Ceram. Int. 2013, 39, 4307. [Google Scholar] [CrossRef]
- Leontsev, S.O.; Eitel, R.E. Dielectric and Piezoelectric Properties in Mn-Modified (1−x)BiFeO3–xBaTiO3 Ceramics. J. Am. Ceram. Soc. 2009, 92, 2957. [Google Scholar] [CrossRef]
- Wang, T.; Jin, L.; Tian, Y.; Shu, L.; Hu, Q.; Wei, X. Microstructure and ferroelectric properties of Nb2O5-modified BiFeO3-BaTiO3 lead-free ceramics for energy storage. Mater. Lett. 2014, 137, 79. [Google Scholar] [CrossRef]
- Zhu, L.-F.; Lei, X.-W.; Zhao, L.; Hussain, M.I.; Zhao, G.-Z.; Zhang, B.-P. Phase structure and energy storage performance for BiFeO3–BaTiO3 based lead-free ferroelectric ceramics. Ceram. Int. 2019, 45, 20266. [Google Scholar] [CrossRef]
- Wang, D.; Fan, Z.; Zhou, D.; Khesro, A.; Murakami, S.; Feteira, A.; Zhao, Q.; Tan, X.; Reaney, I.M. Bismuth ferrite-based lead-free ceramics and multilayers with high recoverable energy density. J. Mater. Chem. A 2018, 6, 4133. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Bai, X.; Wang, H.; Du, J.; Bai, W.; Li, L.; Wen, F.; Zheng, P.; Wu, W.; Zheng, L.; et al. Achieving high-energy storage performance in 0.67Bi1-xSmxFeO3-0.33BaTiO3 lead-free relaxor ferroelectric ceramics. Ceram. Int. 2020, 46, 11549. [Google Scholar] [CrossRef]
- Zheng, D.; Zuo, R.; Zhang, D.; Li, Y. Novel BiFeO3–BaTiO3–Ba(Mg1/3Nb2/3)O3 Lead-Free Relaxor Ferroelectric Ceramics for Energy-Storage Capacitors. J. Am. Ceram. Soc. 2015, 98, 2692. [Google Scholar] [CrossRef]
- Zheng, D.; Zuo, R. Enhanced energy storage properties in La(Mg1/2Ti1/2)O3-modified BiFeO3-BaTiO3 lead-free relaxor ferroelectric ceramics within a wide temperature range. J. Eur. Ceram. Soc. 2017, 37, 413. [Google Scholar] [CrossRef]
- Liu, N.; Liang, R.; Zhou, Z.; Dong, X. Designing lead-free bismuth ferrite-based ceramics learning from relaxor ferroelectric behavior for simultaneous high energy density and efficiency under low electric field. J. Mater. Chem. C 2018, 6, 10211. [Google Scholar] [CrossRef]
- Wang, D.; Fan, Z.; Li, W.; Zhou, D.; Feteira, A.; Wang, G.; Murakami, S.; Sun, S.; Zhao, Q.; Tan, X.; et al. High Energy Storage Density and Large Strain in Bi(Zn2/3Nb1/3)O3-Doped BiFeO3–BaTiO3 Ceramics. ACS Appl. Energy Mater. 2018, 1, 4403. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Zhang, X.; Fan, Z.; Yang, F.; Feteira, A.; Zhou, D.; Sinclair, D.C.; Ma, T.; Tan, X.; et al. Ultrahigh energy storage density lead-free multilayers by controlled electrical homogeneity. Energy Environ. Sci. 2019, 12, 582. [Google Scholar] [CrossRef]
- Liu, N.; Liang, R.; Zhao, X.; Xu, C.; Zhou, Z.; Dong, X. Novel bismuth ferrite-based lead-free ceramics with high energy and power density. J. Am. Ceram. Soc. 2018, 101, 3259. [Google Scholar] [CrossRef]
- Tang, M.; Yu, L.; Wang, Y.; Lv, J.; Dong, J.; Guo, B.; Chen, F.; Ai, Q.; Luo, Y.; Li, Q.; et al. Dielectric, ferroelectric, and energy storage properties of Ba(Zn1/3Nb2/3)O3-modfied BiFeO3–BaTiO3 Pb-Free relaxor ferroelectric ceramics. Ceram. Int. 2021, 47, 3780. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Sun, Q.; Zhang, X.; Ma, Z.; Guo, M.; Sun, B.; Zhu, X.; Liu, Q.; Lou, X. Large energy storage density in BiFeO3-BaTiO3-AgNbO3 lead-free relaxor ceramics. J. Eur. Ceram. Soc. 2020, 40, 2929. [Google Scholar] [CrossRef]
- Yu, Z.; Zeng, J.; Zheng, L.; Rousseau, A.; Li, G.; Kassiba, A. Microstructure effects on the energy storage density in BiFeO3-based ferroelectric ceramics. Ceram. Int. 2021, 47, 12735. [Google Scholar] [CrossRef]
