The Role of Different Alkali Metals in the A15Tl27 Type Structure and the Synthesis and X-ray Structure Analysis of a New Substitutional Variant Cs14.53Tl28.4
Abstract
:1. Introduction
2. Materials and Methods
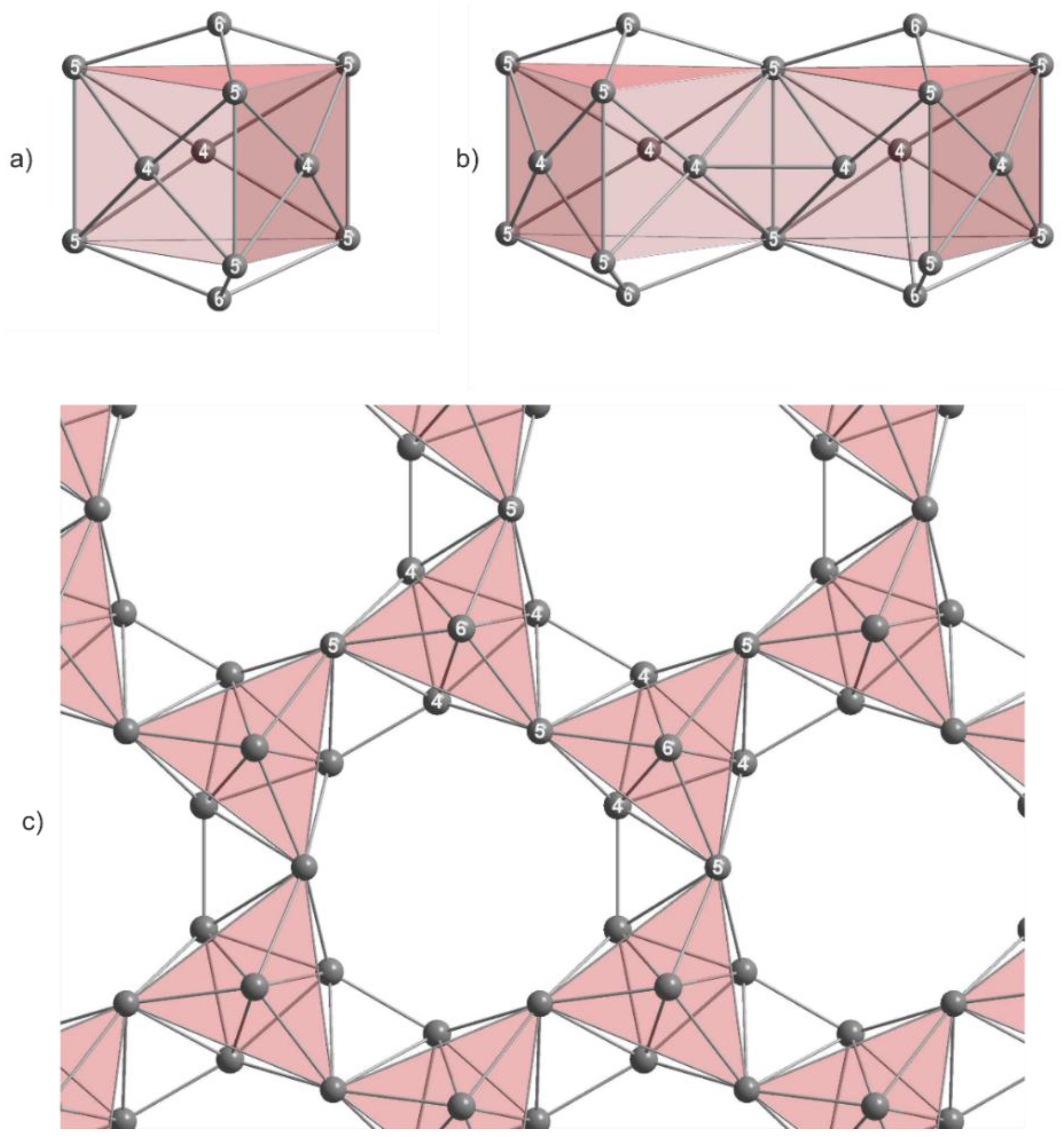
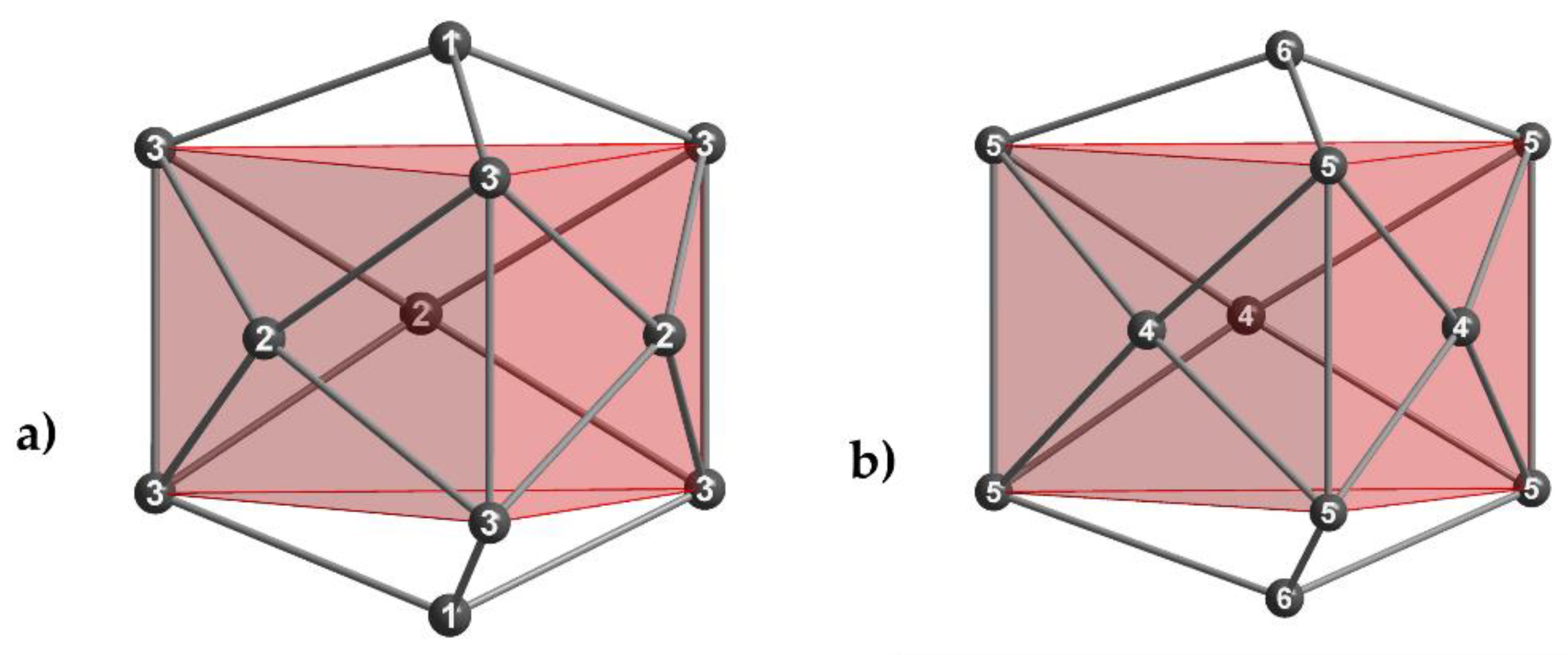
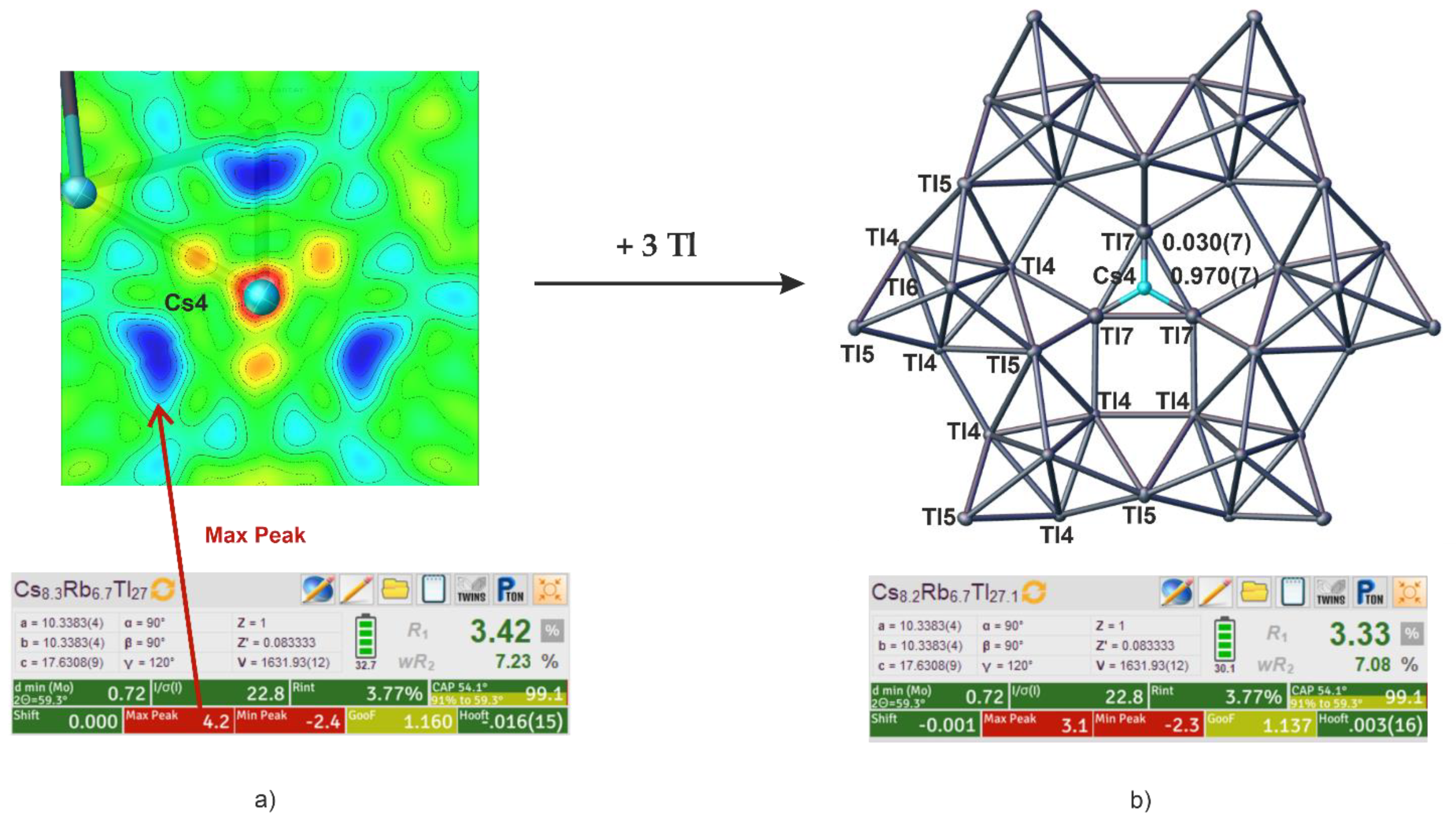
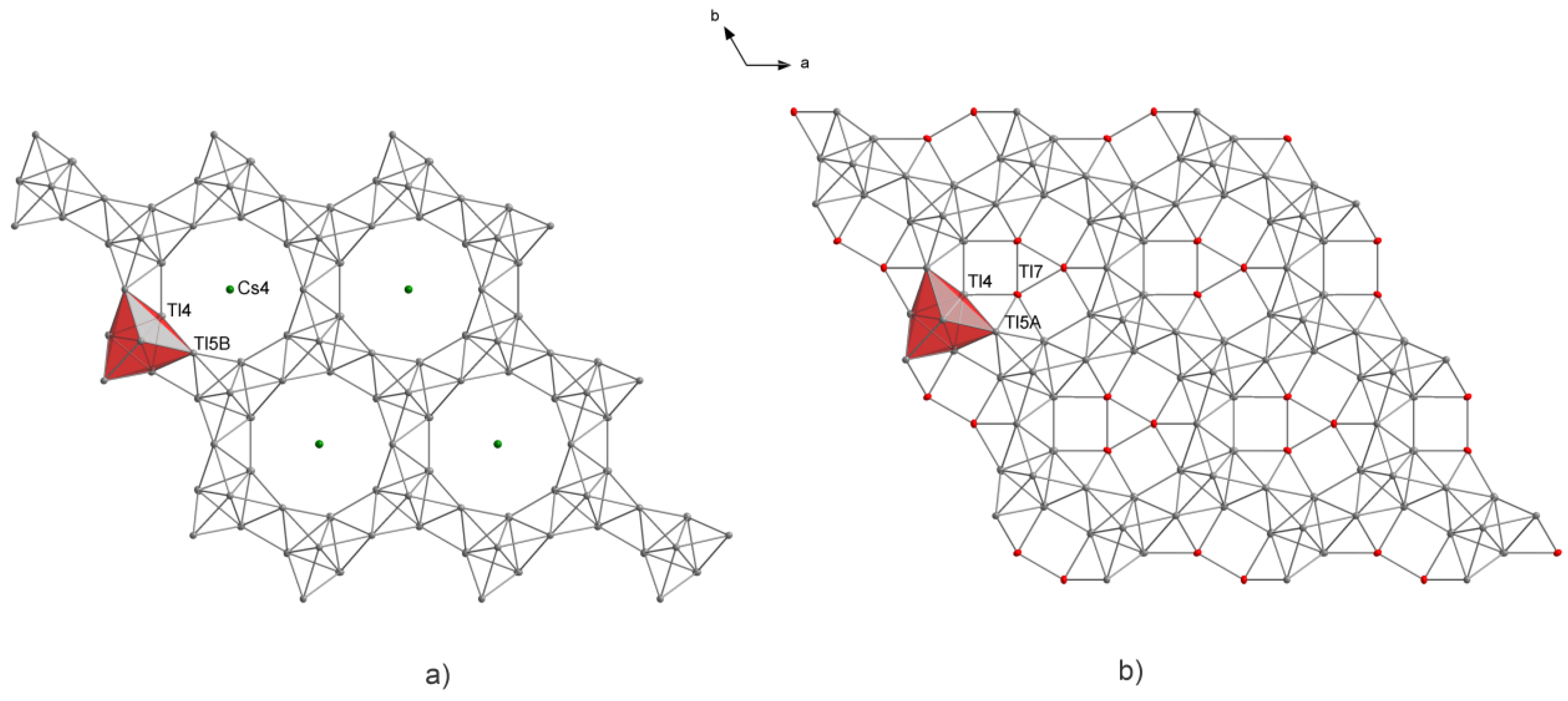
3. Results
4. Discussion
4.1. Occupation Trends of the Alkali Metal Positions
4.2. Influence of Mixed Alkali Metal Sites on the Thallium Substructures
4.3. Effects of Incorporation of Tl in the Two-Dimensional Layers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gärtner, S. Spotlight on Alkali Metals: The Structural Chemistry of Alkali Metal Thallides. Crystals 2020, 10, 1013. [Google Scholar] [CrossRef]
- Nesper, R. The Zintl-Klemm Concept-A Historical Survey. Z. Anorg. Allg. Chem. 2014, 640, 2639–2648. [Google Scholar] [CrossRef]
- Kauzlarich, S.M. (Ed.) Chemistry, Structure and Bonding of Zintl Phases and Ions; VCH Publishers, Inc.: New York, NY, USA; Weinheim, Germany, 1996. [Google Scholar]
- Fässler, T.F. (Ed.) Zintl Phases-Principles and Recent Developments; Springer: Berlin/Heidelberg, Germany, 2011; Volume 139. [Google Scholar]
- Wang, F.; Wedig, U.; Prasad, D.; Jansen, M. Deciphering the Chemical Bonding in Anionic Thallium Clusters. J. Am. Chem. Soc. 2012, 134, 19884–19894. [Google Scholar] [CrossRef] [PubMed]
- Kaskel, S.; Corbett, J.D. Synthesis and structure of K10Tl7: The first binary trielide containing naked pentagonal bipyramidal Tl7 clusters. Inorg. Chem. 2000, 39, 778–782. [Google Scholar] [CrossRef]
- Dong, Z.-C.; Corbett, J.D. A8Tl11 (A = K, Rb, or Cs) Phases with Hypoelectronic Tl117- Cluster Anions: Syntheses, Structure, Bonding and Properties. J. Clust. Sci. 1995, 6, 187–201. [Google Scholar] [CrossRef]
