Waste Aluminum Application as Energy Valorization for Hydrogen Fuel Cells for Mobile Low Power Machines Applications
Abstract
:1. Introduction
2. Materials, Theoretical Models and Tests
2.1. Materials
2.2. Theoretical Kinetic Model
2.2.1. Theoretical Hydrogen Flow Rate
2.2.2. Aluminum Active Surface
2.3. Validation Tests
2.3.1. Test 1: Effect of Molarity
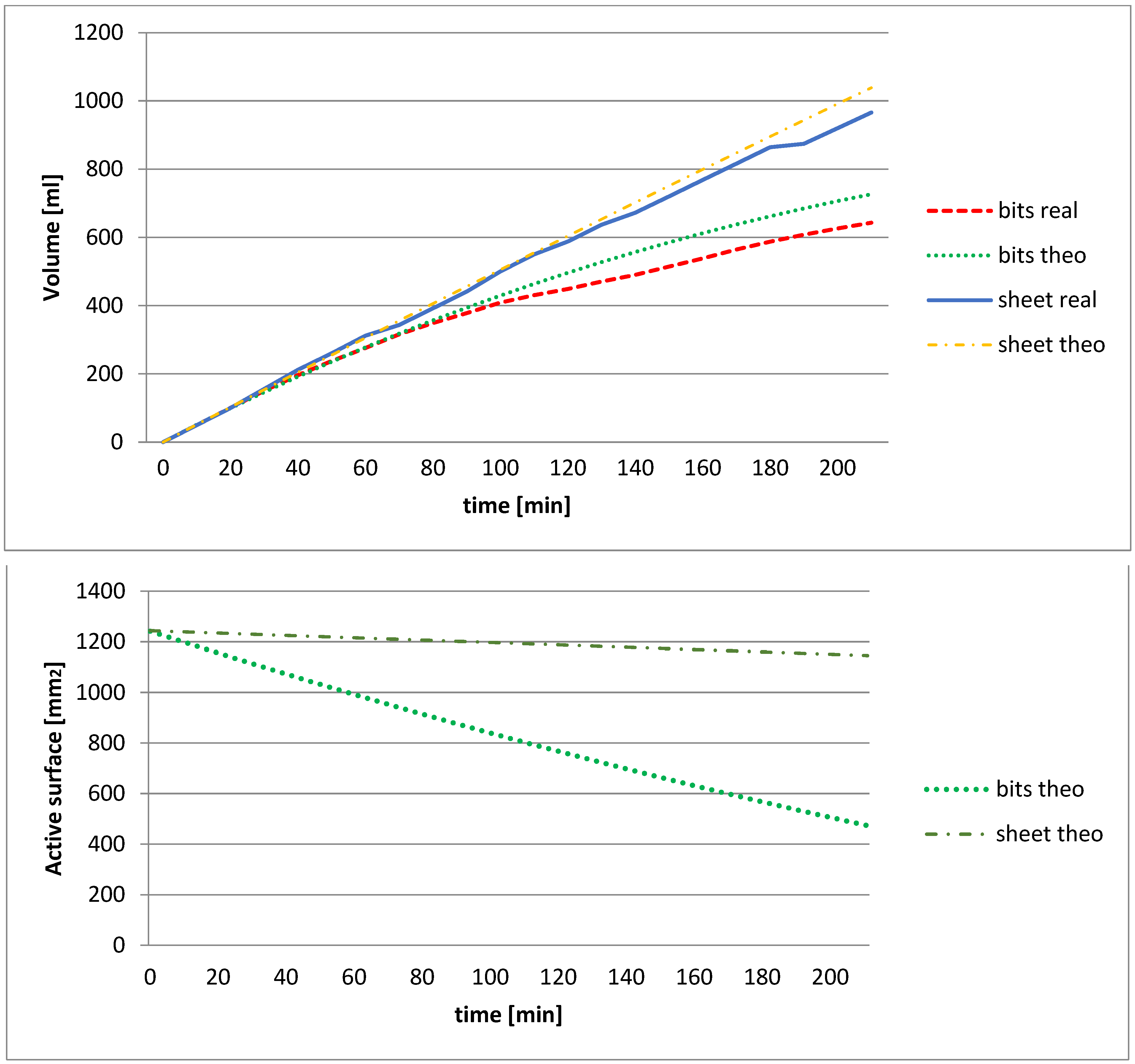
2.3.2. Test 2: Effect of the Dimension of the Sheet
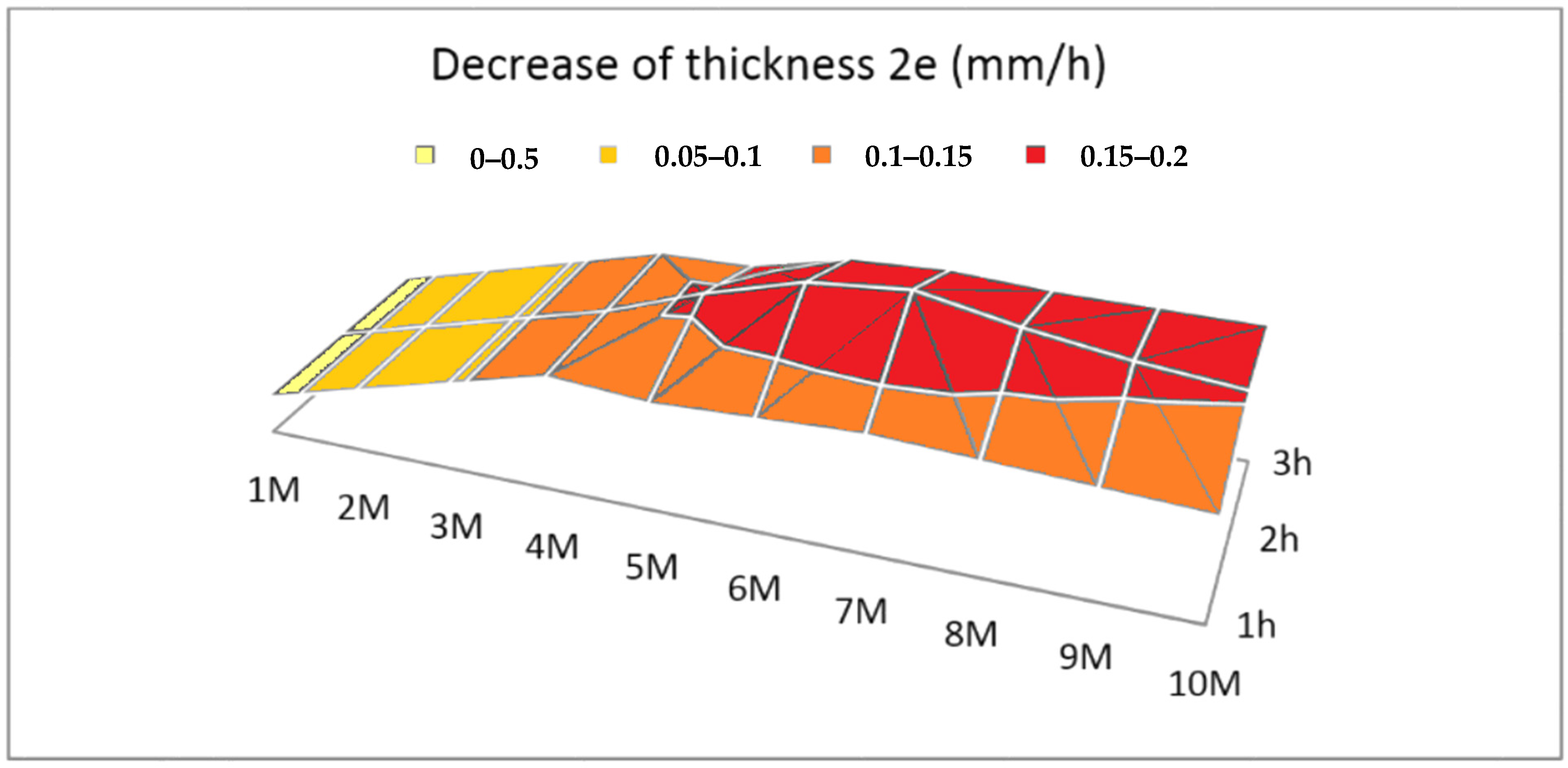
2.3.3. Test 3: Temperature Effect
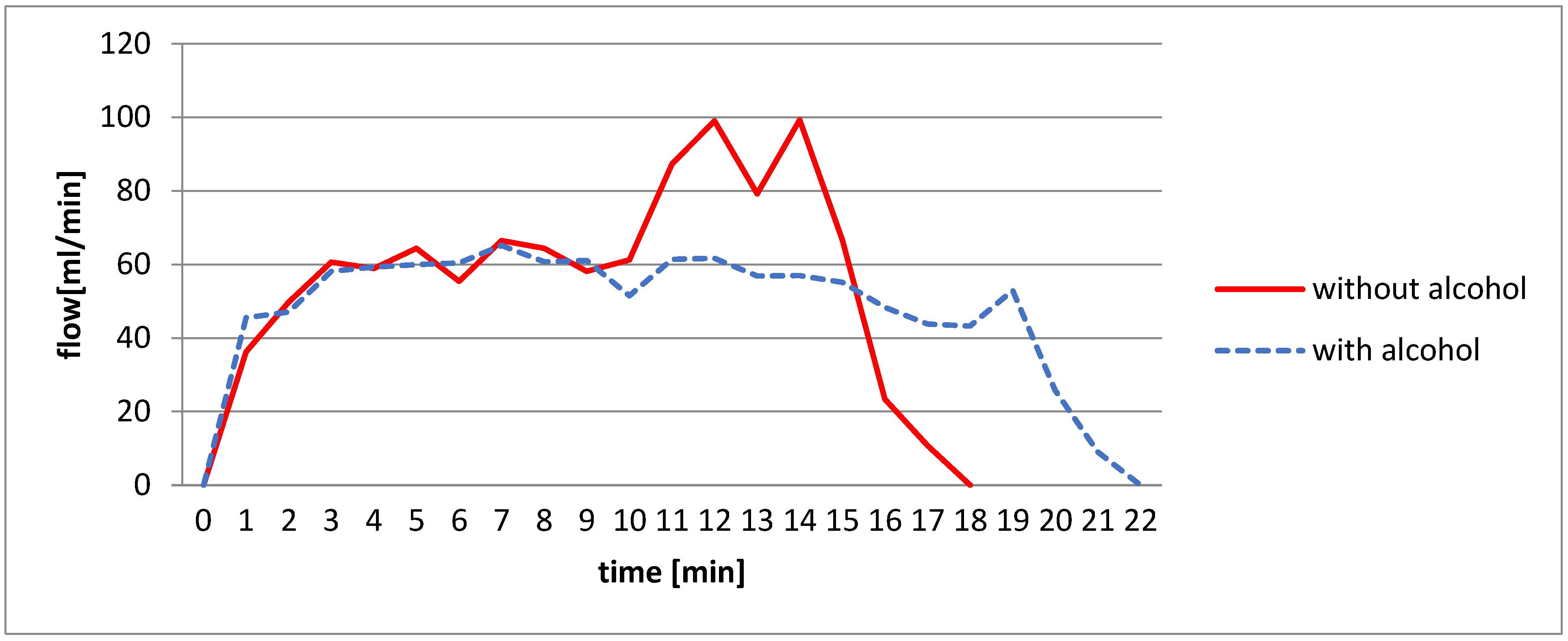
2.3.4. Test 4: Effect of the Addition of Alcohol
2.3.5. Test 5: Hydrogen Purity Analysis
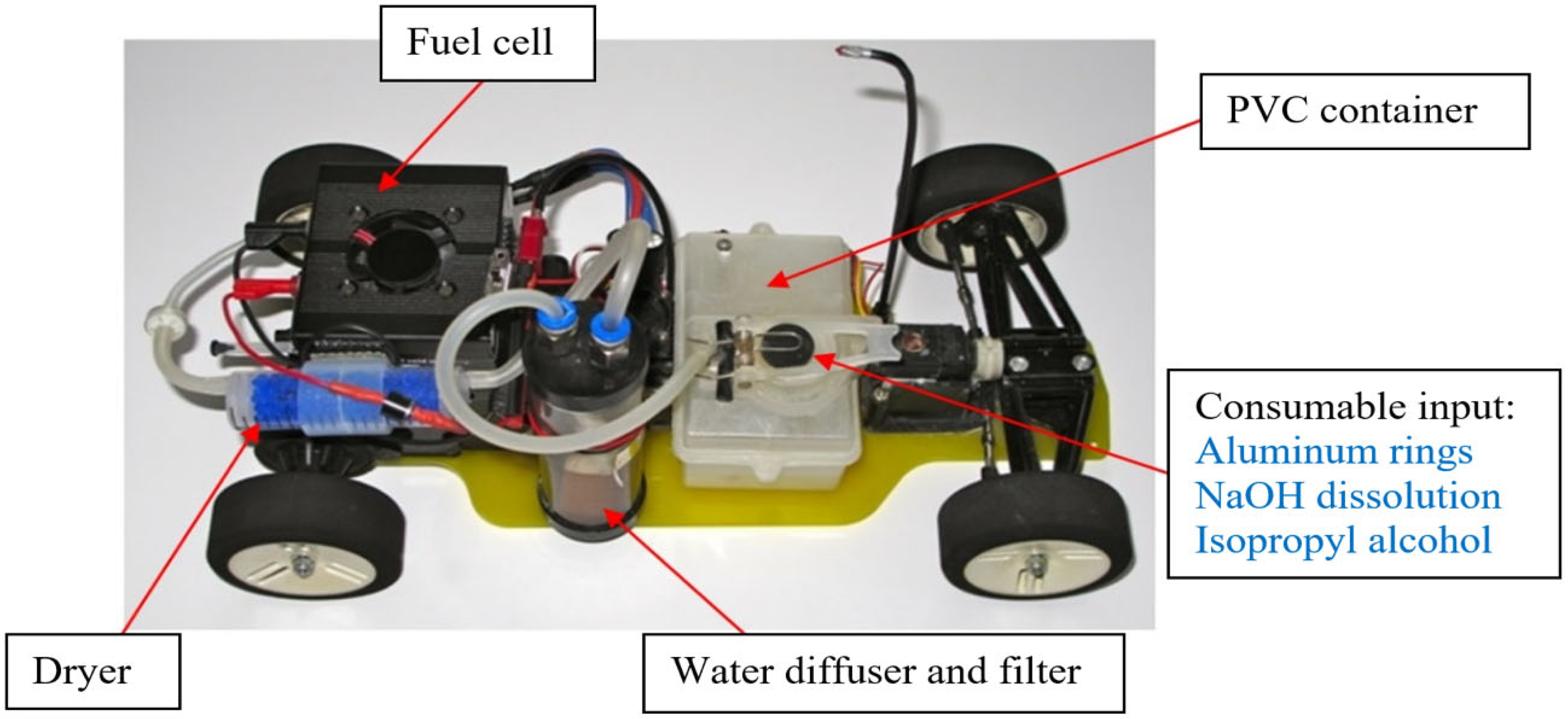
2.3.6. Test 6: Application in a Vehicle Prototype with Hydrogen Fuel Cell
3. Results
3.1. Trials
3.1.1. Effect of Molarity
3.1.2. Effect of Chip Size
3.1.3. Effect of Temperature
3.1.4. Adding Alcohol Effect
3.1.5. Hydrogen Purity
3.1.6. Prototype of a Vehicle with Hydrogen Fuel Cell from Aluminum Soda Rings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tabbi, W.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.G. Advances in stationary and portable fuel cell applications. Int. J. Hydrog. Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef] [Green Version]
- Ramin, M.; Katrina, M.G. Hydrogen storage and delivery: Review of the state of art technologies and risk and reliability analysis. Int. J. Hydrog. Energy 2019, 44, 12254–12269. [Google Scholar]
- Kundn, P.P.; Dutta, K. Hydrogen fuel cells for portable applications. Compend. Hydrog. Energy 2016, 4, 111–131. [Google Scholar] [CrossRef]
