Synthesis and Characterization of TiO2 Nanotubes (TiO2-NTs) Decorated with Platine Nanoparticles (Pt-NPs): Photocatalytic Performance for Simultaneous Removal of Microorganisms and Volatile Organic Compounds
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of TiO2-NTs and Pt/TiO2-NTs Photoelectrodes
2.1.1. Elaboration of Nanotubes by Anodic Oxidation of Ti
2.1.2. Decoration of TiO2 NTs with Platinum Nanoparticles
2.2. Batch Reactor
3. Results
3.1. Characterizations of Pt-NPs/TiO2-NTs
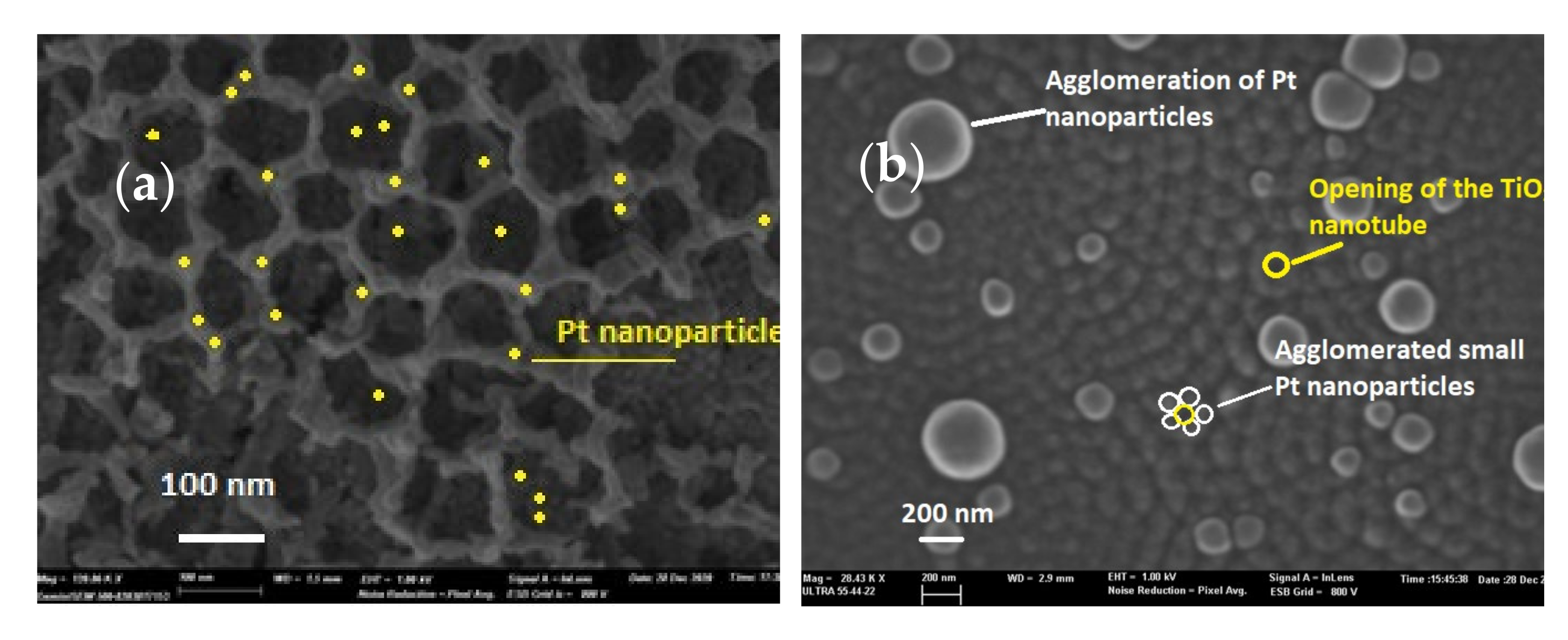
3.1.1. Scanning Electron Microscopy
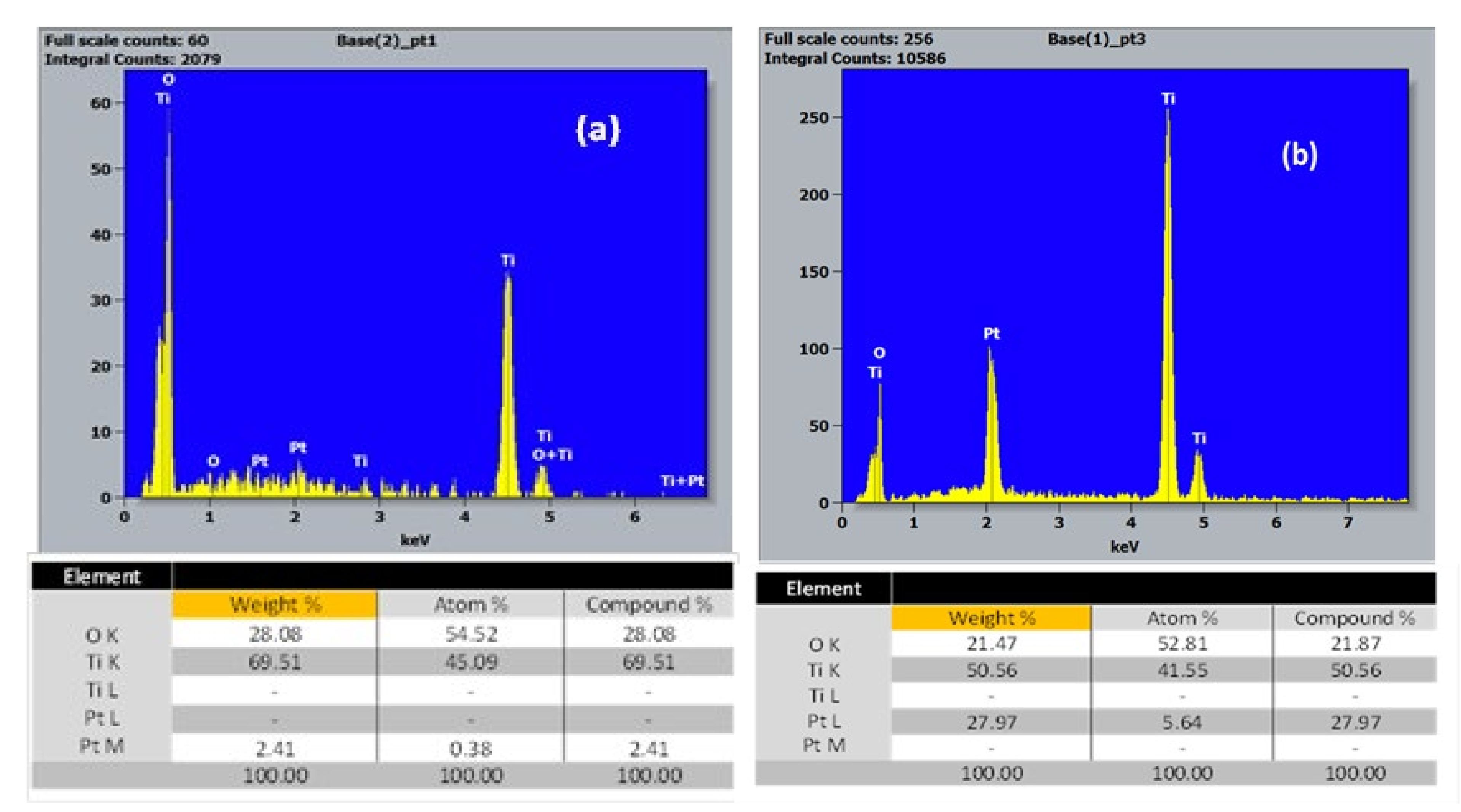
3.1.2. EDX Study of the Chemical Composition of TiO2-NTs Decorated with Pt-NPs