- Dong, Z.C.; Corbett, J.D. CsTl: A new example of tetragonally compressed Tl66− octahedra. Electronic effects and packing requirements in the diverse structures of ATl (A = Li, Na, K, Cs). Inorg. Chem. 1996, 35, 2301–2306. [Google Scholar] [CrossRef]
- Dong, Z.C.; Corbett, J.D. Synthesis, Structure, and Bonding of the Novel Cluster Compound KTl with Isolated Tl66− Ions. J. Am. Chem. Soc. 1993, 115, 11299–11303. [Google Scholar] [CrossRef]
- Smid, S.; Steinberg, S. Probing the Validity of the Zintl-Klemm Concept for Alkaline-Metal Copper Tellurides by Means of Quantum-Chemical Techniques. Materials 2020, 13, 2178. [Google Scholar] [CrossRef] [PubMed]
- Zintl, E.; Dullenkopf, W. Über den Gitterbau von NaTl und seine Beziehung zu den Strukturen des Typus des b-Messings. Z. Phys. Chem. 1932, B16, 195–205. [Google Scholar] [CrossRef]
- Zintl, E.; Goubeau, J.; Dullenkopf, W. Salzartige Verbindungen und intermetallische Phasen des Natriums in flüssigem Ammoniak. Z. Phys. Chem. 1931, 154, 1–46. [Google Scholar] [CrossRef]
- Gärtner, S.; Korber, N.; Reedijk, J.; Poeppelmeier, K. Zintl Anions. In Comprehensive Inorganic Chemistry II; Elsevier: Amsterdam, The Netherlands, 2013; Volume 1, pp. 251–267. [Google Scholar]
- Dong, Z.C.; Corbett, J.D. A15Tl27 (A = Rb,Cs): A structural type containing both isolated clusters and condensed layers based on the Tl11 fragment. Syntheses, structure, properties, and band structure. Inorg. Chem. 1996, 35, 1444–1450. [Google Scholar] [CrossRef]
- Blase, W.; Cordier, G.; Muller, V.; Haussermann, U.; Nesper, R.; Somer, M. Preparation and Crystal-Structures of Rb8In11, K8Tl11, and Rb8Tl11 Band-Strucutre Calculations on K8In11. Z. Naturforsch. B 1993, 48, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Henning, R.W.; Corbett, J.D. Cs8Ga11, a new isolated cluster in a binary gallium compound. A family of valence analogues A8Tr11X: A = Cs, Rb; Tr = Ga, In, Tl; X = Cl, Br, I. Inorg. Chem. 1997, 36, 6045–6049. [Google Scholar] [CrossRef]