- Neda, A.; Ghasem, N.-D. A comprehensive review on biological hydrogen production. Int. J. Hydrog. Energy 2020, 45, 22492–22512. [Google Scholar]
- Atilla, E.; Hayati, O.; Sibel, O. Reforming options for hydrogen production from fossil fuels for PEM fuel cells. J. Power Sources 2006, 154, 67–73. [Google Scholar]
- Sui, J.; Chen, Z.; Wang, C.; Wang, Y.; Liu, J.; Li, W. Efficient hydrogen production from solar energy and fossil fuel via water-electrolysis and methane-steam-reforming hybridization. Appl. Energy 2020, 276, 115409. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Xie, X.; Cui, N.; Wang, B.; Zhang, Y.; Zhao, X.; Lin, L.; Wang, B.; Du, W. Recent advances in hydrogen generation process via hydrolysis of Mg-based materials: A short review. J. Alloys Compd. 2020, 816, 152634. [Google Scholar] [CrossRef]
- Khzouz, M.; Gkanas, E.I. Hydrogen Technologies for Mobility and Stationary Applications: Hydrogen Production, Storage and Infrastructure Development. Renew. Energy Resour. Chall. Appl. 2020, 255. [Google Scholar] [CrossRef]
- Guo, J.; Chen, P. Catalyst: NH3 as an Energy Carrier. Chem 2017, 3, 709–712. [Google Scholar] [CrossRef]
- Tomoda, K.; Hoshi, N.; Haruna, J.; Cao, M.; Yoshizaki, A.; Hirata, K. Hydrolysis Rate Improvement in Hydrogen Generation System Fueled by Powdery Sodium Borohydride for Fuel-Cell Vehicle. IEEE Trans. Ind. Appl. 2013, 50, 2741–2748. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrog. Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Taylor, G.; Jason, R.; Gabl, T.; Pourpoint, L. Portable Power Generation for remote areas using hydrogen generated via maleic acid-promoted hydrolysis of ammonia borane. Molecules 2019, 24, 4045. [Google Scholar]