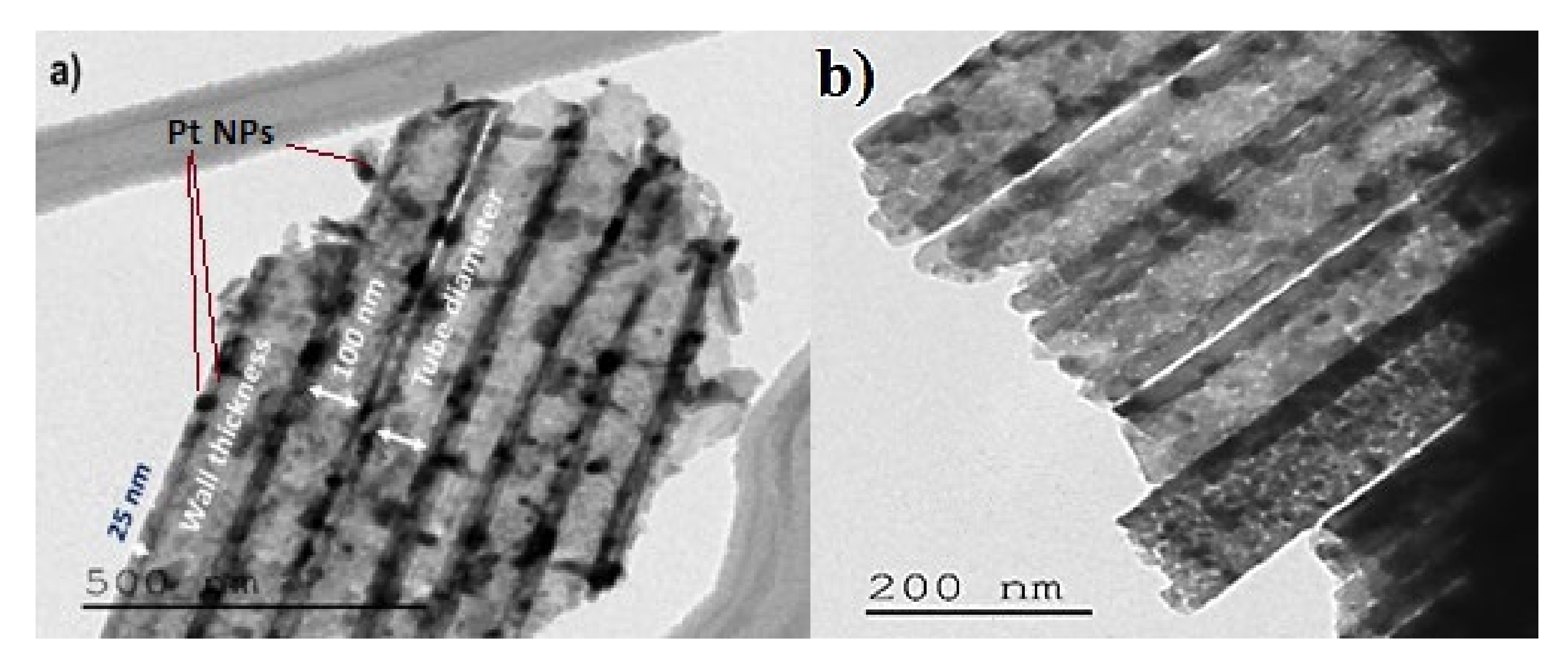
3.1.3. Transmission Electron Microscopy Characterization
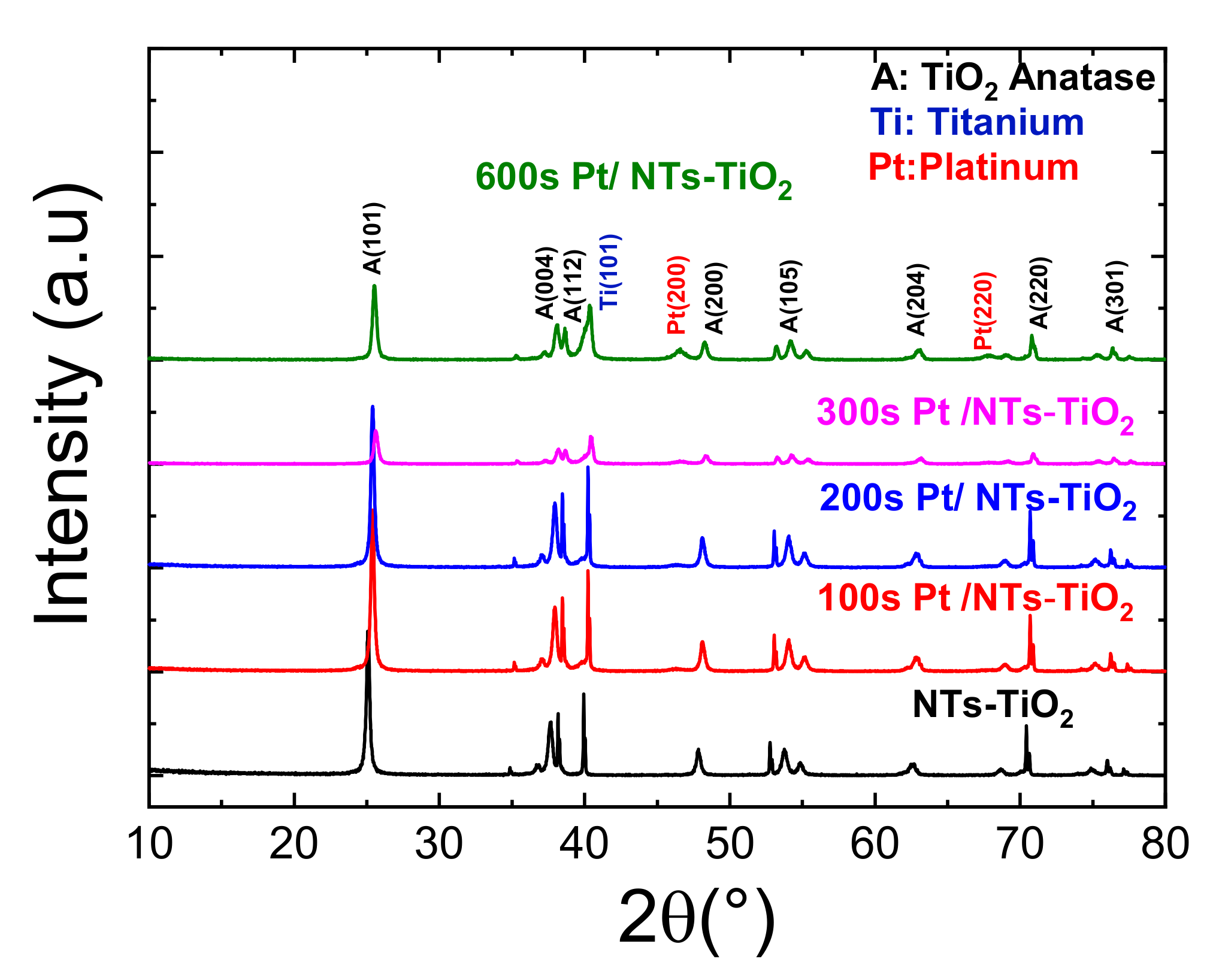
3.1.4. X-ray Diffraction Characterization
3.1.5. Reflectivity Properties Investigations
3.1.6. Photoluminescence Properties Investigations
3.2. Catalyst Application for Pollution Treatment
3.2.1. Effect of Decoration Time
3.2.2. Effect of Inlet Concentration
3.2.3. Simultaneous Oxidation of Cyclohexane and Bacteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abidi, M.; Hajjaji, A.; Bouzaza, A.; Lamaa, L.; Peruchon, L.; Brochier, C.; Rtimi, S.; Wolbert, D.; Bessais, B.; Assadi, A.A. Modeling of indoor air treatment using an innovative photocatalytic luminous textile: Reactor compactness and mass transfer enhancement. Chem. Eng. J. 2022, 430, 132636. [Google Scholar]
- Costarramone, N.; Cantau, C.; Desauziers, V. Photocatalytic air purifiers for indoor air: European standard and pilot room experiments. Environ. Sci. Pollut. Res. 2017, 24, 12538–12546. [Google Scholar] [CrossRef]
- European Commission. Programme Pilote de Vérification des Technologies Environnementales de l ’UE Protocole Général de Verification; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- World Health Organization (WHO). Environmental and Social Determinants of Health (PHE), Geneva. 2014. Available online: https://www.who.int/data/gho/data/themes/world-health-statistics (accessed on 17 November 2021).