- Gärtner, S.; Tiefenthaler, S. Single Crystal X-ray Structure Analyses of Thallides: Halide Incorporation and Mixed Alkali Sites in A8Tl11X (A = K, Rb, Cs; X = Cl, Br). Proceedings 2018, 2, 1124. [Google Scholar] [CrossRef] [Green Version]
- Gärtner, S.; Tiefenthaler, S.; Korber, N.; Stempfhuber, S.; Hischa, B. Structural Chemistry of Halide including Thallides A8Tl11X1-n (A = K, Rb, Cs; X = Cl, Br; n = 0.1–0.9). Crystals 2018, 8, 319. [Google Scholar] [CrossRef]
- Falk, M.; El Addad, A.; Röhr, C. Crystal and electronic structure of alklai triele halogenides of the K8In11-type structure. In Proceedings of the 25th Annual Conference of the German Crystallographic Society, Karlsruhe, Germany, 27–30 March 2017; p. 112. [Google Scholar]
- Häussermann, U.; Svensson, C.; Lidin, S. Tetrahedral stars as flexible basis clusters in sp-bonded intermetallic frameworks and the compound BaLi7Al6 with the NaZn13 structure. J. Am. Chem. Soc. 1998, 120, 3867–3880. [Google Scholar] [CrossRef]
- Dong, Z.C.; Corbett, J.D. K18Tl20Au3-A Novel Derivative of K8Tl11 with the Unprecedented Polyanion Tl9Au29−, the Parent Tl117−, and an Isolated Au− Ion. Inorg. Chem. 1995, 34, 5042–5048. [Google Scholar] [CrossRef]
- Tillard-Charbonnel, M.; Chahine, A.; Belin, C.; Rousseau, R.; Canadell, E. Structure and chemical bonding in K14Cd9Tl21, a compound containing both isolated Tl117− clusters and ∞ 2 [Cd9Tl107−] metallic layers. Chem. Eur. J. 1997, 3, 799–806. [Google Scholar] [CrossRef]
- Cordier, G.; Müller, V. Preparation and crystal structure of K49Tl108. Z. Naturforsch. B 1993, 48, 1035–1040. [Google Scholar] [CrossRef]