- Tekade, S.P.; Pednekar, A.S.; Jadhav, G.R.; Kalekar, S.E.; Shende, D.Z.; Wasewar, K.L. Hydrogen generation through water splitting reaction using waste aluminum in presence of gallium. Int. J. Hydrog. Energy 2020, 45, 23954–23965. [Google Scholar] [CrossRef]
- Dudoladov, A.; Buryakovskaya, O.; Vlaskin, M.; Zhuk, A.; Shkolnikov, E. Generation of hydrogen by aluminium oxidation in aquaeous solutions at low temperatures. Int. J. Hydrog. Energy 2016, 41, 2230–2237. [Google Scholar] [CrossRef]
- Lin, Y.; Genzer, J.; Dickey, M.D. Attributes, Fabrication, and Applications of Gallium-Based Liquid Metal Particles. Adv. Sci. 2020, 7, 2000192. [Google Scholar] [CrossRef] [Green Version]
- Macanás, J.; Soler, L.; Candela, A.M.; Muñoz, M.; Casado, J. Hydrogen generation by aluminum corrosion in aqueous alkaline solutions of inorganic promoters: The AlHidrox process. Energy 2011, 36, 2493–2501. [Google Scholar] [CrossRef]
- Bolt, A.; Dincer, I.; Agelin-Chaab, M. A Review of Unique Aluminum–Water Based Hydrogen Production Options. Energy Fuels 2021, 35, 1024–1040. [Google Scholar] [CrossRef]
- Trowell, K.A.; Goroshin, S.; Frost, D.L.; Bergthorson, J.M. Aluminum and its role as a recyclable, sustainable, carrier of renewable energy. Appl. Energy 2020, 275, 115112. [Google Scholar] [CrossRef]