- Laurence, G. Traitement de L’air Interieur par Photocatalyse—Évaluation de L’innocuité des Systèmes de Traitement de L’air; Rapport de Recherche -ADEME- 2017; Agence de la Transition Écologique: Montrouge, France, 2017. [Google Scholar]
- Saoud, W.A.; Assadi, A.A.; Kane, A.; Jung, A.V.; Le Cann, P.; Gerard, A.; Wolbert, D. Integrated process for the removal of indoor VOCs from food industry manufacturing: Elimination of Butane-2, 3-dione and Heptan-2-one by cold plasma-photocatalysis combination. J. Photochem. Photobiol. A Chem. 2020, 386, 112071. [Google Scholar] [CrossRef]
- Hajjaji, A.; Elabidi, M.; Trabelsi, K.; Assadi, A.A.; Bessais, B.; Rtimi, S. Bacterial adhesion and inactivation on Ag decorated TiO2-nanotubes under visible light: Effect of the nanotube’s geometry on the photocatalytic activity. Colloids Surf. B Biointerfaces 2018, 170, 92–98. [Google Scholar] [CrossRef]
- Elfalleh, W.; Assadi, A.A.; Bouzaza, A.; Wolbert, D.; Kiwi, J.; Rtimi, S. Innovative and stable TiO2 supported catalytic surfaces removing aldehydes under UV-light irradiation. J. Photochem. Photobiol. A Chem. 2017, 343, 96–102. [Google Scholar] [CrossRef]
- Anitha, V.C.; Goswami, A.; Sopha, H.; Nandan, D.; Gawande, M.B.; Cepe, K.; Macak, J.M. Pt nanoparticles decorated TiO2 nanotubes for the reduction of olefins. Appl. Mater. Today 2018, 10, 86–92. [Google Scholar]
- Pradhan, S.K.; Reucroft, P.J.; Yang, F.; Dozier, A. Growth of TiO2 nanorods by metalorganic chemical vapor deposition. J. Cryst. Growth 2003, 256, 83–88. [Google Scholar] [CrossRef]
- Pérez-Jiménez, L.E.; Solis-Cortazar, J.C.; Rojas-Blanco, L.; Perez-Hernandez, G.; Martinez, O.S.; Palomera, R.C.; Morales, E.R. Enhancement of optoelectronic properties of TiO2 films containing Pt nanoparticles. Results Phys. 2019, 12, 1680–1685. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, E.D.; Zhu, L.; Wen, Y.; Tan, Q.W.; Feng, W. Influence of annealing temperature of TiO2 nanotubes via hydrothermal method on Ti foil for photocatalytic degradation. Digest J. Nanomater. Biostruct. 2019, 14, 463–470. [Google Scholar]
- Naduvath, J.; Bhargava, P.; Mallick, S. Multipodal formation of TiO2 nanotubes using anodization. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; p. 2082. [Google Scholar]
- Liang, Y.; Guan, Z.C.; Wang, H.P.; Du, R.G. Enhanced photoelectrochemical anticorrosion performance of WO3/TiO2 nanotube composite films formed by anodization and electrodeposition. Electrochem. Commun. 2017, 77, 120–123. [Google Scholar] [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Schmuki, P. High-aspect-ratio TiO2 nanotubes by anodization of titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef]
