A Chemical-Transport-Mechanics Numerical Model for Concrete under Sulfate Attack
Abstract
:1. Introduction
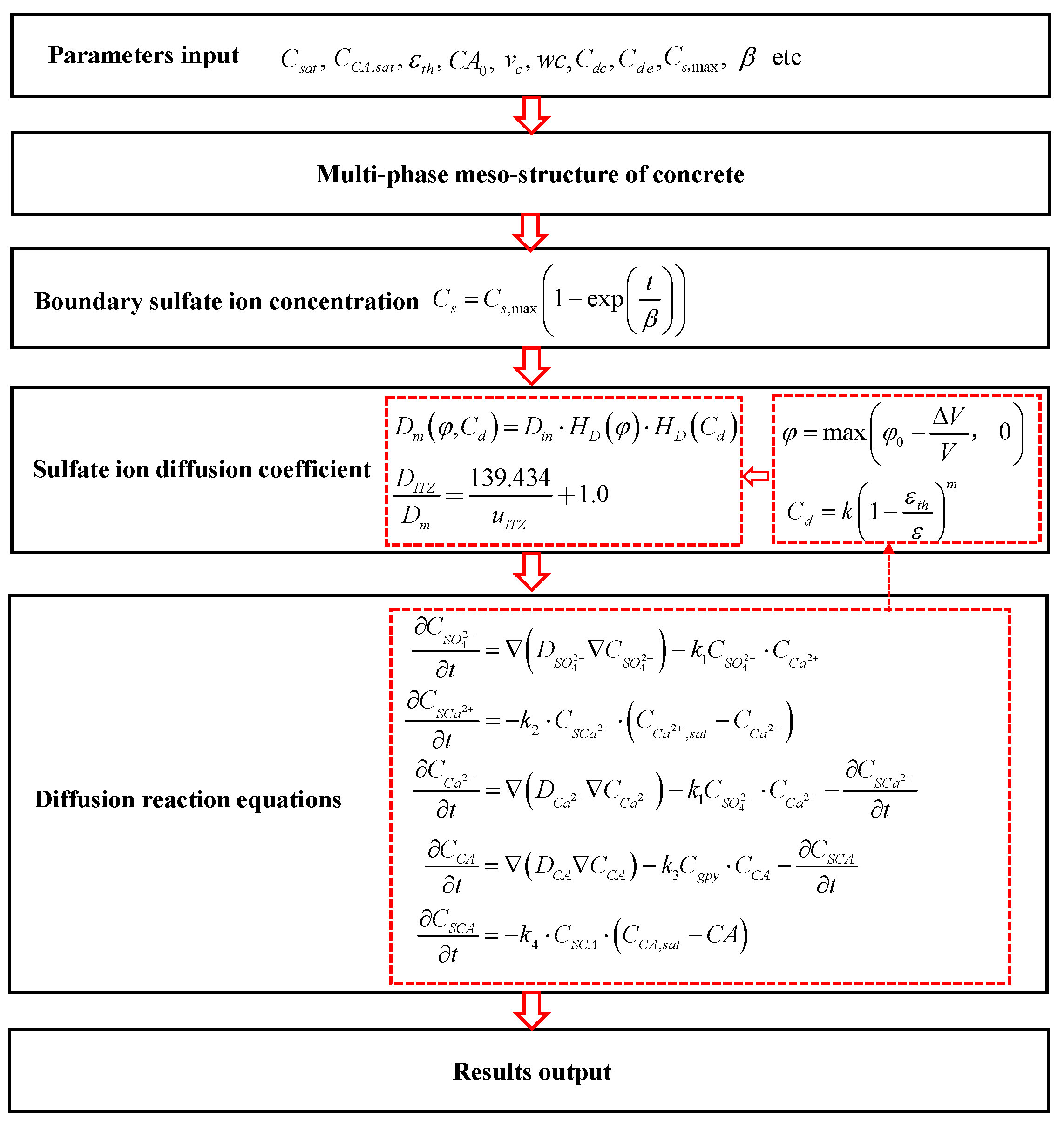
2. Chemo-Transport-Mechanical Model for Concrete under Sulfate Attack
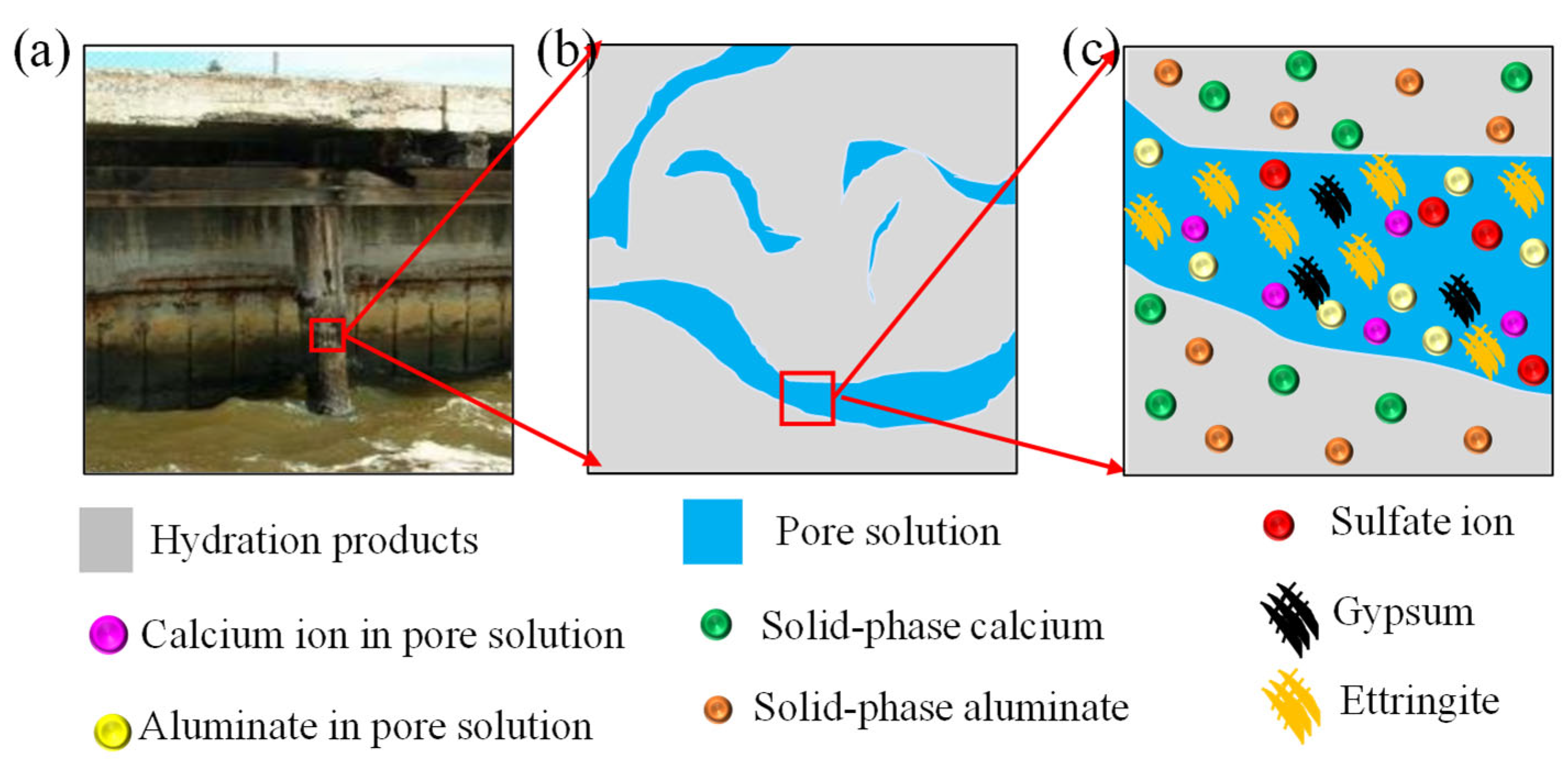
2.1. Sulfate Ion Diffusion-Reaction
2.2. Calcium Ion Reaction-Leaching-Diffusion
2.3. Gypsum and Aluminate Chemical Kinetic Reaction