- Baden, W.; Schmidt, P.C.; Weiss, A. The Intermetallic System LiCd1-xTlx. Phys. State Sol. A 1979, 51, 183–190. [Google Scholar] [CrossRef]
- Tiefenthaler, S.M.; Schlosser, M.; Pielnhofer, F.; Shenderovich, I.G.; Pfitzner, A.; Gärtner, S. Investigations on Tetragonally Distorted Sodium Thallide NaTl-tI8. Z. Anorg. Allg. Chem. 2020, 646, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Tiefenthaler, S.; Korber, N.; Gärtner, S. Synthesis of the Tetragonal Phase of Zintl’s NaTl and Its Structure Determination from Powder Diffraction Data. Materials 2019, 12, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evers, J.; Oehlinger, G. After more than 60 years, a new NaTl type Zintl phase: KTl at high pressure. Inorg. Chem. 2000, 39, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Evers, J. High Pressure Investigations of AIBIII Zintl Compounds (AI = Li to Cs; BIII = Al to Tl) up to 30 GPa. In Zintl Phases-Principles and Recent Developments, 1st ed.; Structure and Bonding; Fässler, T.F., Mingos, D.M.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 139, pp. 57–96. [Google Scholar]
- Cordier, G.; Müller, V.; Fröhlich, R. Crystal structure of potasium thallide (49/108), K49Tl108. Z. Kristallogr. 1993, 203, 148–149. [Google Scholar] [CrossRef]
- Hackspill, L. Sur quelques properiétés des métaux alcalins. Helv. Chim. Acta 1928, 11, 1003–1026. [Google Scholar] [CrossRef]
- Schneider, M.W.; Oppel, I.M.; Griffin, A.; Mastalerz, M. CrysAlisPRO, 171.41.93a; Oxford Diffraction/Agilent Technologies UK Ltd.: Yarnton, UK, 2020. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C-Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Brandenburg, K. Diamond, 4.6.6; Crystal Impact GbR: Bonn, Germany, 2021. [Google Scholar]
- Toombs, A. STOE WinXPOW, 3.4.6; STOE & Cie GmbH: Darmstadt, Germany, 2016. [Google Scholar]