- Lerch, S.; Reinhard, B.M. Effect of interstitial palladium on plasmon-driven charge transfer in nanoparticle dimers. Nat. Commun. 2018, 9, 1608. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, L.; Zang, D.; Wu, H. A Competitive Reaction Strategy toward Binary Metal Sulfides for Tailoring Electromagnetic Wave Absorption. Adv. Funct. Mater. 2021, 31, 2105018. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Wu, H.; Zang, D. Boosted electromagnetic wave absorption performance from vacancies, defects and interfaces engineering in Co (OH) F/Zn0. 76Co0. 24S/Co3S4 composite. Chem. Eng. J. 2021, 411, 128601. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Zhang, L.; Zang, D.; Liu, H.; Liotta, L.F.; Wu, H. Tunable sulfur vacancies and hetero-interfaces of FeS2-based composites for high-efficiency electromagnetic wave absorption. J. Colloid Interface Sci. 2021, 591, 148–160. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Wu, H. Electromagnetic wave absorbing performance of carbons, carbides, oxides, ferrites and sulfides: Review and perspective. J. Phys. D Appl. Phys. 2021, 54, 203001. [Google Scholar] [CrossRef]
- Arkusz, K.; Paradowska, E.; Nycz, M.; Krasicka-Cydzik, E. Influence of Thermal Modification and Morphology of TiO2 nanotubes on their electrochemical properties for biosensors applications. J. Nanosci. Nanotechnol. 2018, 18, 3713–3721. [Google Scholar] [CrossRef]
- Kim, W.T.; Choi, W.Y. Interferometric Biosensor Using TiO2 Nanotube Arrays. Appl. Mech. Mater. Trans. Tech. Publ. 2017, 864, 212–217. [Google Scholar] [CrossRef]
- Schmuki, P.; Liu, N.; Altomare, M. TiO2 Nanotube Arrays: Photoelectrochemical and Photocatalytic Applications. In ECS Meeting Abstracts; IOP Publishing: Bristol, UK, 2019; Volume MA2019-01, p. 2061. [Google Scholar]
- Zghab, E.; Hamandi, M.; Dappozze, F.; Kochkar, H.; Zina, M.S.; Guillard, C.; Berhault, G. Influence of graphene and copper on the photocatalytic response of TiO2 nanotubes. Mater. Sci. Semicond. Process. 2020, 107, 104847. [Google Scholar] [CrossRef]
- Çırak, B.B.; Caglar, B.; Kılınç, T.; Karadeniz, S.M.; Erdoğan, Y.; Kılıç, S.; Çırak, Ç. Synthesis and characterization of ZnO nanorice decorated TiO2 nanotubes for enhanced photocatalytic activity. Mater. Res. Bullet. 2019, 109, 160–167. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Zhou, D.; Zhao, W.; Qian, X.; Yang, Q.; Shen, C. Ultra-low Au–Pt Co-decorated TiO2 nanotube arrays: Construction and its improved visible-light-induced photocatalytic properties. Solar Energy Mater. Solar Cells 2019, 201, 110065. [Google Scholar] [CrossRef]