2.4. Chemical Damage
2.5. Effective Diffusion Coefficient of Sulfate Ion
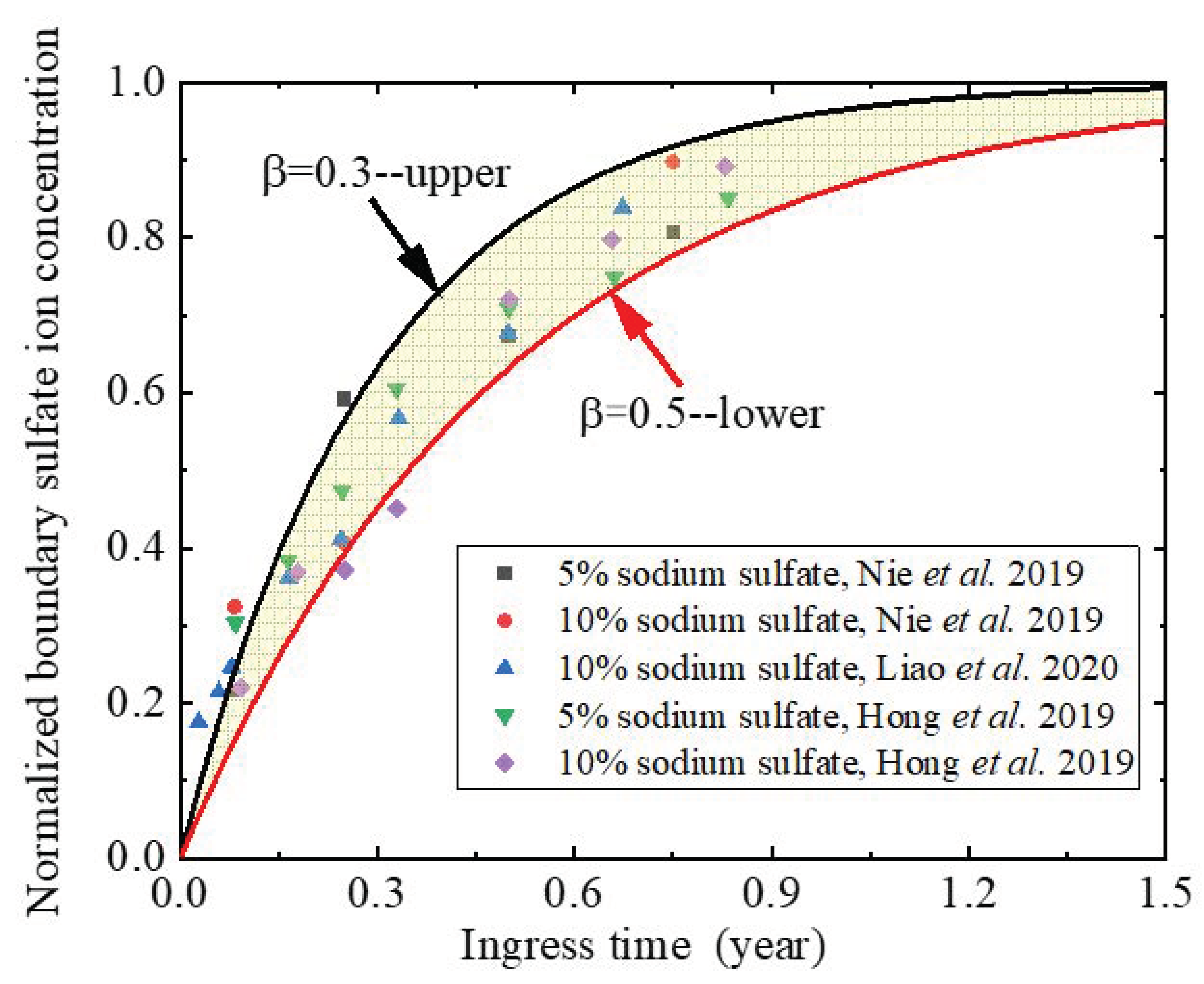
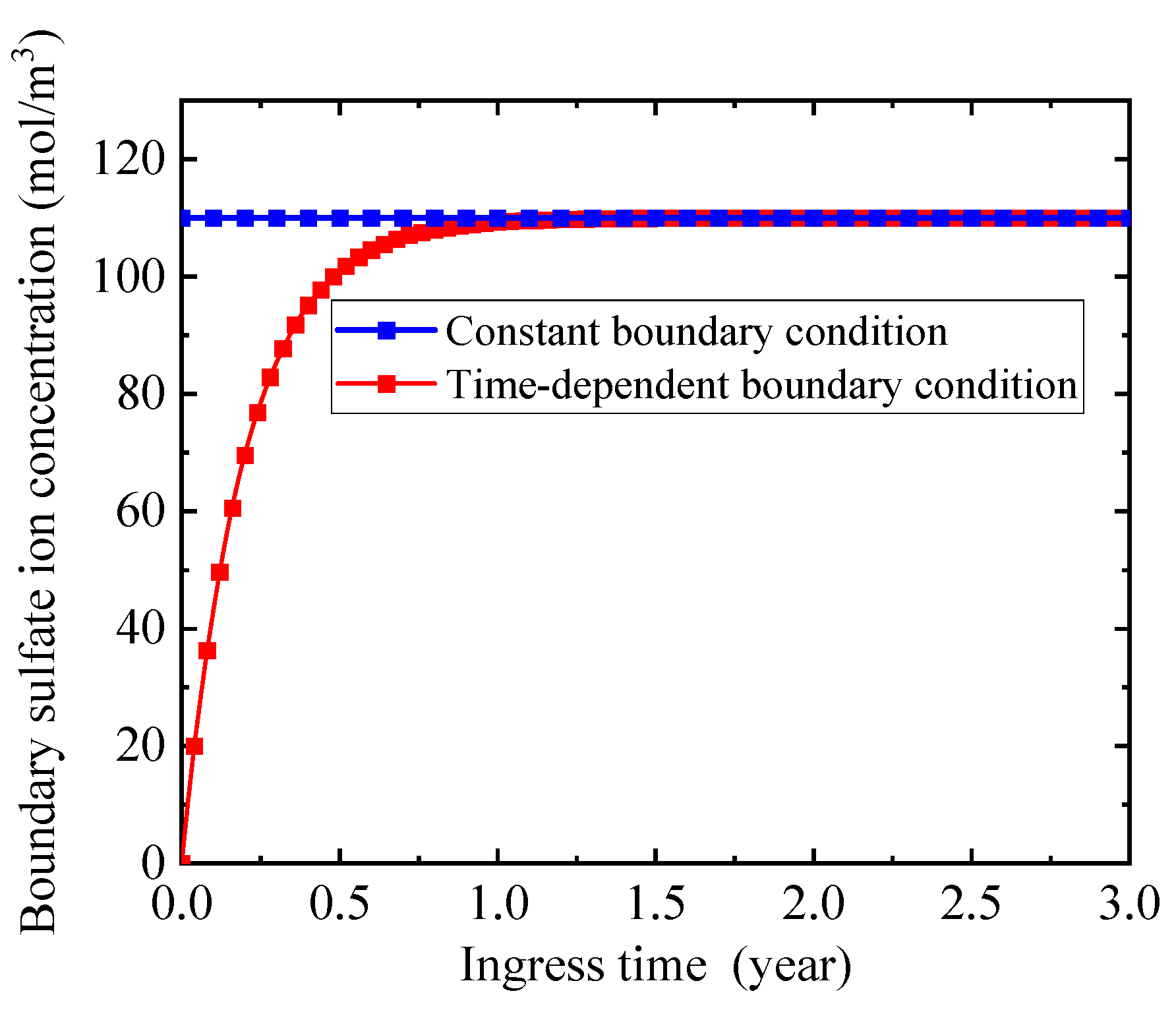
2.6. Boundary Sulfate Ion Concentration
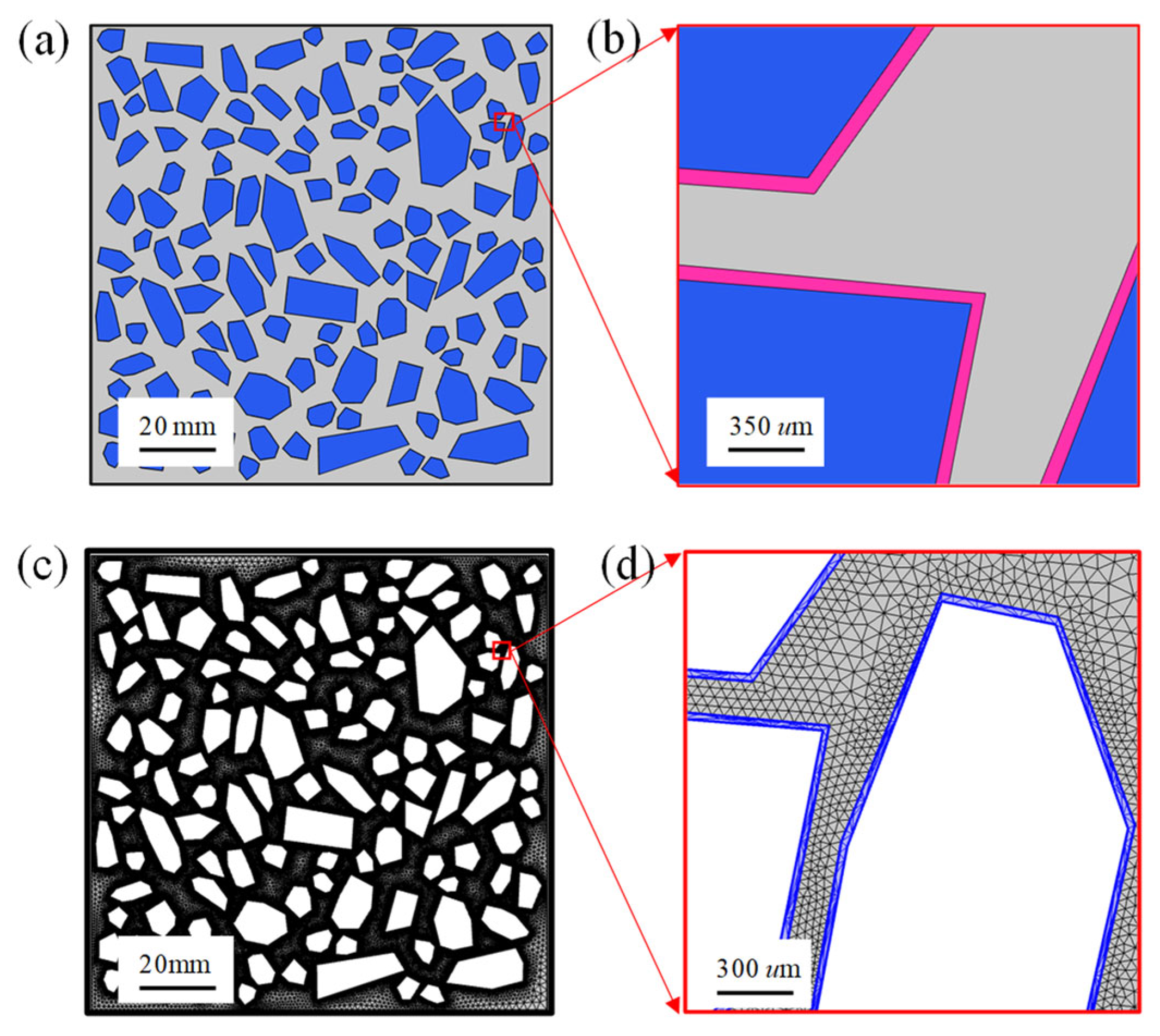
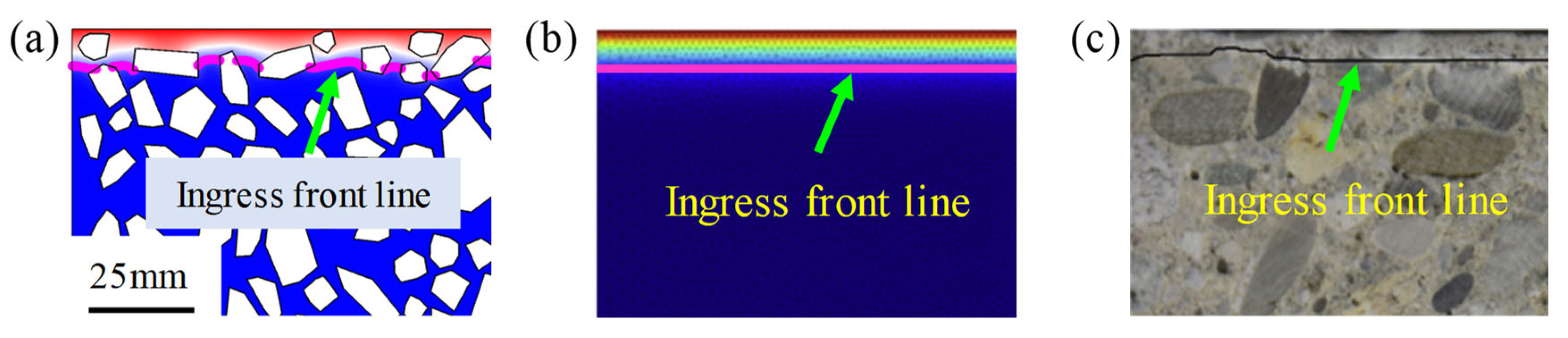
3. Numerical Simulation
4. Model Validation
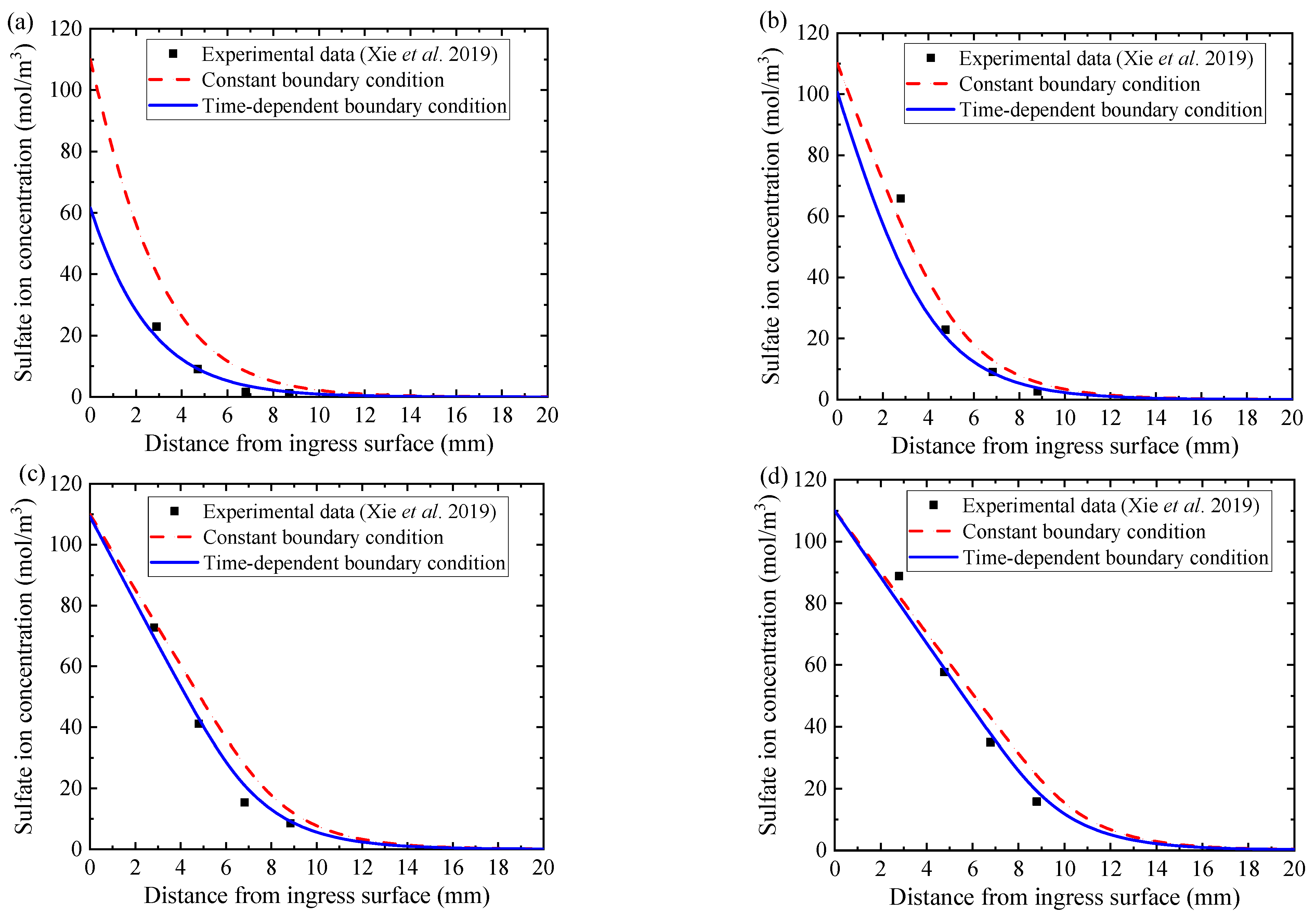
4.1. Distribution of Sulfate Ion Concentration
