Effect of Al Concentration on Microstructure and Properties of AlNbTiZr Medium-Entropy Alloy Coatings
Abstract
:1. Introduction
2. Experimental Details
2.1. Coating Deposition
2.2. Coating Characterization
3. Results and Discussion
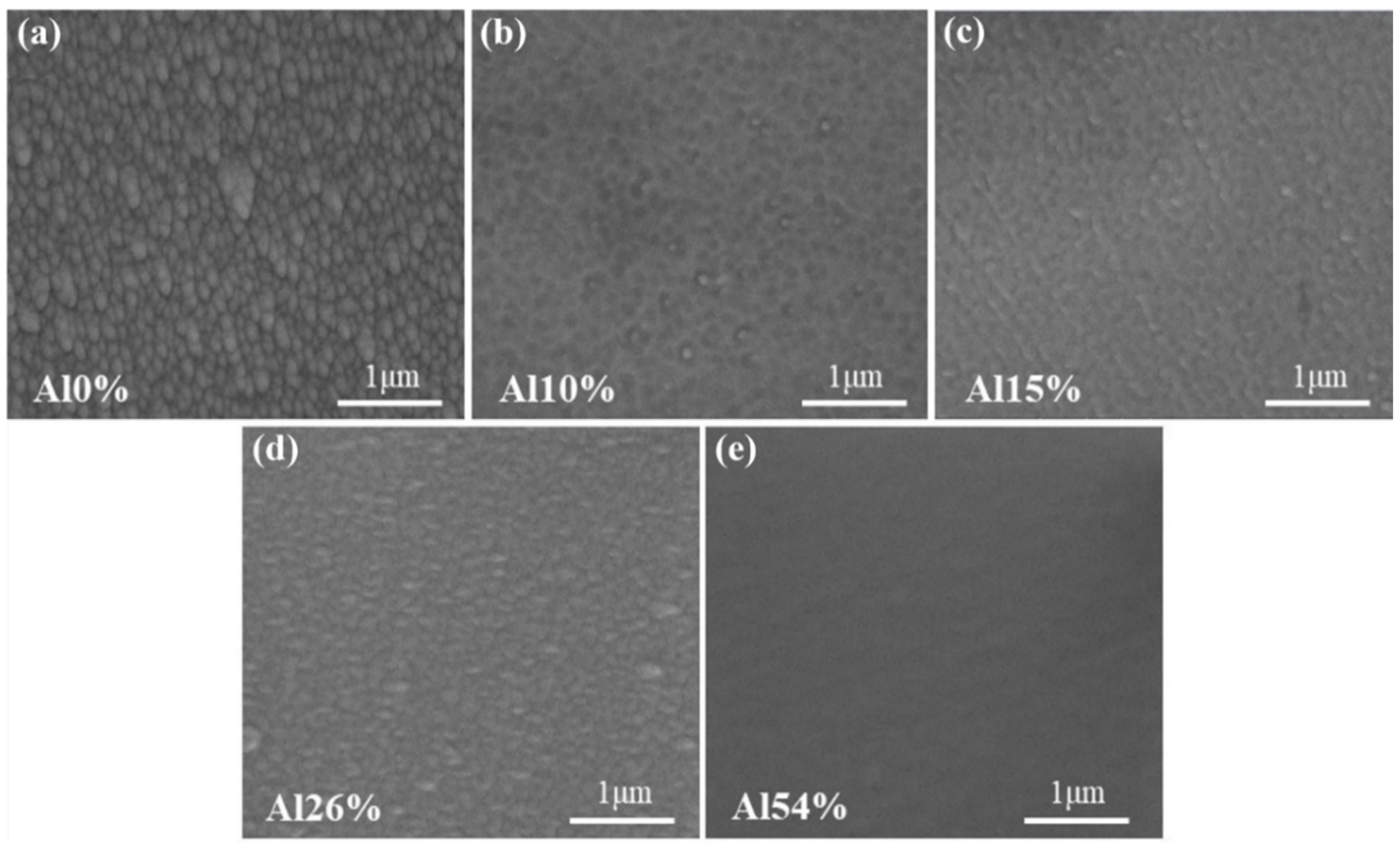
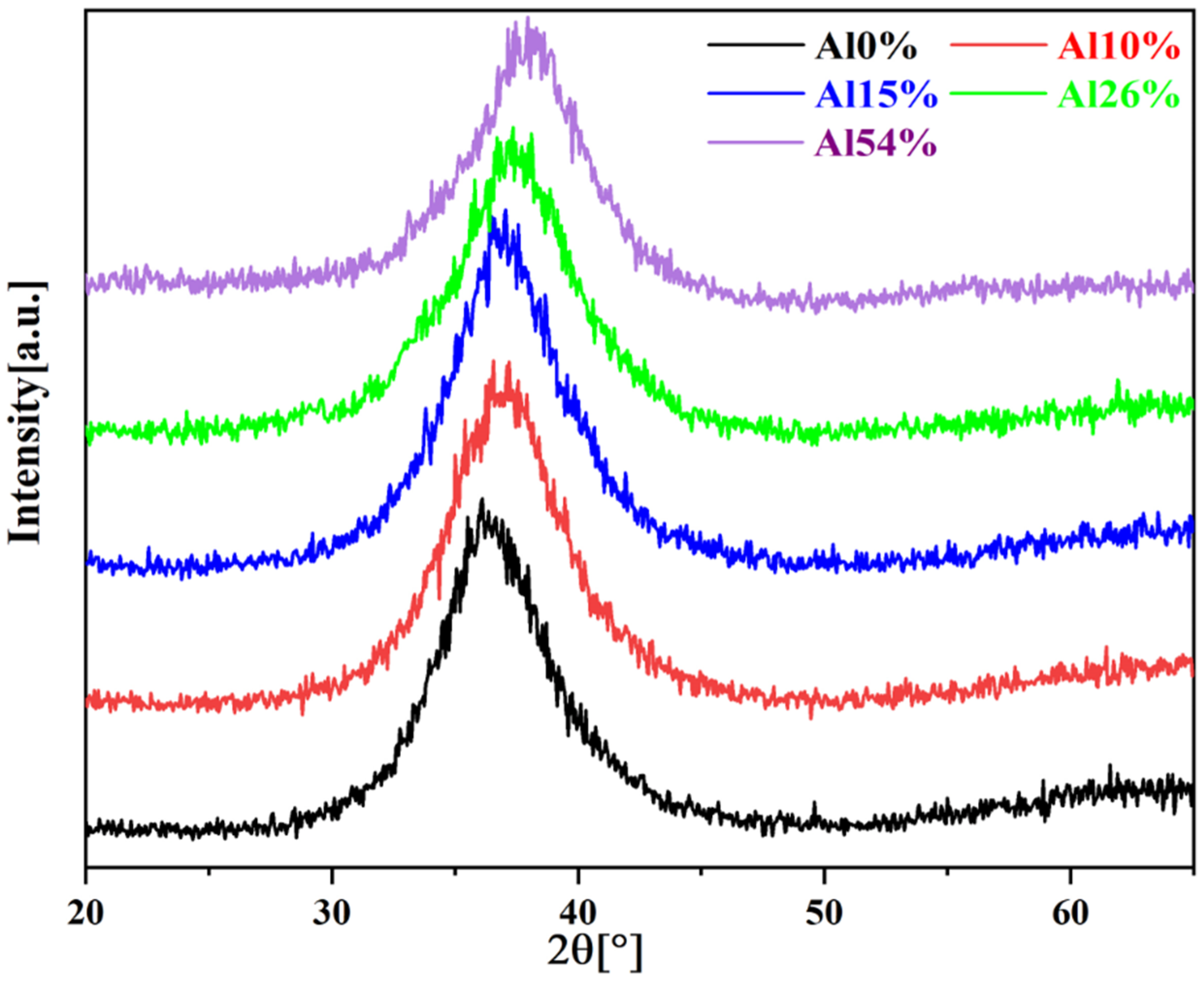
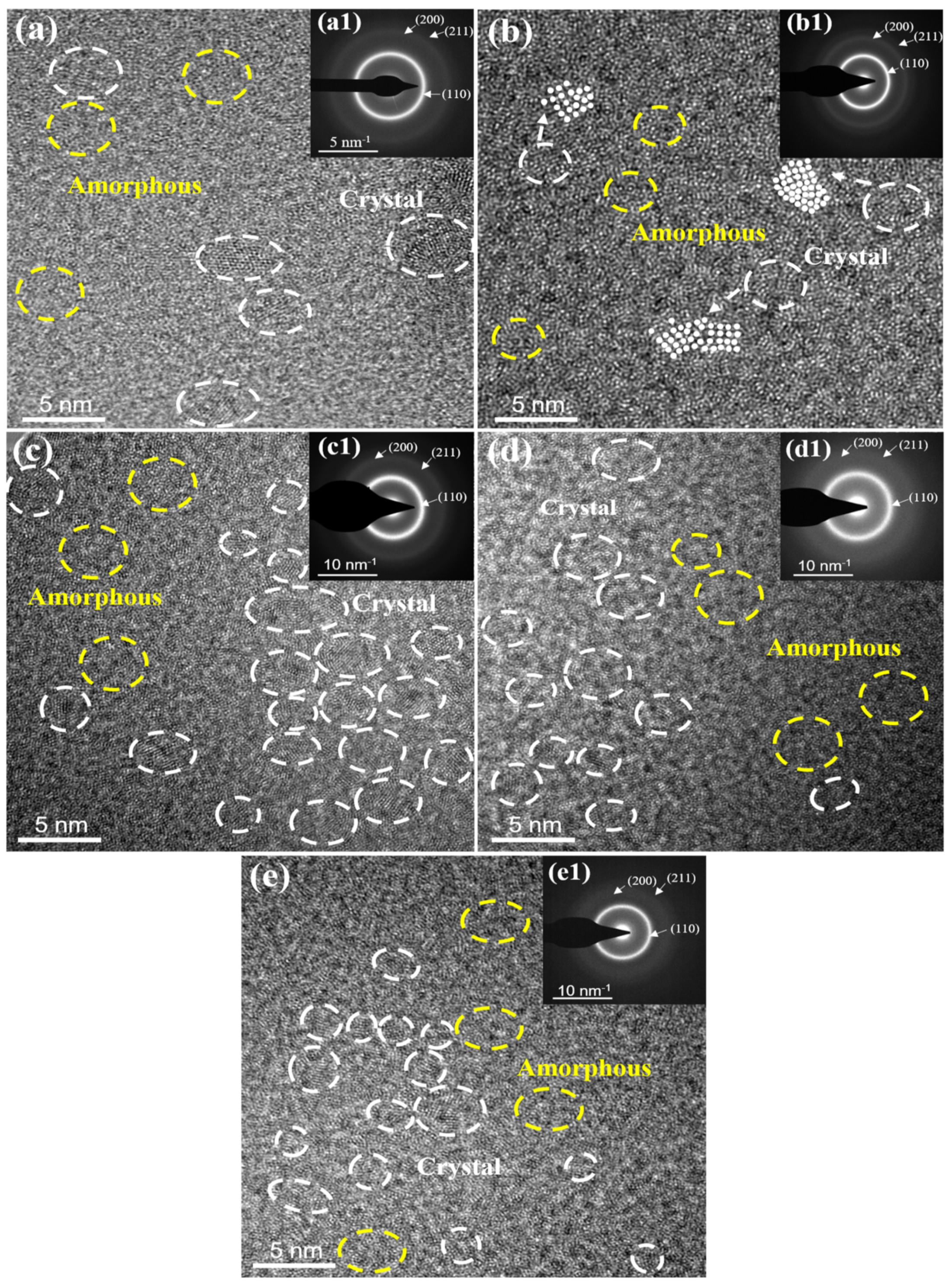
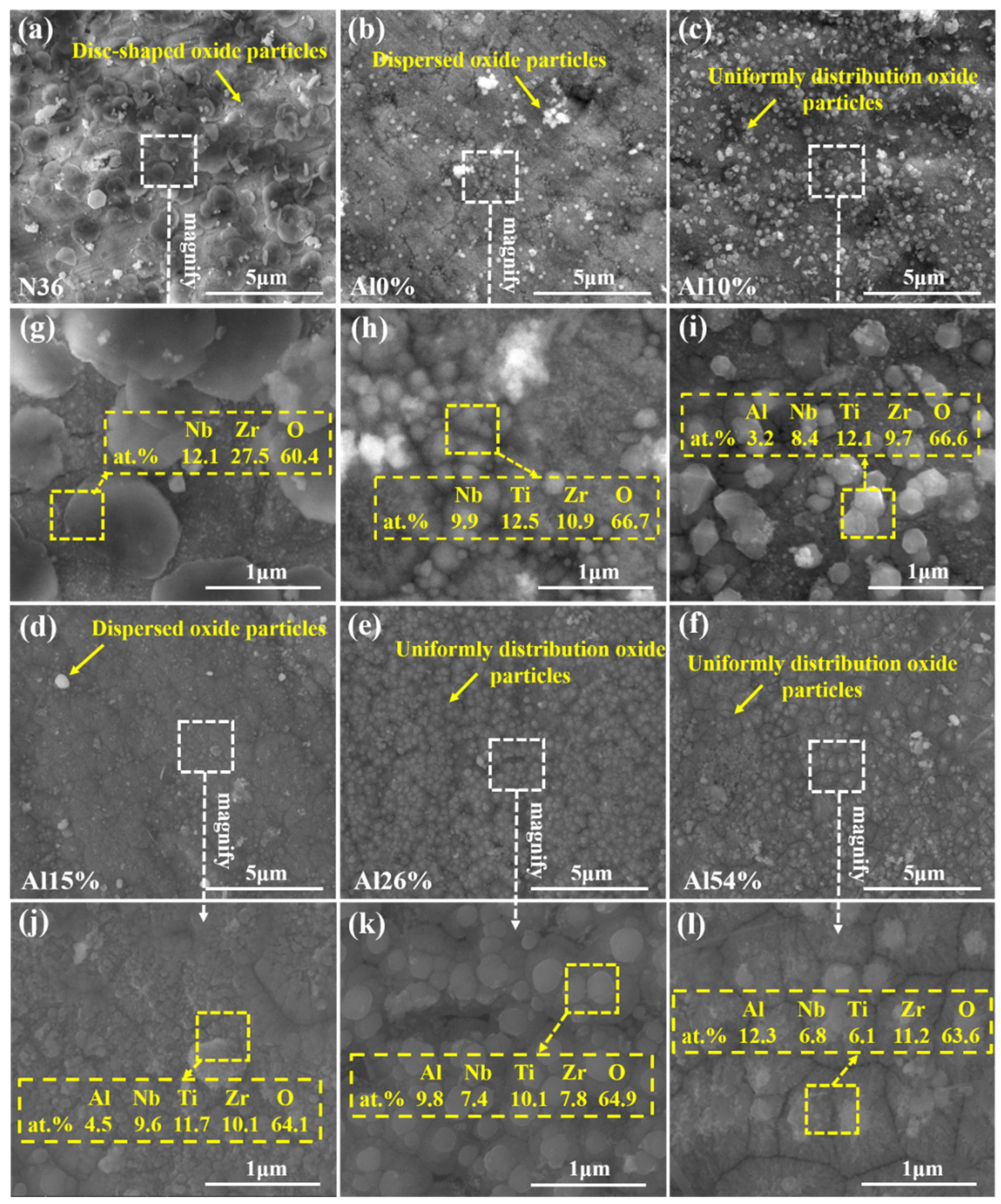
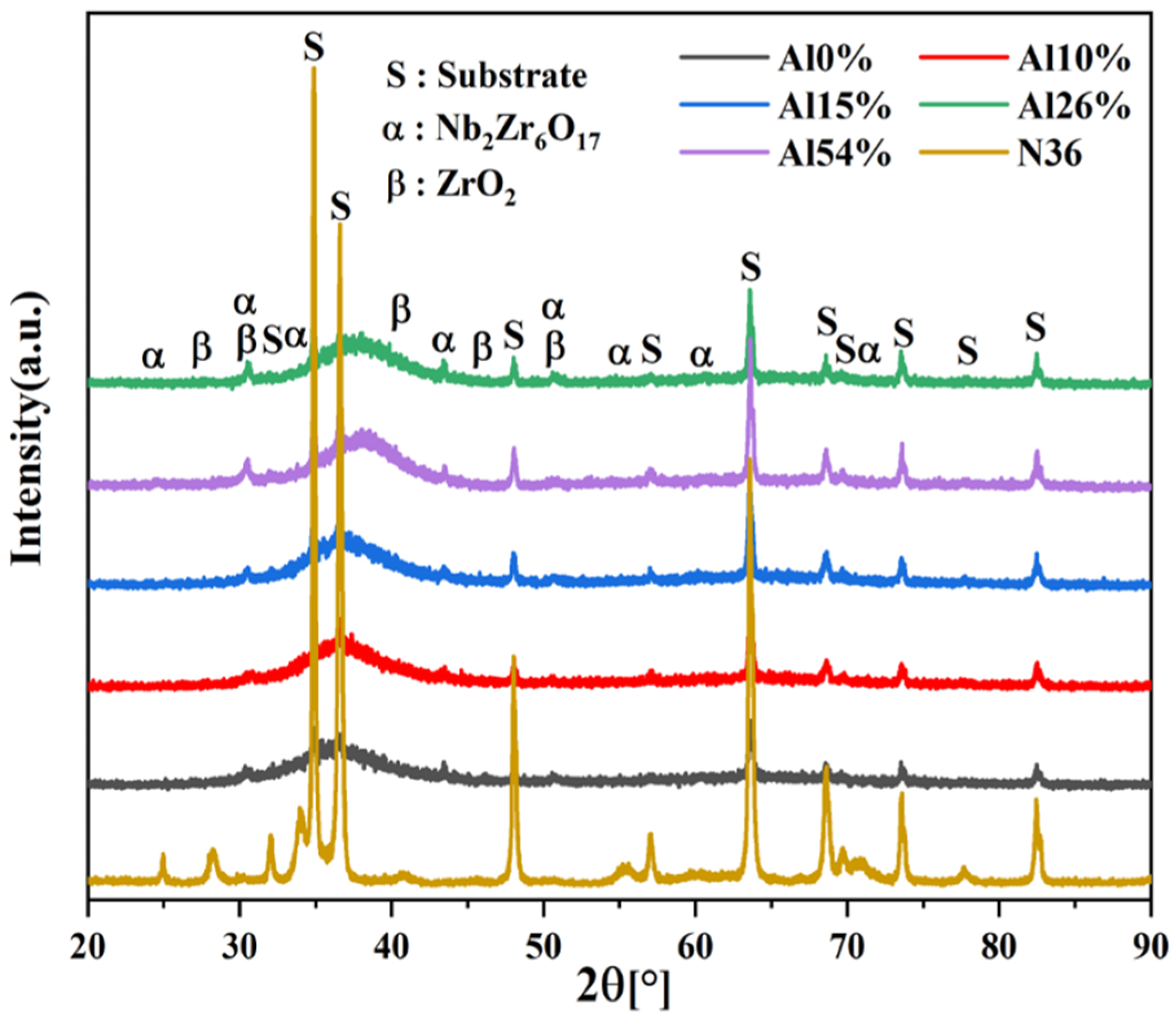
3.1. Chemical Composition, Phase and Microstructure