- Gogoi, D.; Namdeo, A.; Golder, A.K.; Peela, N.R. Ag-doped TiO2 photocatalysts with effective charge transfer for highly efficient hydrogen production through water splitting. Int. J. Hydrog. Energy 2020, 45, 2729–2744. [Google Scholar] [CrossRef]
- Photharin, S.; Tipparach, U. Preparation and microstructure of Ag-doped TiO2 nanotubes by anodization method. Int. J. Appl. Eng. Res. 2017, 12, 1350–1354. [Google Scholar]
- Gaidi, M.; Trabelsi, K.; Hajjaji, A.; Chourou, M.L.; Alhazaa, A.N.; Bessais, B.; El Khakani, M.A. Optimizing the photochemical conversion of UV–vis light of silver-nanoparticles decorated TiO2 nanotubes based photoanodes. Nanotechnology 2017, 29, 015703. [Google Scholar] [CrossRef]
- Trabelsi, K.; Hajjaji, A.; Gaidi, M.; Bessais, B.; El Khakani, M.A. Enhancing the photoelectrochemical response of TiO2 nanotubes through their nanodecoration by pulsed-laser-deposited Ag nanoparticles. J. Appl. Phys. 2017, 122, 064503. [Google Scholar] [CrossRef]
- Hajjaji, A.; Jemai, S.; Rebhi, A.; Trabelsi, K.; Gaidi, M.; Alhazaa, A.N.; Bessais, B. Enhancement of photocatalytic and photoelectrochemical properties of TiO2 nanotubes sensitized by SILAR-Deposited PbS nanoparticles. J. Mater. 2020, 6, 62–69. [Google Scholar] [CrossRef]
- Hajjaji, A.; Jemai, S.; Trabelsi, K.; Kouki, A.; Assaker, I.B.; Ka, I.; El Khakani, M.A. Study of TiO2 nanotubes decorated with PbS nanoparticles elaborated by pulsed laser deposition: Microstructural, optoelectronic and photoelectrochemical properties. J. Mater. Sci. Mater. Electron. 2019, 30, 20935–20946. [Google Scholar] [CrossRef]
- Abidi, M.; Assadi, A.A.; Bouzaza, A.; Hajjaji, A.; Bessais, B.; Rtimi, S. Photocatalytic indoor/outdoor air treatment and bacterial inactivation on CuxO/TiO2 prepared by HiPIMS on polyester cloth under low intensity visible light. Appl. Catal. B Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Khaliq, N.; Rasheed, M.A.; Cha, G.; Khan, M.; Karim, S.; Schmuki, P.; Ali, G. Development of non-enzymatic cholesterol bio-sensor based on TiO2 nanotubes decorated with Cu2O nanoparticles. Sens. Actuators B Chem. 2020, 302, 127200. [Google Scholar] [CrossRef]
- Liu, Y.; Su, D.; Zhang, Y.; Wang, L.; Yang, G.; Shen, F.; Zhang, X.; Zhang, S. Anodized TiO2 nanotubes coated with Pt nanoparticles for enhanced photoelectrocatalytic activity. J. Mater. Res. 2017, 32, 757–765. [Google Scholar] [CrossRef]