| Empirical Formula | K6.96Rb8.04Tl27 | Cs5.92K9.08Tl27 | Cs2.27K12.73Tl27 | Cs3.57K4.55Rb6.92Tl27 |
|---|---|---|---|---|
| CSD number * | 2088508 | 2093385 | 2093386 | 2093391 |
| Formula weight | 6477.30 | 6659.84 | 6318.00 | 6761.84 |
| Temperature (K) | 123 | 123 | 123 | 123 |
| Crystal system | hexagonal | hexagonal | hexagonal | hexagonal |
| Space group | P-62m | P-62m | P-62m | P-62m |
| a (Å) | 10.1835(2) | 10.2542(4) | 10.20330(10) | 10.30543(11) |
| c (Å) | 17.1041(4) | 17.0278(12) | 16.7702(2) | 17.2475(2) |
| α (°) | 90 | 90 | 90 | 90 |
| γ (°) | 120 | 120 | 120 | 120 |
| Volume (Å3) | 1536.12(7) | 1550.57(17) | 1511.99(3) | 1586.31(4) |
| c/a | 1.68 | 1.66 | 1.64 | 1.67 |
| Z | 1 | 1 | 1 | 1 |
| ρcalc (g/cm3) | 7.002 | 7.132 | 6.938 | 7.078 |
| µ (mm−1) | 77.292 | 73.869 | 73.847 | 75.853 |
| F(000) | 2617.0 | 2685.0 | 2554.0 | 2726.0 |
| Crystal size (mm3) | 0.12 × 0.09 × 0.08 | 0.183 × 0.113 × 0.044 | 0.093 × 0.058 × 0.04 | 0.105 × 0.051 × 0.039 |
| Radiation | MoKα | MoKα | MoKα | MoKα |
| (λ = 0.71073) | (λ = 0.71073) | (λ = 0.71073) | (λ = 0.71073) | |
| 2Θ range for data collection (°) | 6.636 to 59.01 | 6.63 to 59.508 | 6.698 to 70.162 | 6.57 to 72.68 |
| Index ranges | −10 ≤ h ≤ 12 | −13 ≤ h ≤ 14 | −16 ≤ h ≤ 16 | −16 ≤ h ≤ 17 |
| −13 ≤ k ≤ 5 | −11 ≤ k ≤ 12 | −16 ≤ k ≤ 16 | −17 ≤ k ≤ 17 | |
| −11 ≤ l ≤ 23 | −23 ≤ l ≤ 23 | −27 ≤ l ≤ 26 | −28 ≤ l ≤ 28 | |
| Reflections collected | 3380 | 7018 | 97696 | 103808 |
| Independent reflections | 1431 | 1526 | 2479 | 2828 |
| Data/restraints/parameters | 1431/0/48 | 1526/0/47 | 2479/0/47 | 2828/3/52 |
| Goodness-of-fit on F2 | 1.079 | 1.111 | 1.359 | 1.235 |
| Rint | Rint = 0.0352 | Rint = 0.0660 | Rint = 0.0481 | Rint = 0.0611 |
| Final R indexes | R1 = 0.0419 | R1 = 0.0345 | R1 = 0.0173 | R1 = 0.0208 |