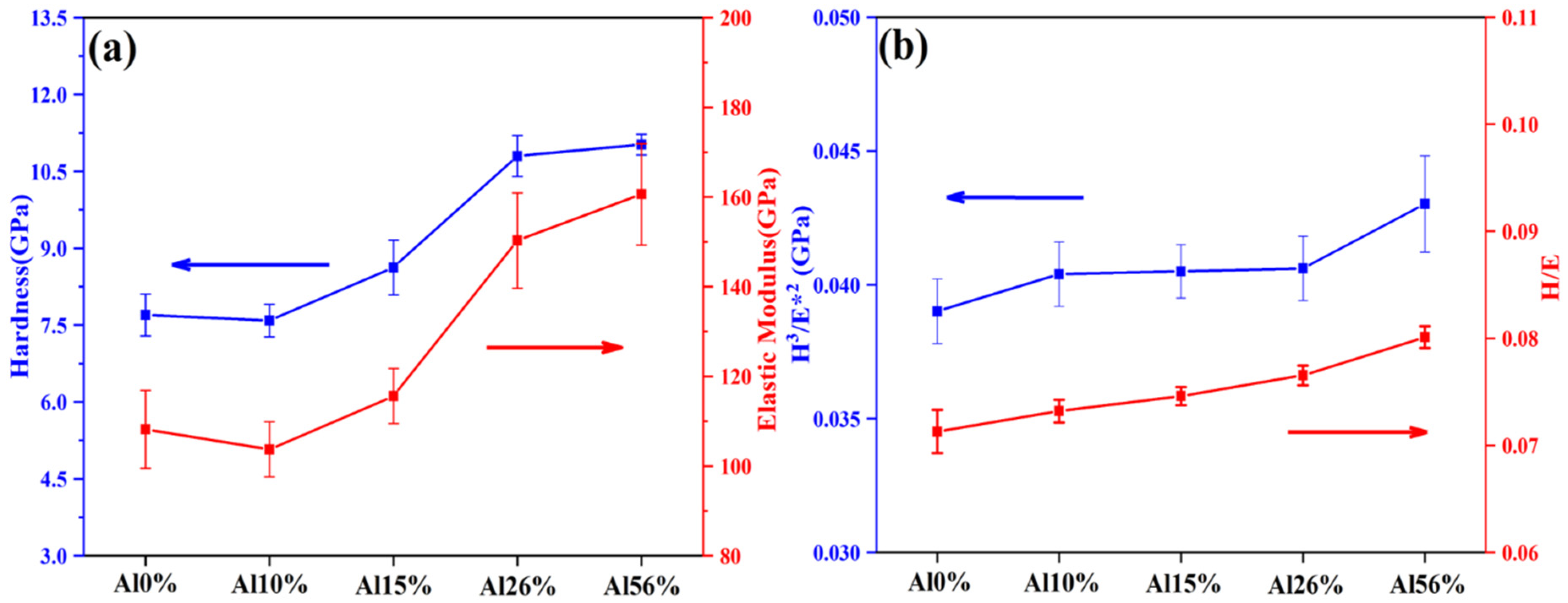
3.2. Mechanical Properties
3.3. Surface Wettability
3.4. Autoclave Test
4. Conclusions
- The XRD and TEM analyses showed that all the coatings were composed of amorphous phase and tiny bcc nanocrystals; the number of nanocrystals in the coatings tends to increase with increasing Al content.
- With the increase of Al content in the coatings, the hardness and modulus of elasticity decreased first and then increased, while the cracking resistance and plastic deformation resistance increased slightly.
- Compared to the uncoated N36 substrate, the AlNbTiZr MEA coatings exhibited excellent corrosion resistance with a much thinner oxide layer and enhanced hydrophobicity.