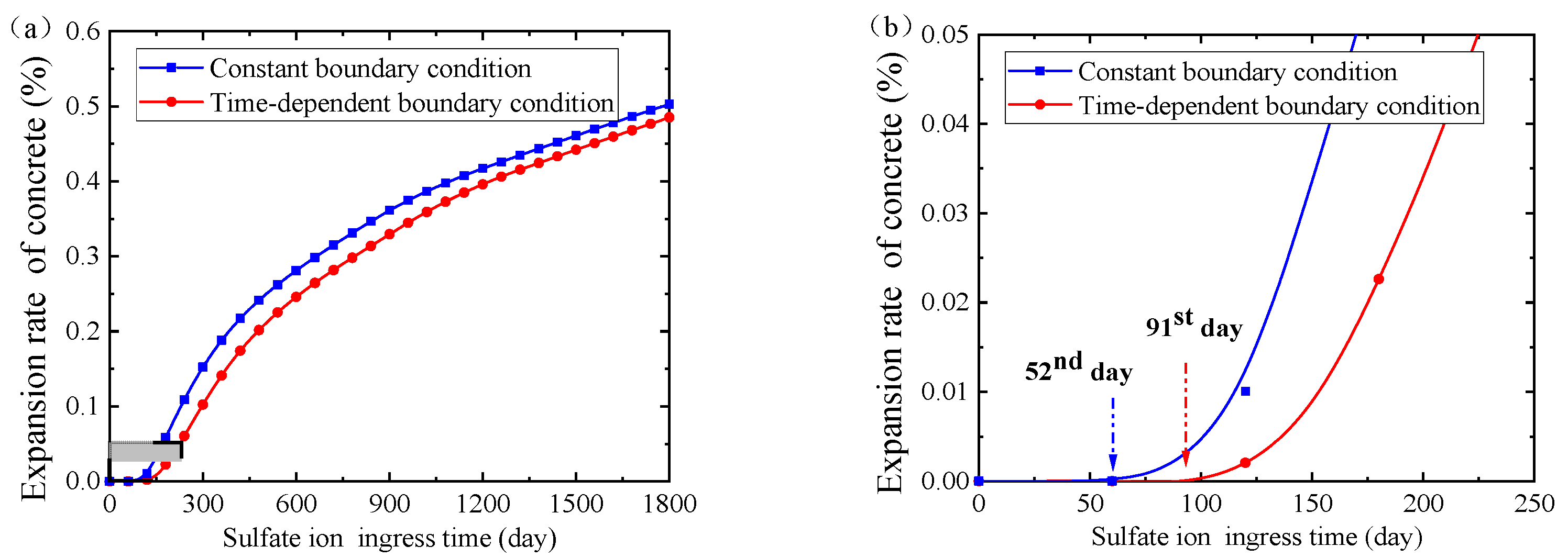
4.2. Expansion Rate of Concrete
5. Result Analysis and Discussion
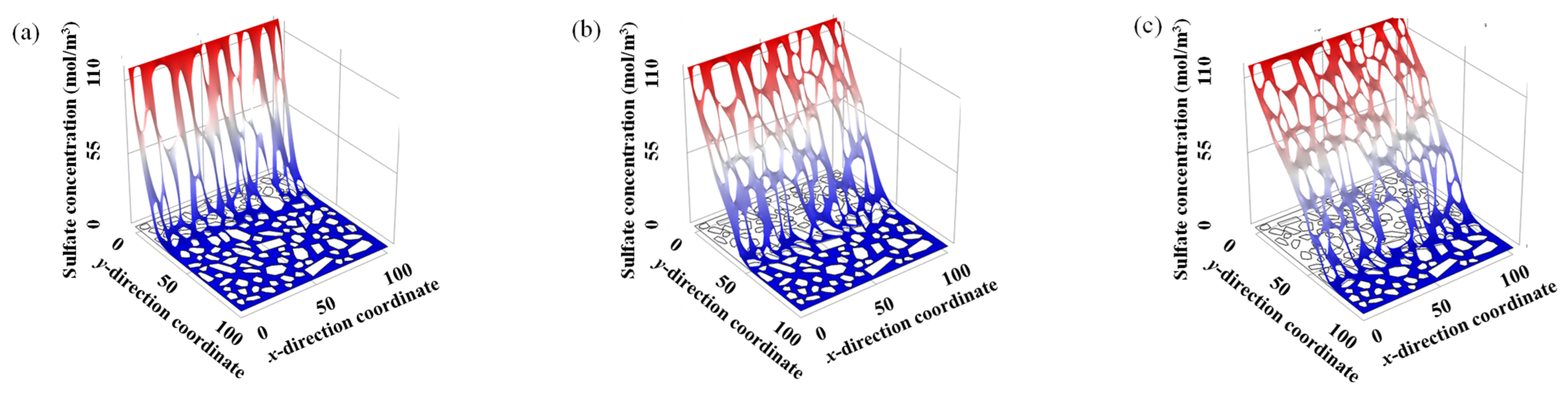
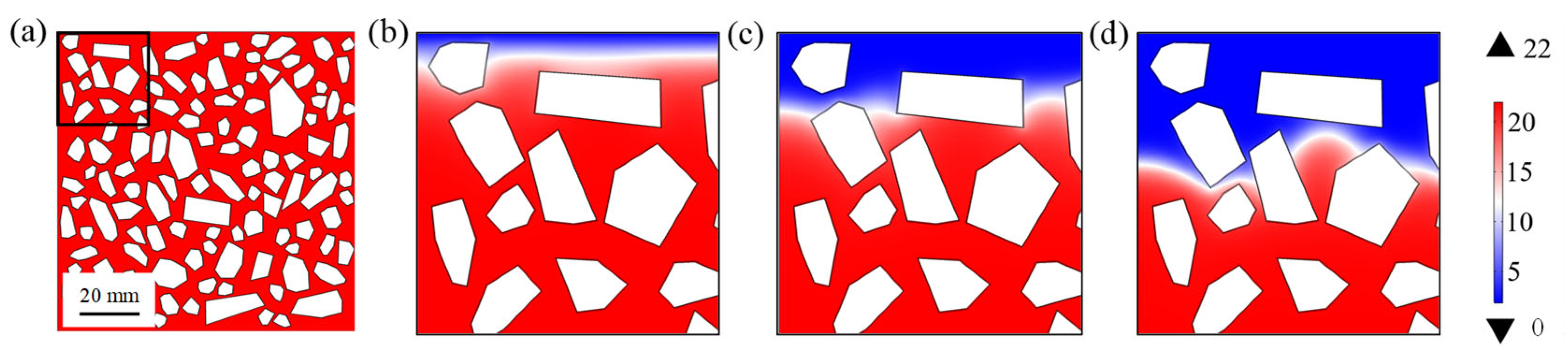
5.1. Sulfate Ion Diffusion
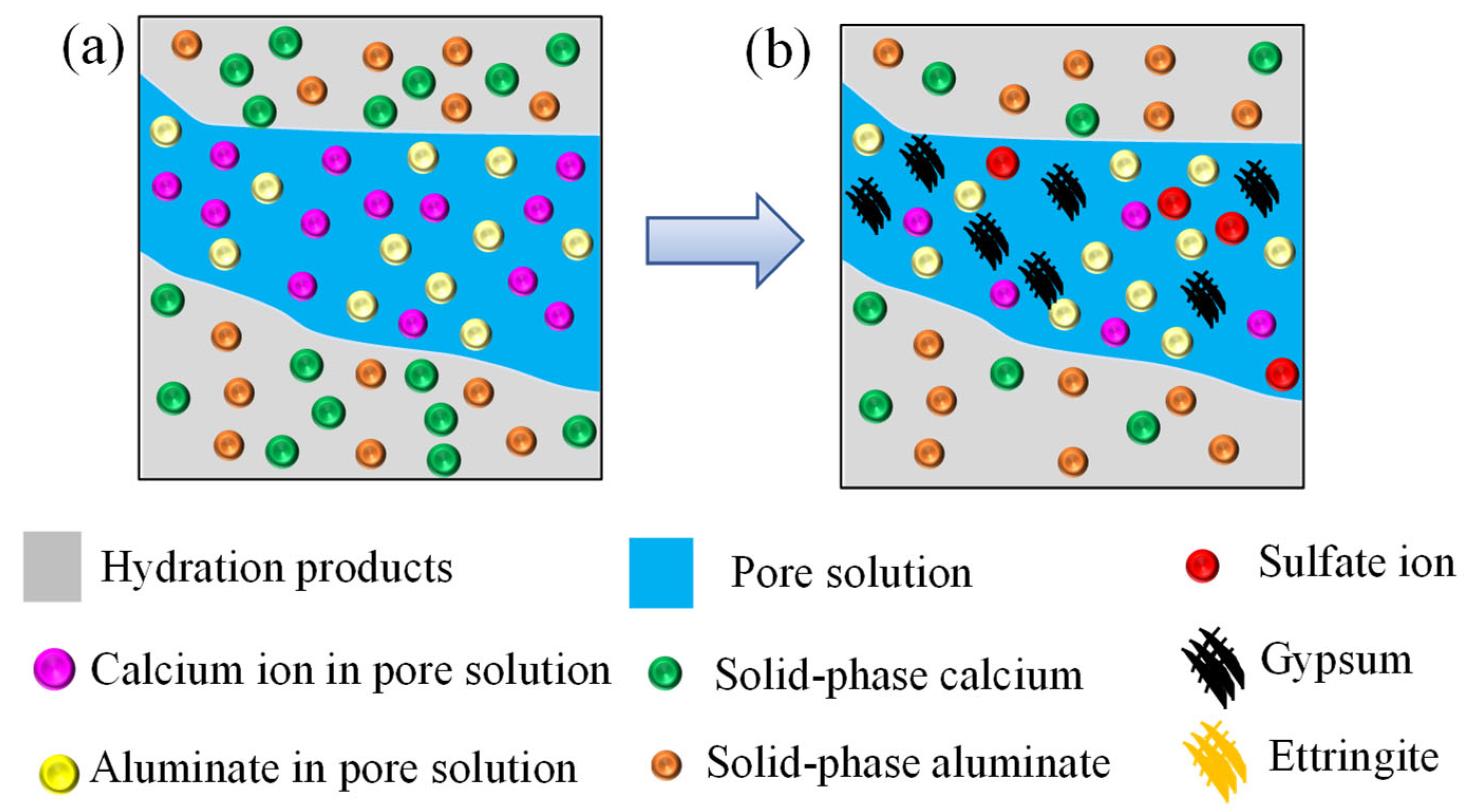
5.2. Influence of Solid-Phase Calcium Leaching
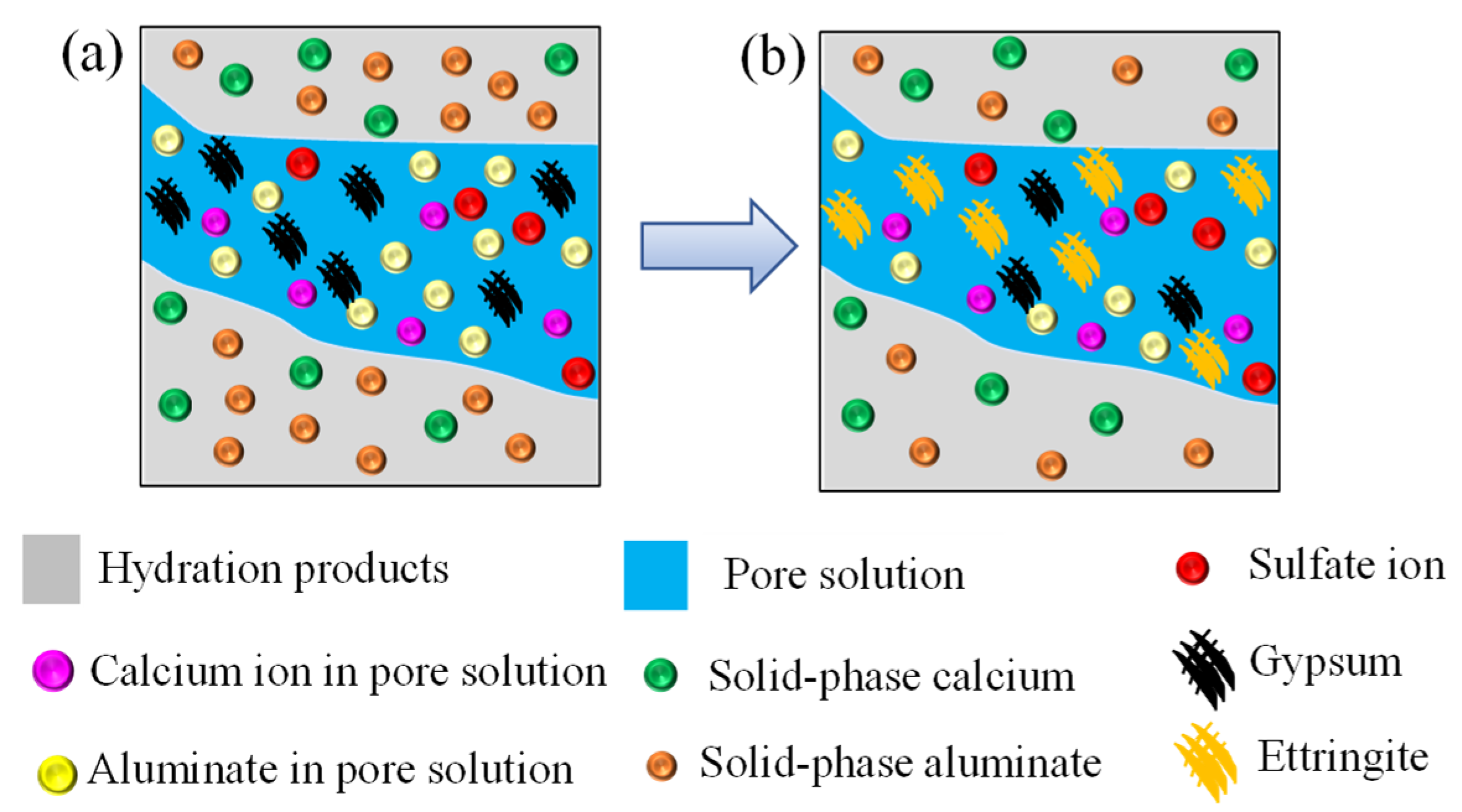
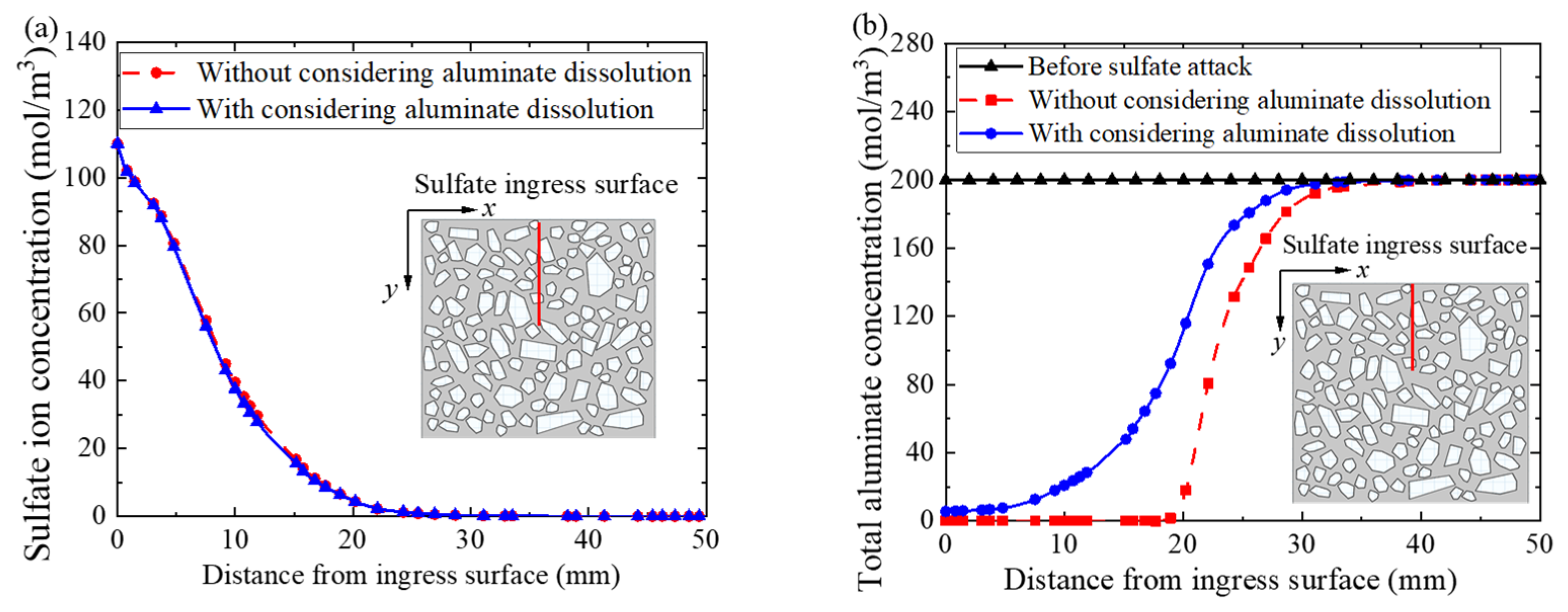