- Xiang, C.; She, Z.; Zou, Y.; Cheng, J.; Chu, H.; Qiu, S.; Xu, F. A room-temperature hydrogen sensor based on Pd nanoparticles doped TiO2 nanotubes. Ceram. Int. 2014, 40, 16343–16348. [Google Scholar] [CrossRef]
- Pearson, A.; Jani, H.; Kalantar-Zadeh, K.; Bhargava, S.K.; Bansal, V. Gold nanoparticle-decorated keggin ions/TiO2 photococatalyst for improved solar light photocatalysis. Langmuir 2011, 27, 6661–6667. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, L.; Xie, K.; Lai, Y.; Liu, B.; Ren, B.; Lin, C. SERS study of Ag nanoparticles electrodeposited on patterned TiO2 nanotube films. J. Raman Spectrosc. 2011, 42, 986–991. [Google Scholar] [CrossRef]
- Li, W.; Sun, Z.; Dongliang, T.; Nevirkovets, I.P.; Dou, S.-X. Platinum dendritic nanoparticles with magnetic behavior. J. Appl. Phys. 2014, 116, 033911. [Google Scholar] [CrossRef] [Green Version]
- Yuwono, A.H.; Sofyan, N.; Kartini, I.; Ferdiansyah, A.; Pujianto, T.H. Nanocrystallinity Enhancement of TiO2 Nanotubes by Post-Hydrothermal Treatment. Adv. Mater. Res. 2011, 277, 90–99. [Google Scholar] [CrossRef]
- Essalhi, Z.; Hartiti, B.; Lfakir, A.; Siadat, M.; Thevenin, P. Optical properties of TiO2 Thin films prepared by Sol Gel method. J. Mater. Environ. Sci. 2016, 7, 1328–1333. [Google Scholar]
- Wang, X.; Blackford, M.; Prince, K.; Caruso, R.A. Preparation of Boron-Doped Porous Titania Networks Containing Gold Nanoparticles with Enhanced Visible-Light Photocatalytic Activity. ACS Appl. Mater. Interfaces 2012, 4, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Chen, L.; Yuan, L.; Cai, Q. Visible light-induced efficiently oxidative decomposition of p-Nitrophenol by CdTe/TiO2 nanotube arrays. Chem. Eng. J. 2013, 215, 591–599. [Google Scholar] [CrossRef]
- Palau, J.; Assadi, A.A.; Penya-Roja, J.M.; Bouzaza, A.; Wolbert, D.; Martínez-Soria, V. Isovaleraldehyde degradation using UV photocatalytic and dielectric barrier discharge reactors, and their combinations. J. Photochem. Photobiol. A Chem. 2015, 299, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Maxime, G.; Amine, A.A.; Abdelkrim, B.; Dominique, W. Removal of gas-phase ammonia and hydrogen sulfide using photocatalysis, nonthermal plasma, and combined plasma and photocatalysis at pilot scale. Environ. Sci. Pollut. Res. 2014, 21, 13127–13137. [Google Scholar] [CrossRef]