- Compared with other coatings, the best corrosion resistance of AlNbTiZr MEA coating was obtained by the addition of 10.2% Al; because of its more reasonable composition ratio, it can greatly reduce the formation probability of the boehmite phase.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, Z.; Yang, H.; Satoh, Y.; Murakami, K.; Kano, S.; Zhao, Z.; Shen, J.; Abe, H. Current status of materials development of nuclear fuel cladding tubes for light water reactors. Nucl. Eng. Des. 2017, 316, 131–150. [Google Scholar] [CrossRef]
- Hirano, M.; Yonomoto, T.; Ishigaki, M.; Watanabe, N.; Maruyama, Y.; Sibamoto, Y.; Watanabe, T.; Moriyama, K. Insights from review and analysis of the Fukushima Daiichi accident. J. Nucl. Sci. Technol. 2012, 49, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Zinkle, S.J.; Terrani, K.A.; Gehin, J.C. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Shimoda, K.; Hinoki, T.; Katoh, Y. Development of the tailored SiC/SiC composites by the combined fabrication process of ICVI and NITE methods. J. Nucl. Mater. 2009, 384, 103–108. [Google Scholar] [CrossRef]
- Pint, B.A.; Unocic, K.A.; Terrani, K.A. Effect of steam on high temperature oxidation behaviour of alumina-forming alloys. Mater. High Temp. 2015, 32, 28–35. [Google Scholar] [CrossRef]
- Pint, B.A. Performance of FeCrAl for accident-tolerant fuel cladding in high-temperature steam. Corros. Rev. 2017, 35, 232–240. [Google Scholar] [CrossRef]
- Unocic, K.A.; Yamamoto, Y.; Pint, B.A. Effect of Al and Cr Content on Air and Steam Oxidation of FeCrAl Alloys and Commercial APMT Alloy. Oxid. Met. 2017, 87, 431–441. [Google Scholar] [CrossRef]
- Cheng, B.; Kim, Y.-J.; Chou, P. Improving Accident Tolerance of Nuclear Fuel with Coated Mo-alloy Cladding. Nucl. Eng. Tech. 2016, 48, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Cheng, B.; Chou, P.; Kim, Y.-J. Evaluations of Mo-alloy for light water reactor fuel cladding to enhance accident tolerance. EPJ Nucl. Sci. Technol. 2016, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Park, J.-H.; Kim, I.-H.; Park, D.-J.; Jung, Y.-I.; Choi, B.-K.; Kim, H.-G. Enhanced wear resistance of CrAl-coated cladding for accident-tolerant fuel. J. Nucl. Mater. 2019, 523, 223–230. [Google Scholar] [CrossRef]
- He, L.X.; Liu, C.H.; Lin, J.H.; Chen, Q.S.; Yang, J.J.; Zhang, R.Q.; Yang, H.Y.; Wang, Y.; Wang, J.; Long, J.P.; et al. Microstructure, oxidation and corrosion properties of FeCrAl coatings with low Al content prepared by magnetron sputtering for accident tolerant fuel cladding. J. Nucl. Mater. 2021, 551, 152966. [Google Scholar] [CrossRef]
- Chen, Q.S.; Liu, C.H.; Zhang, R.Q.; Yang, H.Y.; Wei, T.G.; Wang, Y.; Li, Z.; He, L.X.; Wang, J.; Wang, L.; et al. Microstructure and high-temperature steam oxidation properties of thick Cr coatings prepared by magnetron sputtering for accident tolerant fuel claddings: The role of bias in the deposition process. Corros. Sci. 2020, 165, 108378. [Google Scholar] [CrossRef]
- Wu, J.J.; Shen, M.L.; Hu, M.; Guo, C.; Li, Q.; Zhu, S.L. High vacuum arc ion plating Cr films: Self-ion bombarding effect and oxidation behavior. Corros. Sci. 2021, 187, 109476. [Google Scholar] [CrossRef]
- Brachet, J.C.; Urvoy, S.; Rouesne, E.; Nony, G.; Dumerval, M.; Le Saux, M.; Ott, F.; Michaud, A.; Schuster, F.; Maury, F. DLI-MOCVD CrxCy coating to prevent Zr-based cladding from inner oxidation and secondary hydriding upon LOCA conditions. J. Nucl. Mater. 2021, 550, 152953. [Google Scholar] [CrossRef]
- Hui, R.; Cook, W.; Sun, C.; Xie, Y.; Yao, P.; Miles, J.; Olive, R.; Li, J.; Zheng, W.; Zhang, L. Deposition, characterization and performance evaluation of ceramic coatings on metallic substrates for supercritical water-cooled reactors. Surf. Coat. Technol. 2011, 205, 3512–3519. [Google Scholar] [CrossRef] [Green Version]
- Usui, T.; Sawada, A.; Amaya, M.; Suzuki, A.; Chikada, T.; Terai, T. SiC coating as hydrogen permeation reduction and oxidation resistance for nuclear fuel cladding. J. Nucl. Sci. Technol. 2015, 3131, 1–5. [Google Scholar] [CrossRef]
- Maier, B.R.; Garcia-Diaz, B.L.; Hauch, B.; Olson, L.C.; Sindelar, R.L.; Sridharan, K. Ti2AlC coatings for improved nuclear fuel cladding. J. Nucl. Mater. 2015, 466, 1–6. [Google Scholar] [CrossRef]