- Huang, X.; Liu, S. On-Demand Hydrogen Generator Based on the Reaction between Aluminum Slurry and Alkaline Solution. Adv. Mater. Res. 2012, 347–353, 3242–3245. [Google Scholar] [CrossRef]
- Razavi-Tousi, S.S.; Szpunar, J. Effect of structural evolution of aluminum powder during ball milling on hydrogen generation in aluminum–water reaction. Int. J. Hydrog. Energy 2013, 38, 795–806. [Google Scholar] [CrossRef]
- Hakenjos, A.; Muenter, H.; Wittstadt, U.; Hebling, C. A PEM fuel cell for combined measurement of current and temperature distribution, and flow field flooding. J. Power Sources 2004, 131, 213–216. [Google Scholar] [CrossRef]
- Wu, E.D.; Shi, P.F.; Du, C.D. A mini-type hydrogen generator from aluminium for proton exchange membrane fuel cells. J. Power Sources 2008, 181, 144–148. [Google Scholar]
- Petrovic, J.; Thomas, G. Reaction of Aluminum with Water to Produce Hydrogen. A Study of Issues Related to the Use of Aluminum for On-Board Vehicular Hydrogen Storage. U. S. Dep. Energy 2008, 1, 1–26. [Google Scholar]
- Bolt, A.; Dincer, I.; Agelin-Chaab, M. Energy and exergy analyses of hydrogen production process with aluminum and water chemical reaction. Energy 2020, 205, 117978. [Google Scholar] [CrossRef]
- Parmuzina, A.V.; Kravchenko, O.V.; Bulychev, B.M.; Shkol’Nikov, E.I.; Burlakova, A.G. Oxidation of activated aluminum with water as a method for hydrogen generation. Russ. Chem. Bull. 2009, 58, 493–498. [Google Scholar] [CrossRef]
- Huang, X.; Gao, T.; Pan, X.; Wei, D.; Lv, C.; Qin, L.; Huang, Y. A review: Feasibility of hydrogen generation from the reaction between aluminum and water for fuel cell applications. J. Power Sources 2013, 229, 133–140. [Google Scholar] [CrossRef]