| [I >= 2σ (I)] | wR2 = 0.1003 | wR2 = 0.0631 | wR2 = 0.0504 | wR2 = 0.0592 |
| Final R indexes | R1 = 0.0442 | R1 = 0.0404 | R1 = 0.0185 | R1 = 0.0221 |
| [all data] | wR2 = 0.1031 | wR2 = 0.0655 | wR2 = 0.0506 | wR2 = 0.0596 |
| Largest diff. peak/hole (e Å−3) | 2.04/−1.94 | 2.59/−2.62 | 3.37/−2.07 | 2.78/−2.06 |
| Flack parameter | 0.03(7) | −0.006(16) | 0.005(4) | 0.003(5) |
| Empirical Formula | Cs8.21Rb6.76Tl27.09 | Cs14.53Tl28.4 | Cs15Tl27 |
|---|---|---|---|
| CSD number * | 2088490 | 2088509 | 2088513 |
| Formula weight | 7205.35 | 7735.60 | 7511.64 |
| Temperature (K) | 122.99(10) | 123.00(16) | 123.00(18) |
| Crystal system | hexagonal | hexagonal | hexagonal |
| Space group | P-62m | P-62m | P-62m |
| a (Å) | 10.3383(4) | 10.5007(3) | 10.4240(7) |
| c (Å) | 17.6308(9) | 17.9963(6) | 18.0525(16) |
| α (°) | 90 | 90 | 90 |
| γ (°) | 120 | 120 | 120 |
| Volume (Å3) | 1631.93(15) | 1718.50(11) | 1698.8(3) |
| c/a | 1.71 | 1.71 | 1.73 |
| Z | 1 | 1 | 2 |
| ρcalc (g/cm3) | 7.332 | 7.474 | 14.685 |
| μ (mm−1) | 76.096 | 73.862 | 143.327 |
| F(000) | 2896.0 | 3100.0 | 6024.0 |
| Crystal size (mm3) | 0.06 × 0.05 × 0.04 | 0.051 × 0.045 × 0.036 | 0.05 × 0.032 × 0.016 |
| Radiation | MoKα | MoKα | MoKα |
| (λ = 0.71073) | (λ = 0.71073) | (λ = 0.71073) | |
| 2Θ range for data collection (°) | 6.486 to 59.35 | 7.762 to 66.274 | 7.82 to 56.438 |
| Index ranges | −13 ≤ h ≤ 13, | −16 ≤ h ≤ 15, | −11 ≤ h ≤ 12, |
| −13 ≤ k ≤ 13, | −16 ≤ k ≤ 16, | −13 ≤ k ≤ 13, | |
| −24 ≤ l ≤ 14 | −27 ≤ l ≤ 26 | −21 ≤ l ≤ 24 | |
| Reflections collected | 4416 | 14514 | 8330 |
| Independent reflections | 1536 | 2408 | 1570 |
| Data/restraints/parameters | 1536/0/50 | 2408/6/56 | 1570/0/45 |
| Goodness-of-fit on F2 | 1.178 | 1.062 | 1.069 |
| Rint | Rint = 0.0377 | Rint = 0.0473 | Rint = 0.0881 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0333 | R1 = 0.0281 | R1 = 0.0376 |