- Liu, J.K.; Hao, Z.; Cui, Z.X.; Ma, D.Y.; Lu, J.Q.; Cui, Y.G.; Li, C.; Liu, W.B.; Xie, S.J.; Hu, P.F.; et al. Oxidation behavior, thermal stability, and the coating/substrate interface evolution of CrN-coated Zircaloy under high-temperature steam. Corros. Sci. 2021, 185, 127228. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.H.; Chen, Q.S.; Yang, J.J.; Liu, J.M.; Yang, H.Y.; Zhang, W.; Zhang, R.Q.; He, L.X.; Long, J.P.; et al. Microstructure, high-temperature corrosion and steam oxidation properties of Cr/CrN multilayer coatings prepared by magnetron sputtering. Corros. Sci. 2021, 191, 109755. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Shi, K.; Zhang, W.; Chen, Q.; Ning, Z.; Zhu, C.; Liao, J.; Yang, Y.; Liu, N.; Zhang, W.; et al. A novel AlCrFeMoTi high-entropy alloy coating with a high corrosion-resistance in lead-bismuth eutectic alloy. Corros. Sci. 2021, 187, 109524. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.B.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Preparation, structure, and properties of an AlCrMoNbZr high-entropy alloy coating for accident-tolerant fuel cladding. Surf. Coat. Technol. 2018, 347, 13–19. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.B.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Preparation, structure, and properties of high-entropy alloy multilayer coatings for nuclear fuel cladding: A case study of AlCrMoNbZr/(AlCrMoNbZr)N. J. Nucl. Mater. 2018, 512, 15–24. [Google Scholar] [CrossRef]
- Chen, Q.S.; Liu, C.H.; Long, J.P.; Wang, J.; Zhang, R.Q.; Yang, H.Y.; Zhang, W.; Yao, F.Y.; Zhao, S.; Zhang, Q. Microstructure and corrosion characteristics of CrCuFeMoNi HEA coatings with different compositions in high-temperature and high-pressure water. Mater. Res. Express 2019, 6, 086511. [Google Scholar] [CrossRef]
- Guan, H.T.; Chai, L.J.; Wang, Y.Y.; Xiang, K.; Wu, L.; Pan, H.C.; Yang, M.B.; Teng, C.Q.; Zhang, W. Microstructure and hardness of NbTiZr and NbTaTiZr refractory medium-entropy alloy coatings on Zr alloy by laser cladding. Appl. Surf. Sci. 2021, 549, 149338. [Google Scholar] [CrossRef]
- Cao, F.Y.; Munroe, P.; Zhou, Z.F.; Xie, Z.H. Microstructure and mechanical properties of a multilayered CoCrNi/Ti coating with varying crystal structure. Surf. Coat. Technol. 2018, 350, 596–602. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, G.X.; Barr, C.M.; Jin, K.; Bei, H.B.; Hattar, K.; Weber, W.J.; Zhang, Y.W.; More, K.L. Defect evolution in Ni and NiCoCr by in situ 2.8 MeV Au irradiation. J. Nucl. Mater. 2019, 523, 502–509. [Google Scholar]
- Feng, K.; Zhang, Y.; Li, Z.; Yao, C.; Yao, L.; Fan, C. Corrosion properties of laser cladded CrCoNi medium entropy alloy coating. Surf. Coat. Technol. 2020, 397, 126004. [Google Scholar] [CrossRef]
- Chai, L.; Wang, C.; Xiang, K.; Wang, Y.; Wang, T.; Ma, Y. Phase constitution, microstructure and properties of pulsed laser-clad ternary CrNiTi medium-entropy alloy coating on pure titanium. Surf. Coat. Technol. 2020, 402, 126503. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Ceramic coating for corrosion (c3) resistance of nuclear fuel cladding. Surf. Coat. Technol. 2015, 281, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Sun, Q.; Fang, R.; Lin, H.; Chen, Z.; Zhang, X.; Zhang, J. Microstructure and properties of AlCoCrCuFeNi high-entropy alloy laser processing. J. Netshape Form. Eng. 2019, 11, 178–188. (In Chinese) [Google Scholar]
- Xiang, C.; Han, E.H.; Zhang, Z.M.; Fu, H.M.; Wang, J.Q.; Zhang, H.F.; Hu, G.D. Design of single-phase high-entropy alloys composed of low thermal neutron absorption cross-section elements for nuclear power plant application. Intermetallics 2019, 104, 143–153. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, C.H.; Yang, J.J.; Zhang, W.; He, L.X.; Zhang, R.Q.; Yang, H.Y.; Wang, J.; Long, J.P.; Chang, H. Mechanical and high-temperature corrosion properties of AlTiCrNiTa high entropy alloy coating prepared by magnetron sputtering for accident-tolerant fuel cladding. Surf. Coat. Technol. 2021, 417, 127228. [Google Scholar] [CrossRef]