- Yang, H.; Qi, H.; Zuo, R. Enhanced breakdown strength and energy storage density in a new BiFeO3-based ternary lead-free relaxor ferroelectric ceramic. J. Eur. Ceram. Soc. 2019, 39, 2673. [Google Scholar] [CrossRef]
- Liu, G.; Tang, M.; Hou, X.; Guo, B.; Lv, J.; Dong, J.; Wang, Y.; Li, Q.; Yu, K.; Yan, Y.; et al. Energy storage properties of bismuth ferrite based ternary relaxor ferroelectric ceramics through a viscous polymer process. Chem. Eng. J. 2020, 412, 127555. [Google Scholar] [CrossRef]
- Li, Q.; Ji, S.; Wang, D.; Zhu, J.; Li, L.; Wang, W.; Zeng, M.; Hou, Z.; Gao, X.; Lu, X.; et al. Simultaneously enhanced energy storage density and efficiency in novel BiFeO3-based lead-free ceramic capacitors. J. Eur. Ceram. Soc. 2021, 41, 387. [Google Scholar] [CrossRef]
- Ji, S.; Li, Q.; Wang, D.; Zhu, J.; Zeng, M.; Hou, Z.; Fan, Z.; Gao, X.; Lu, X.; Li, Q.; et al. Enhanced energy storage performance and thermal stability in relaxor ferroelectric (1-x)BiFeO3-x(0.85BaTiO3-0.15Bi(Sn0.5Zn0.5)O3) ceramics. J. Am. Ceram. Soc. 2021, 104, 2426. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, G.; Bao, W.; Li, J.; Li, L.; Mostaed, A.; Yang, H.; Ji, H.; Li, D.; Feteira, A.; et al. Superior energy density through tailored dopant strategies in multilayer ceramic capacitors. Energy Environ. Sci. 2020, 13, 2938. [Google Scholar] [CrossRef]
- Qi, H.; Xie, A.; Tian, A.; Zuo, R. Superior Energy-Storage Capacitors with Simultaneously Giant Energy Density and Efficiency Using Nanodomain Engineered BiFeO3-BaTiO3-NaNbO3 Lead-Free Bulk Ferroelectrics. Adv. Energy Mater. 2020, 10, 1903338. [Google Scholar] [CrossRef]
- Correia, T.M.; McMillen, M.; Rokosz, M.K.; Weaver, P.M.; Gregg, J.M.; Viola, G.; Cain, M.G. A Lead-Free and High-Energy Density Ceramic for Energy Storage Applications. J. Am. Ceram. Soc. 2013, 96, 2699. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Zeng, Y.; Shen, Y.; Lin, Y.-H.; Ma, J.; Li, L.; Nan, C.-W. BiFeO3–SrTiO3 thin film as a new lead-free relaxor-ferroelectric capacitor with ultrahigh energy storage performance. J. Mater. Chem. A 2017, 5, 5920. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, B.; Koritala, R.E.; Balachandran, U. Temperature dependent energy storage properties of antiferroelectric Pb0. 96La0.04Zr0.98Ti0.02O3 thin films. Appl. Phys. Lett. 2014, 104, 263902. [Google Scholar]
- Pan, H.; Ma, J.; Ma, J.; Zhang, Q.; Liu, X.; Guan, B.; Gu, L.; Zhang, X.; Zhang, Y.-J.; Li, L.; et al. Giant energy density and high efficiency achieved in bismuth ferrite-based film capacitors via domain engineering. Nat. Commun. 2018, 9, 1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; Li, F.; Liu, Y.; Zhang, Q.; Wang, M.; Lan, S.; Zheng, Y.; Ma, J.; Gu, L.; Shen, Y.; et al. Ultrahigh–energy density lead-free dielectric films via polymorphic nanodomain design. Science 2019, 365, 578. [Google Scholar] [CrossRef] [PubMed]
- Kursumovic, A.; Li, W.-W.; Cho, S.; Curran, P.J.; Tjhe, D.H.L.; MacManus-Driscoll, J.L. Lead-free relaxor thin films with huge energy density and low loss for high temperature applications. Nano Energy 2020, 71, 104536. [Google Scholar] [CrossRef]
- Yan, F.; Shi, Y.; Zhou, X.; Zhu, K.; Shen, B.; Zhai, J. Optimization of polarization and electric field of bismuth ferrite-based ceramics for capacitor applications. Chem. Eng. J. 2020, 417, 127945. [Google Scholar] [CrossRef]