- Wang, K.; Eom, S.; Cho, E.A.; Kwon, H. Feasibility of on-board hydrogen production from hydrolysis of AlFe alloy for PEMFCs. Int. J. Hydrog. Energy 2011, 36, 12338–12342. [Google Scholar]
- Zhang, Q.; Schulze, M.; Gazdzicki, P.; Friedrich, K.A. Comparison of different performance recovery procedures for polymer electrolyte membrane fuel cells. Appl. Energy 2021, 302, 117490. [Google Scholar] [CrossRef]
- Salueña-Berna, X.; Mujal-Rosas, R.; Dagà-Monmany, J.M.; Martínez-López, J. Aprovechamiento de residuos de aluminio industrial para la obtención controlada de hidrógeno mediante la reacción aluminio-agua. Afinidad Rev. Quím. Teór. Apl. 2016, 49, 269–277. [Google Scholar]
- Razavi-Tousi, S.; Szpunar, J. Mechanism of Corrosion of Activated Aluminum Particles by Hot Water. Electrochim. Acta 2014, 127, 95–105. [Google Scholar] [CrossRef]
- Shaytura, N.S.; Laritchev, N.M.; Laritcheva, O. Study of the texture of hydroxides formed by aluminum oxidation with liquid water at various activation techniques Current. Appl. Phys. J. 2010, 10, S66–S68. [Google Scholar]
- Teng, H.-T.; Lee, T.-Y.; Chen, Y.-K.; Wang, H.-W.; Cao, G. Effect of Al (OH)3 on the hydrogen generation of aluminum–water system. J. Power Sources 2012, 219, 16–21. [Google Scholar] [CrossRef]
- Hu, H.; Qiao, M.; Pei, Y.; Fan, K.; Li, H. Kinetics of hydrogen evolution in alkali leaching of rapidly quenched Ni-Al alloys. Appl. Catal. A Gen. 2003, 252, 173–183. [Google Scholar] [CrossRef]