- Niu, P.; Li, R.; Yuan, T.; Wang, M.; Liu, Y. Research progress of high-entropy Alloys by Additive Manufacturing. J. Netshape Form. Eng. 2019, 11, 51–57. (In Chinese) [Google Scholar]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Tsai, D.C.; Shieu, F.S.; Chang, S.Y.; Yao, H.C.; Deng, M.J. Structures and characterizations of TiVCr and TiVCrZrY films deposited by magnetron sputtering under different Bias powers. J. Electrochem. Soc. 2010, 157, K52–K58. [Google Scholar] [CrossRef]
- Vishwanadh, B.; Sarkar, N.; Gangil, S.; Singh, S.; Tewari, R.; Dey, G.K.; Banerjee, S. Synthesis and microstructural characterization of a novel multicomponent equiatomic ZrNbAlTiV high entropy alloy. Scr. Mater. 2016, 124, 146–150. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Z.; Dou, D.; Li, J. Microstructure and properties of coating of FeAlCuCrCoMn high entropy alloy deposited by direct current magnetron sputtering. Mater. Res. 2016, 19, 802–806. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than entropy in high-entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Inoue, A. High strength bulk amorphous alloys with low critical cooling rates. Mater. Trans. JIM 1995, 36, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.W.; Wang, W.; Zhou, Y.C. Investigation of the relationship between elastic modulus and hardness based on depth-sensing indentation measurements. Acta Mater. 2004, 52, 5397–5404. [Google Scholar] [CrossRef]
- Berlind, T.; Hellgren, N.; Johansson, M.P.; Hultman, L. Microstructure, mechanical properties, and wetting behavior of Si-C-N thin films grown by reactive magnetron sputtering. Surf. Coat. Technol. 2001, 141, 145–155. [Google Scholar] [CrossRef]
- Barin, I. Thermochemical Data of Pure Substances, 3rd ed.; VCH: New York, NY, USA, 1995; pp. 48–1692. [Google Scholar]
- Shang, P.; Xiong, S.; Li, L.; Tian, D.; Ai, W. Investigation on thermal stability of Ta2O5, TiO2 and Al2O3 coatings for application at high temperature. Appl. Surf. Sci. 2013, 285, 713–720. [Google Scholar] [CrossRef]
- Hassan, A.M.; Naga, S.M.; Awaad, M. Toughening and strengthening of Nb2O5 doped zirconia/alumina (ZTA) composites. Int. J. Refract. Met. Hard Mater. 2015, 48, 338–345. [Google Scholar] [CrossRef]
- Su, N.K.; Banjuraizah, J.; Idris, M.A.; Ahmad, Z.A. The role of Nb2Zr6O17 phase on the hardness and fracture toughness of ZTA/Nb2O5 by cold isostatic pressing. In Materials Science Forum; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2017; Volume 888, pp. 126–130. ISSN 1662-9752. [Google Scholar]




| Base Pressure (Pa) | Working Pressure (Pa) | Working Temperature (°C) | Ar Flow Rate (sccm) | Substrate Bias Voltage(V) | Sputtering Power (W) | Deposition Time (min) |
|---|---|---|---|---|---|---|
| 7.5 × 10−4 | 0.51 | 300 | 51 | −100 | 200 | 150 |
| Designated Name | Chemical Composition (at.%) | Coating Thickness (μm) | Mixing Entropy (J∙K−1 mol−1) | |||
|---|---|---|---|---|---|---|
| Al | Nb | Ti | Zr | |||
| Al0% | 0 | 32.4 | 33.5 | 34.1 | 5.4 | 1.10R |
| Al10% | 10.2 | 29.7 | 29.4 | 30.7 | 5.7 | 1.32R |
| Al15% | 15.6 | 28.1 | 28.9 | 27.4 | 5.8 | 1.36R |
| Al26% | 26.1 | 24.4 | 24.1 | 25.4 | 5.7 | 1.39R |
| Al54% | 54.4 | 15.4 | 15.3 | 14.9 | 6.1 | 1.19R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, H.; Yang, J.; Mao, J.; Chen, Q.; Yang, J.; Zhang, W.; Ning, Z.; Teng, C.; Ma, C.; Wu, L.; et al. Effect of Al Concentration on Microstructure and Properties of AlNbTiZr Medium-Entropy Alloy Coatings. Materials 2021, 14, 7661. https://doi.org/10.3390/ma14247661
Xin H, Yang J, Mao J, Chen Q, Yang J, Zhang W, Ning Z, Teng C, Ma C, Wu L, et al. Effect of Al Concentration on Microstructure and Properties of AlNbTiZr Medium-Entropy Alloy Coatings. Materials. 2021; 14(24):7661. https://doi.org/10.3390/ma14247661
Chicago/Turabian StyleXin, Hongyang, Jijun Yang, Jianjun Mao, Qingsong Chen, Jiaqi Yang, Wei Zhang, Zhien Ning, Changqing Teng, Cong Ma, Lu Wu, and et al. 2021. "Effect of Al Concentration on Microstructure and Properties of AlNbTiZr Medium-Entropy Alloy Coatings" Materials 14, no. 24: 7661. https://doi.org/10.3390/ma14247661
APA StyleXin, H., Yang, J., Mao, J., Chen, Q., Yang, J., Zhang, W., Ning, Z., Teng, C., Ma, C., Wu, L., & Wu, X. (2021). Effect of Al Concentration on Microstructure and Properties of AlNbTiZr Medium-Entropy Alloy Coatings. Materials, 14(24), 7661. https://doi.org/10.3390/ma14247661