- Wang, G.; Lu, Z.; Li, J.; Ji, H.; Yang, H.; Li, L.; Sun, S.; Feteira, A.; Yang, H.; Zuo, R.; et al. Lead-free (Ba,Sr)TiO3—BiFeO3 based multilayer ceramic capacitors with high energy density. J. Eur. Ceram. Soc. 2020, 40, 1779. [Google Scholar] [CrossRef]
- Wang, G.; Lu, Z.; Yang, H.; Ji, H.; Mostaed, A.; Li, L.; Wei, Y.; Feteira, A.; Sun, S.; Sinclair, D.C.; et al. Fatigue resistant lead-free multilayer ceramic capacitors with ultrahigh energy density. J. Mater. Chem. A 2020, 8, 11414. [Google Scholar] [CrossRef]
- Qi, X.; Dho, J.; Tomov, R.; Blamire, M.G.; MacManus-Driscoll, J.L. Greatly reduced leakage current and conduction current mechanism in aliovalent-ion-doped BiFeO3. Appl. Phys. Lett. 2005, 86, 062903. [Google Scholar] [CrossRef]
- Hu, G.D.; Fan, S.H.; Yang, C.H.; Wu, W.B. Low leakage and current and enhanced ferroelectric properties of Ti and Zn codoped BiFeO3 thin films. Appl. Phys. Lett. 2008, 92, 192905. [Google Scholar] [CrossRef]
- Singh, S.K.; Maruyama, K.; Ishiwara, H. Reduced leakage current in La and Na codoped BiFeO3 thin films. Appl. Phys. Lett. 2007, 91, 112913. [Google Scholar] [CrossRef]
- Hu, G.D.; Cheng, X.; Wu, W.B.; Yang, C.H. Effect of Gd substitution on structure and ferroelectric properties of BiFeO3 thin films prepared using metal organic decomposition. Appl. Phys. Lett. 2007, 91, 232909. [Google Scholar] [CrossRef]
- Veerapandian, V.; Benes, F.; Gindel, T.; Deluca, M. Strategies to develop energy storage properties of perovskite lead free relaxor ferroelectrics: A review. Materials 2020, 13, 5742. [Google Scholar] [CrossRef]
- Ray, S. An Introduction to High Voltage Engineering; PHI Learning Pvt. Ltd.: Delhi, India, 2013. [Google Scholar]
- Chen, I.W.; Wang, X.H. Sintering dense nanocrystalline ceramics without final-stage grain-growth. Nature 2000, 404, 168. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Pilania, G.; Ramprasad, R. From organized high-throughput data to phenomenological theory using machine learning: The example of dielectric breakdown. Chem. Mater. 2016, 28, 1304. [Google Scholar] [CrossRef]
- Moubah, R.; Schmerber, G.; Rousseau, O.; Colson, D.; Viret, M. Photoluminescence Investigation of Defects and Optical Band Gap in Multiferroic BiFeO3 Single Crystals. Appl. Phys. Express 2012, 5, 035802. [Google Scholar] [CrossRef]
- Walker, J.; Simons, H.; Alikin, D.O.; Turygin, A.P.; Shur, V.Y.; Kholkin, A.L.; Ursic, H.; Bencan, A.; Malic, B.; Nagarajan, V.; et al. Dual strain mechanisms in a leadfree morphotropic phase boundary ferroelectric. Sci. Rep. 2016, 6, 19630. [Google Scholar] [CrossRef] [PubMed]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deka, B.; Cho, K.-H. BiFeO3-Based Relaxor Ferroelectrics for Energy Storage: Progress and Prospects. Materials 2021, 14, 7188. https://doi.org/10.3390/ma14237188
Deka B, Cho K-H. BiFeO3-Based Relaxor Ferroelectrics for Energy Storage: Progress and Prospects. Materials. 2021; 14(23):7188. https://doi.org/10.3390/ma14237188
Chicago/Turabian StyleDeka, Bipul, and Kyung-Hoon Cho. 2021. "BiFeO3-Based Relaxor Ferroelectrics for Energy Storage: Progress and Prospects" Materials 14, no. 23: 7188. https://doi.org/10.3390/ma14237188
APA StyleDeka, B., & Cho, K.-H. (2021). BiFeO3-Based Relaxor Ferroelectrics for Energy Storage: Progress and Prospects. Materials, 14(23), 7188. https://doi.org/10.3390/ma14237188