- Saoud, W.A.; Kane, A.; le Cann, P.; Gerard, A.; Lamaa, L.; Peruchon, L.; Brochier, C.; Bouzaza, A.; Wolbert, D.; Assadi, A.A. Innovative photocatalytic reactor for the degradation of VOCs and microorganism under simulated indoor air conditions: Cu-Ag/TiO2-based optical fibers at a pilot scale. Chem. Eng. J. 2021, 411, 128622. [Google Scholar] [CrossRef]
- Assadi, A.A.; Bouzaza, A.; Soutrel, I.; Petit, P.; Medimagh, K.; Wolbert, D. A study of pollution removal in exhaust gases from animal quartering centers by combining photocatalysis with surface discharge plasma: From pilot to industrial scale. Chem. Eng. Process. Process Intensif. 2017, 111, 1–6. [Google Scholar] [CrossRef]

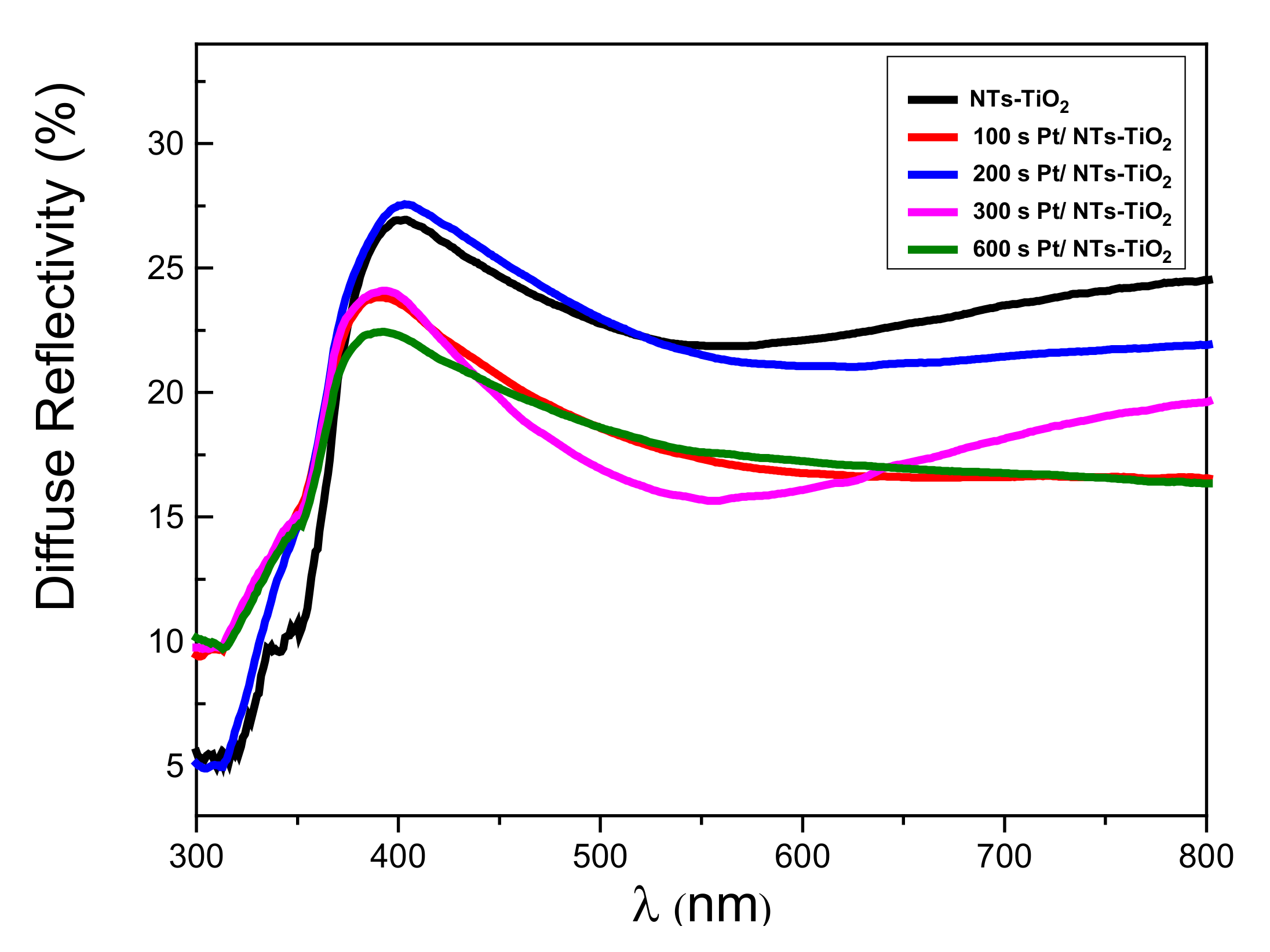
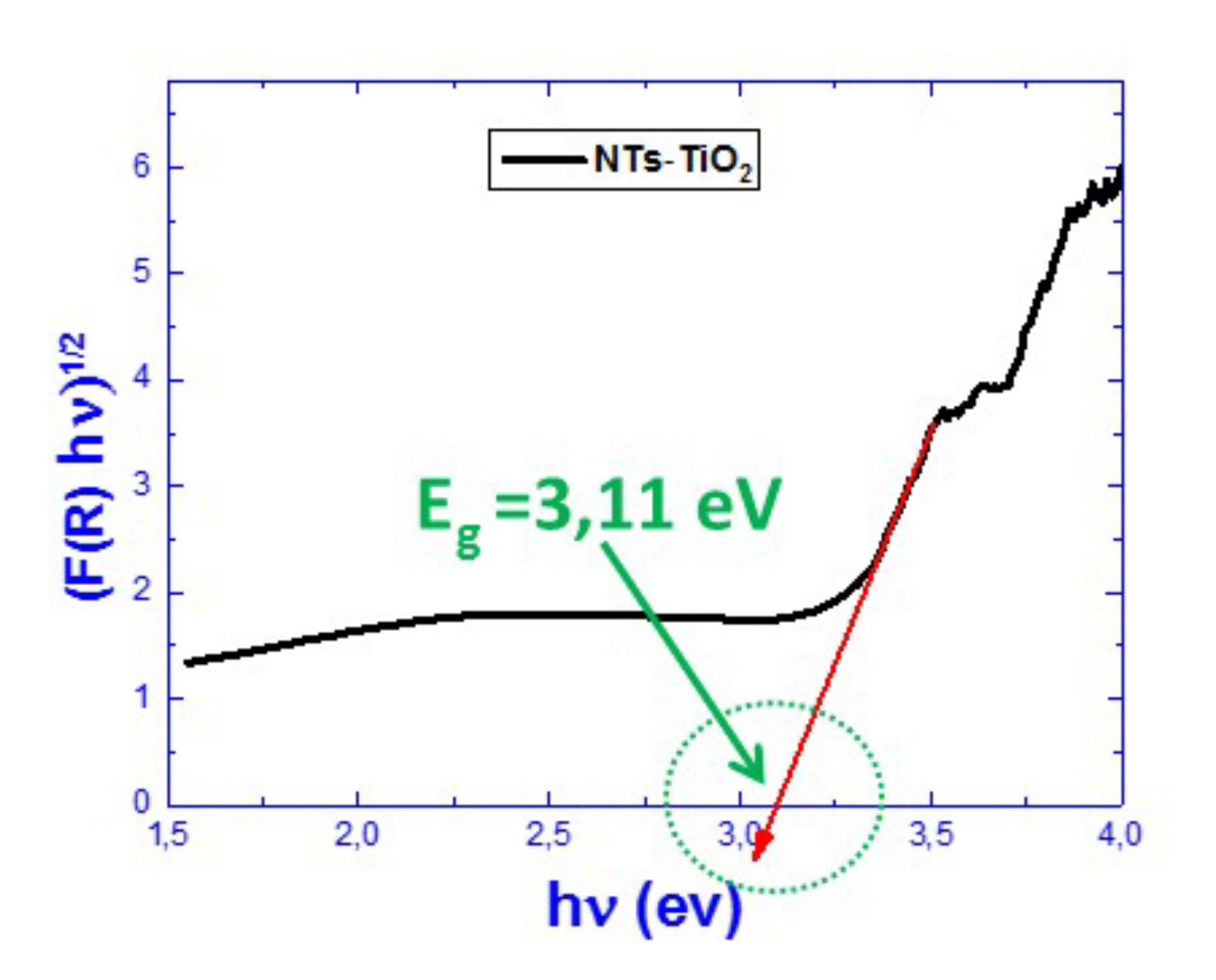

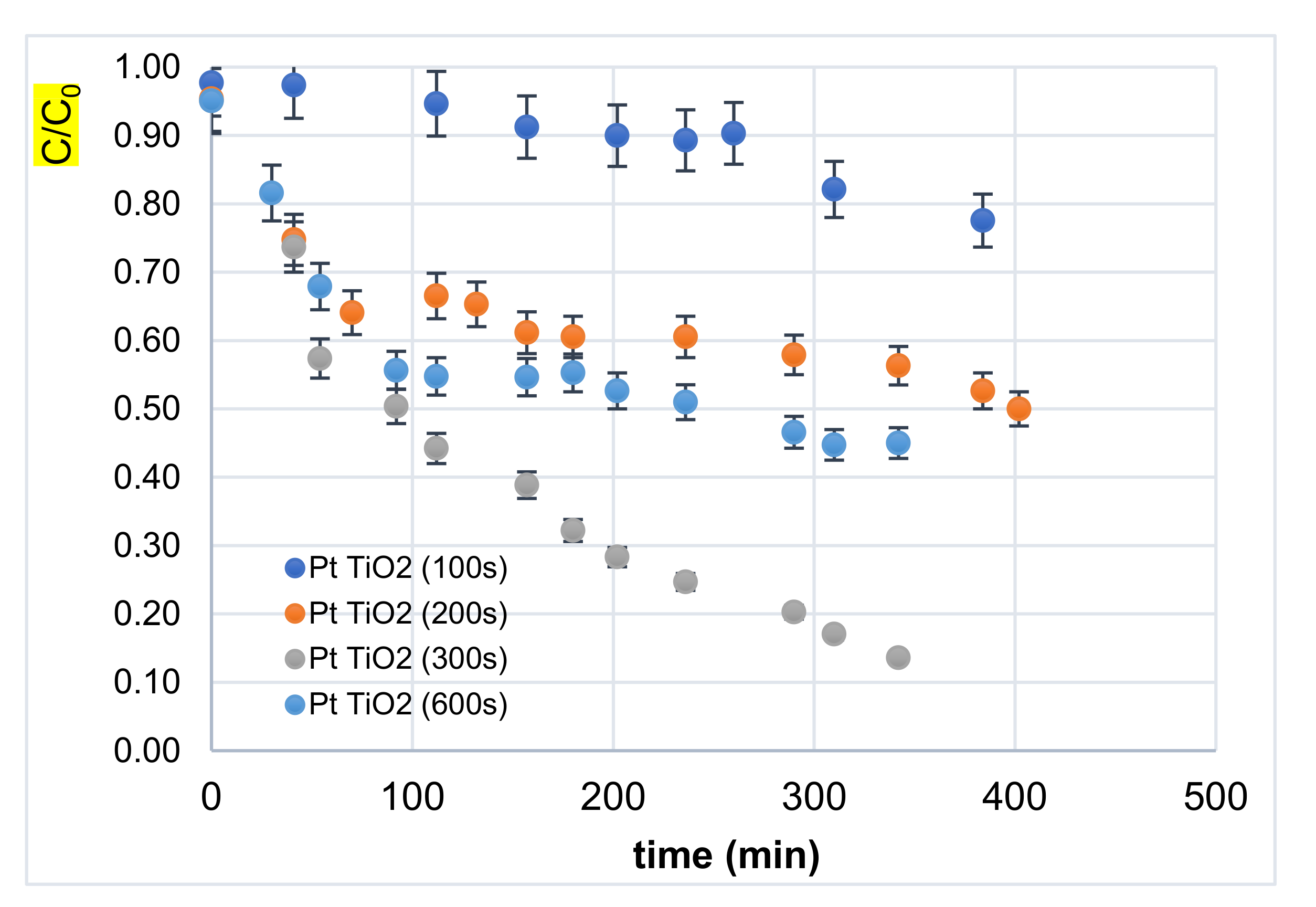

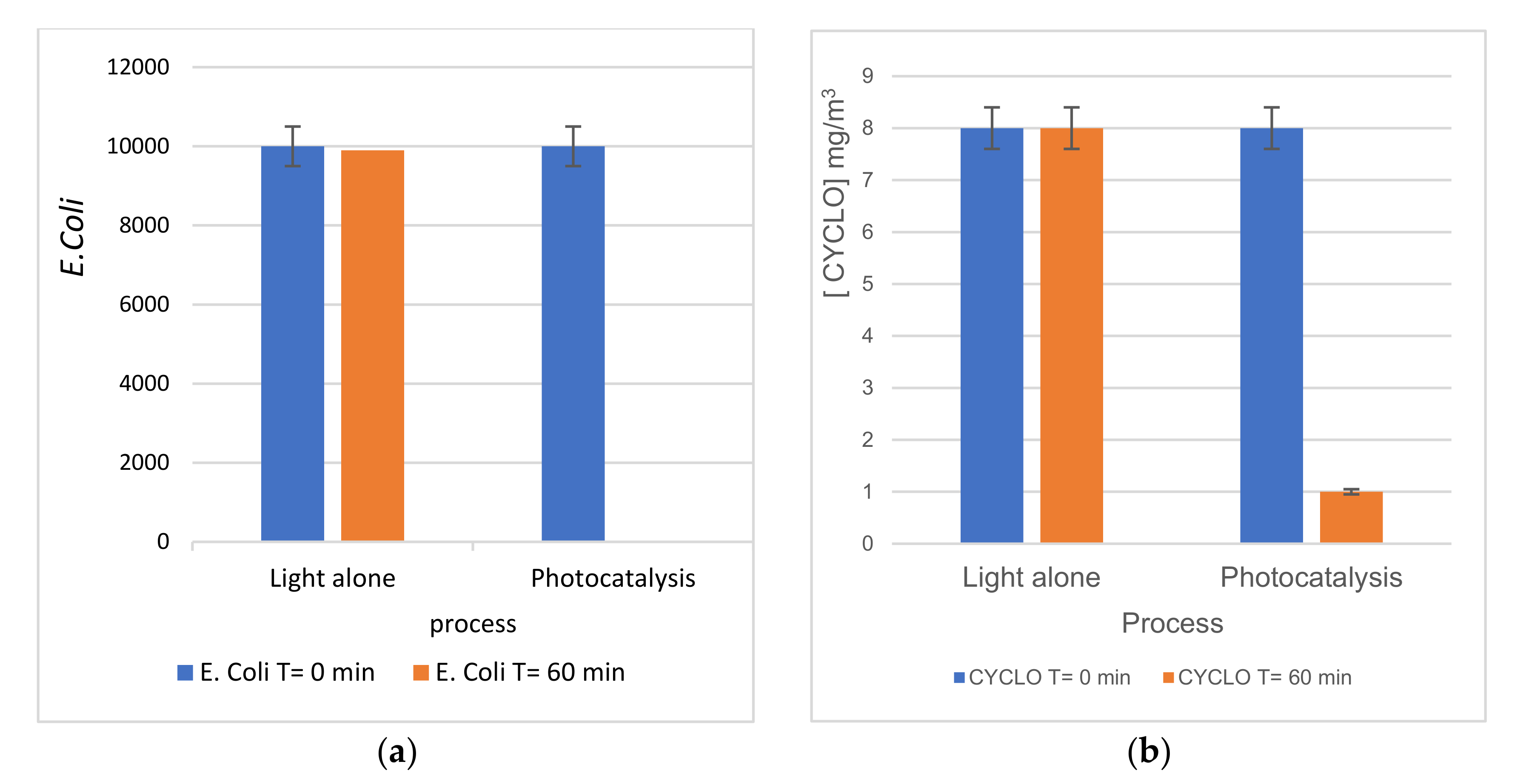
| Sample | TiO2-NTs Pure | 100 s Pt/TiO2 NTs | 200 s Pt/TiO2 NTs | 300 s Pt/TiO2 NTs | 600 s Pt/TiO2 NTs |
|---|---|---|---|---|---|
| Band Gap (eV) | 3.11 | 2.85 | 2.91 | 2.91 | 2.85 |
| kc: Kinetic Constant of L–H (mg.m−3. min−1) | K: Adsorption Constant of L–H (m3. mg−1) |
|---|---|
| 0.064 | 5.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khezami, L.; Lounissi, I.; Hajjaji, A.; Guesmi, A.; Assadi, A.A.; Bessais, B. Synthesis and Characterization of TiO2 Nanotubes (TiO2-NTs) Decorated with Platine Nanoparticles (Pt-NPs): Photocatalytic Performance for Simultaneous Removal of Microorganisms and Volatile Organic Compounds. Materials 2021, 14, 7341. https://doi.org/10.3390/ma14237341
Khezami L, Lounissi I, Hajjaji A, Guesmi A, Assadi AA, Bessais B. Synthesis and Characterization of TiO2 Nanotubes (TiO2-NTs) Decorated with Platine Nanoparticles (Pt-NPs): Photocatalytic Performance for Simultaneous Removal of Microorganisms and Volatile Organic Compounds. Materials. 2021; 14(23):7341. https://doi.org/10.3390/ma14237341
Chicago/Turabian StyleKhezami, Lotfi, Imen Lounissi, Anouar Hajjaji, Ahlem Guesmi, Aymen Amine Assadi, and Brahim Bessais. 2021. "Synthesis and Characterization of TiO2 Nanotubes (TiO2-NTs) Decorated with Platine Nanoparticles (Pt-NPs): Photocatalytic Performance for Simultaneous Removal of Microorganisms and Volatile Organic Compounds" Materials 14, no. 23: 7341. https://doi.org/10.3390/ma14237341
APA StyleKhezami, L., Lounissi, I., Hajjaji, A., Guesmi, A., Assadi, A. A., & Bessais, B. (2021). Synthesis and Characterization of TiO2 Nanotubes (TiO2-NTs) Decorated with Platine Nanoparticles (Pt-NPs): Photocatalytic Performance for Simultaneous Removal of Microorganisms and Volatile Organic Compounds. Materials, 14(23), 7341. https://doi.org/10.3390/ma14237341