- Mahmoodi, K.; Alineiad, B. Enhancement of hydrogen generation rate in reaction of aluminum with water. Int. J. Hydrog. Energy 2010, 35, 5227–5232. [Google Scholar] [CrossRef]
- Miadoková, M.; Molnárová-Plchová, M. kinetics and mechanism of the reaction of aluminum in aqueous solution of sodium hydroxide. Chem. Pap. 1985, 39, 229–235. [Google Scholar]
- Aleksandrov, Y.A.; Tsyganova, E.I.; Pisarev, A.L. Reaction of Aluminum with Dilute Aqueous NaOH Solutions. Russ. J. Gen. Chem. 2003, 73, 689–694. [Google Scholar] [CrossRef]
- Gomma, G.K.; Wahdan, M.H. Schiff bases as corrosion inhibitors for aluminum in hydrochloric acid solution. Mater. Chem. Phys. 1995, 39, 209–213. [Google Scholar] [CrossRef]
- Hiraki, T.; Takeuchi, M.; Hisa, M.; Akiyama, T. Hydrogen Production from Waste Aluminum at Different Temperatures, with LCA. Mater. Trans. 2005, 46, 1052–1057. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.K.; Tadd, E.H.; Zubris, M.; Tannenbaum, R. Size-controlled synthesis of alumina nanoparticles from aluminum alkoxides. Mater. Res. Bull. 2005, 40, 1506–1512. [Google Scholar] [CrossRef]
- Berna, X.S.; Marín-Genescà, M.; Dagà-Monmany, J. Analysis of Valorization Process of Aluminum Breakage Scraps to Obtain Green Hydrogen. Metals 2021, 11, 598. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, L.; Zhang, L.; Zhang, X.; Wang, X.; Ge, M.; Li, X.; Zou, M. Preparation of Al-3Ga-3In-3Sn Alloy Powder by Coupling Alloying and Ball Milling and Its Application on High-Rate Hydrogen Generation at Room Temperature. Metals 2021, 11, 1704. [Google Scholar] [CrossRef]