| wR2 = 0.0656 | wR2 = 0.0591 | wR2 = 0.0634 | |
| Final R indexes [all data] | R1 = 0.0384 | R1 = 0.0325 | R1 = 0.0495 |
| wR2 = 0.0675 | wR2 = 0.0606 | wR2 = 0.0671 | |
| Largest diff. peak/hole (e Å−3) | 3.12/−2.33 | 1.48/−1.51 | 2.42/−2.30 |
| Flack parameter | 0.002(16) | −0.004(9) | −0.017(19) |
| Compound | A1 (6i) | A2 (2c) | A3 (6i) | A4 (1b) | d(Tl4-A4) (Å) d(Tl5-A4) (Å) |
|---|---|---|---|---|---|
| Cs8.21Rb6.76Tl27.09 | Cs 0.57(2) | Cs | Cs 0.31(2) | Cs 0.970(7) | 4.2212(11) |
| Rb 0.43(2) | Rb 0.69(2) | Tl 0.030(7) | 4.2995(12) | ||
| K6.96Rb8.04Tl27 | K 0.45(3) | Rb | K 0.68(3) | K 0.18(6) | 4.1369(13) |
| Rb 0.55(3) | Rb 0.32(3) | Rb 0.82(6) | 4.2369(15) | ||
| Cs5.85K9.15Tl27 | Cs 0.368(10) | Cs | Cs 0.119(9) | Cs | 4.1786(9) |
| K 0.632(10) | K 0.881(9) | 4.2623(11) | |||
| Cs2.27K12.73Tl27 | K | Cs 0.665(13) | K | Cs | 4.1475(5) |
| K 0.335(13) | 4.2584(5) | ||||
| Cs3.57K4.55Rb6.92Tl27 | Cs 0.18(3) Rb 0.56(5) K 0.26(3) | Cs 0.75(3) Rb 0.25(4) | Rb 0.508(16) K 0.49(3) | Cs | 4.1996(5) 4.2885(6) |
| Compound | d(Tl2-Tl2) (Å) | d(Tl3-Tl3) (Å) | d(Tl2-Tl3) (Å) |
|---|---|---|---|
| Cs8.21Rb6.76Tl27.09 | 3.749(3) | 3.202(2) | 3.0710(10) |
| K6.96Rb8.04Tl27 | 3.753(3) | 3.198(2) | 3.0855(11) |
| Cs5.85K9.15Tl27 | 3.721(2) | 3.197(2) | 3.0731(9) |
| Cs2.27K12.73Tl27 | 3.7333(12) | 3.1908(10) | 3.0926(4) |
| Cs14.53Tl28.4 | 3.7697(16) | 3.2146(13) | 3.0833(6) |
| Cs15Tl27 | 3.774(3) | 3.212(3) | 3.0816(12) |
| Compound | d(Tl6-Tl5) (Å) | d(Tl5-Tl5) (Å) | d(Tl4-Tl4) (Å) | d(Tl4-Tl5) (Å) | d(Tl4-Tl5#) (Å) | d(Tl4-Tl6) (Å) | c/a | |
|---|---|---|---|---|---|---|---|---|
| Cs8.21Rb6.76Tl27.09 | 3.3735(7) | 3.463(2) | 3.2924(19) 3.088(2) | 3.2122(11) | 3.3917(14) | 3.3078(13) | 5.44 | 1.71 |