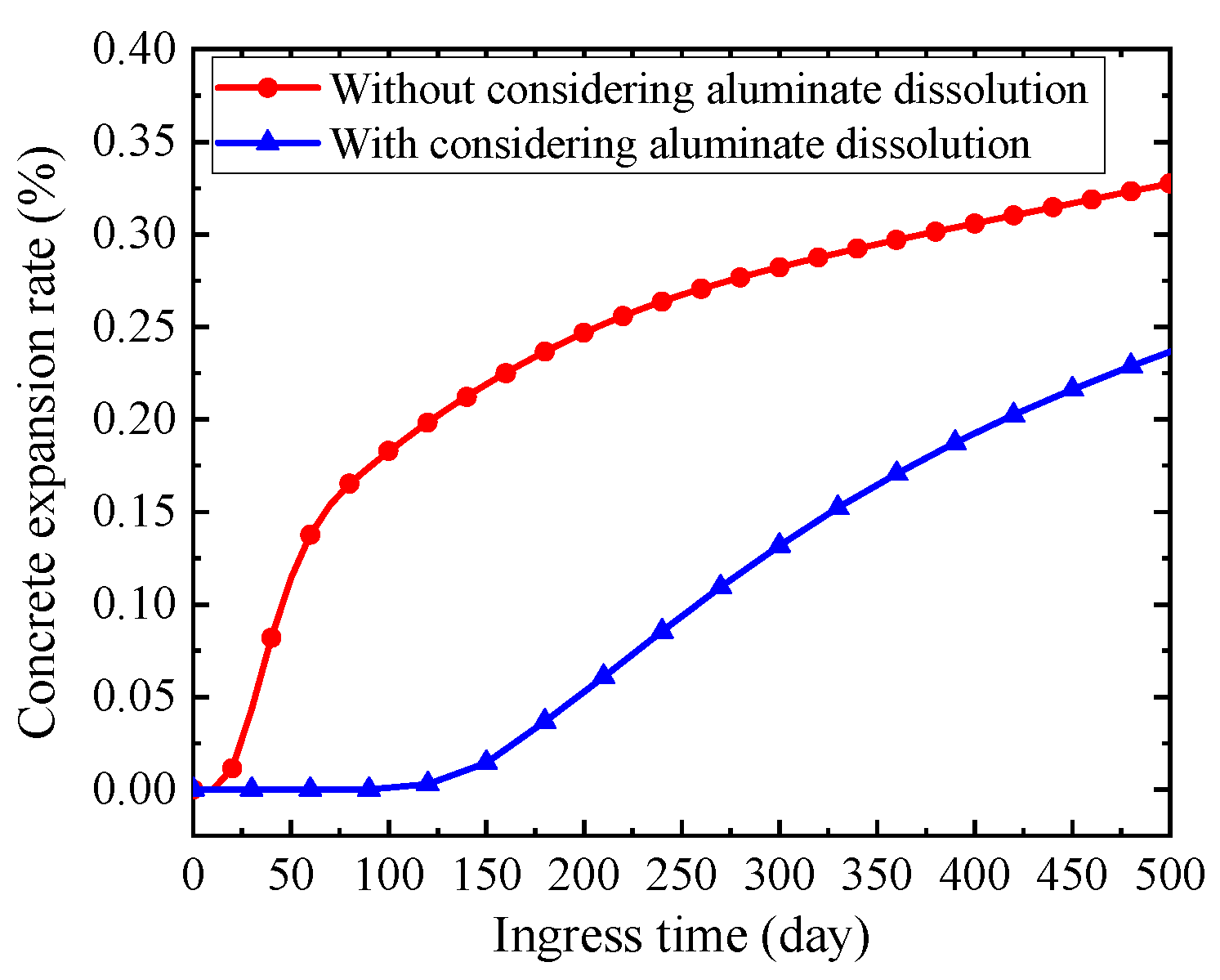
5.3. Influence of Solid-Phase Aluminate Dissolution
5.4. Influence of Boundary Sulfate Ion Concentration
6. Conclusions
- (1)
- With the increase of sulfate ingress time, the calcium ion and the solid-phase calcium near the sulfate ingress surface are completely consumed, which promote the diffusion of sulfate ion.
- (2)