| Temperature (°C) | Theoretical Reduction of Average Thickness (mm/min) | Experimental Reduction of Average Thickness (mm/min) |
|---|---|---|
| 25 | 0.00125 | 0.00117 ± 0.00003 |
| 40 | 0.00370 | 0.00365 ± 0.00003 |
| 60 | 0.01410 | 0.01473 ± 0.0003 |
| Temperature (°C) | Theoretical Thickness Reduction (mm/min) | Experimental Thickness Reduction (mm/min) |
|---|---|---|
| 50 | 0.00515 | 0.00516 ± 0.00003 |
| 60 | 0.00986 | 0.00987 ± 0.00003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salueña-Berna, X.; Marín-Genescà, M.; Massagués Vidal, L.; Dagà-Monmany, J.M. Waste Aluminum Application as Energy Valorization for Hydrogen Fuel Cells for Mobile Low Power Machines Applications. Materials 2021, 14, 7323. https://doi.org/10.3390/ma14237323
Salueña-Berna X, Marín-Genescà M, Massagués Vidal L, Dagà-Monmany JM. Waste Aluminum Application as Energy Valorization for Hydrogen Fuel Cells for Mobile Low Power Machines Applications. Materials. 2021; 14(23):7323. https://doi.org/10.3390/ma14237323
Chicago/Turabian StyleSalueña-Berna, Xavier, Marc Marín-Genescà, Lluís Massagués Vidal, and José M. Dagà-Monmany. 2021. "Waste Aluminum Application as Energy Valorization for Hydrogen Fuel Cells for Mobile Low Power Machines Applications" Materials 14, no. 23: 7323. https://doi.org/10.3390/ma14237323
APA StyleSalueña-Berna, X., Marín-Genescà, M., Massagués Vidal, L., & Dagà-Monmany, J. M. (2021). Waste Aluminum Application as Energy Valorization for Hydrogen Fuel Cells for Mobile Low Power Machines Applications. Materials, 14(23), 7323. https://doi.org/10.3390/ma14237323