| K6.96Rb8.04Tl27 | 3.3423(8) | 3.533(2) | 3.270(2) 3.040(3) | 3.1796(13) | 3.4068(17) | 3.3450(15) | 6.90 | 1.68 |
| Cs5.85K9.15Tl27 | 3.3622(7) | 3.475(2) | 3.2721(16) 3.0713(19) | 3.1860(10) | 3.3923(12) | 3.3329(13) | 6.27 | 1.66 |
| Cs2.27K12.73Tl27 | 3.3493(3) | 3.5211(9) | 3.2665(8) 3.0606(9) | 3.1798(5) | 3.3981(6) | 3.3611(5) | 6.64 | 1.64 |
| Cs14.53Tl28.4 | 3.3093(15) (A) 3.4628(19) (B) | 3.627(10) (A) 3.457(11) (B) | 3.2860(14) 3.1423(15) | 3.242(4) (A) 3.259(3) (B) | 3.385(3) (A) 3.465(4) (B) | 3.3170(8) | 4.32 6.13 | 1.71 |
| Cs15Tl27 | 3.3940(8) | 3.457(3) | 3.322(2) 3.102(3) | 3.2361(14) | 3.4039(17) | 3.3026(16) | 5.05 | 1.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwinghammer, V.F.; Tiefenthaler, S.M.; Gärtner, S. The Role of Different Alkali Metals in the A15Tl27 Type Structure and the Synthesis and X-ray Structure Analysis of a New Substitutional Variant Cs14.53Tl28.4. Materials 2021, 14, 7512. https://doi.org/10.3390/ma14247512
Schwinghammer VF, Tiefenthaler SM, Gärtner S. The Role of Different Alkali Metals in the A15Tl27 Type Structure and the Synthesis and X-ray Structure Analysis of a New Substitutional Variant Cs14.53Tl28.4. Materials. 2021; 14(24):7512. https://doi.org/10.3390/ma14247512
Chicago/Turabian StyleSchwinghammer, Vanessa F., Susanne M. Tiefenthaler, and Stefanie Gärtner. 2021. "The Role of Different Alkali Metals in the A15Tl27 Type Structure and the Synthesis and X-ray Structure Analysis of a New Substitutional Variant Cs14.53Tl28.4" Materials 14, no. 24: 7512. https://doi.org/10.3390/ma14247512
APA StyleSchwinghammer, V. F., Tiefenthaler, S. M., & Gärtner, S. (2021). The Role of Different Alkali Metals in the A15Tl27 Type Structure and the Synthesis and X-ray Structure Analysis of a New Substitutional Variant Cs14.53Tl28.4. Materials, 14(24), 7512. https://doi.org/10.3390/ma14247512