- The dissolution of solid-phase aluminate has little influence on the distribution of sulfate ion concentration. However, the concrete expansion rate is overestimated if the dissolution of solid-phase aluminate is not modeled in the simulation.
- (3)
- For short-term material performance assessment, the sulfate attack ability and the concrete expansion rate are overestimated if the time-dependent boundary of sulfate concentration is not taken into consideration.
- (4)
- The sulfate ion in the mortar zone tends to diffuse into the ITZ, rather than directly diffuses in the mortar zone, indicating that ITZ is a fast channel for sulfate ion diffusion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulzar, M.A.; Rahim, A.; Ali, B.; Khan, A.H. An investigation on recycling potential of sulfur concrete. J. Build. Eng. 2021, 38, 102175. [Google Scholar] [CrossRef]
- Bankir, M.B.; Korkut Sevim, U. Performance optimization of hybrid fiber concretes against acid and sulfate attack. J. Build. Eng. 2020, 32, 101443. [Google Scholar] [CrossRef]
- Yin, G.-J.; Zuo, X.-B.; Sun, X.-H.; Tang, Y.-J. Macro-microscopically numerical analysis on expansion response of hardened cement paste under external sulfate attack. Constr. Build. Mater. 2019, 207, 600–615. [Google Scholar] [CrossRef]
- Nakarai, K.; Ishida, T.; Maekawa, K. Modeling of Calcium Leaching from Cement Hydrates Coupled with Micro-Pore Formation. J. Adv. Concr. Technol. 2006, 4, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Samson, E.; Marchand, J. Modeling the transport of ions in unsaturated cement-based materials. Comput. Struct. 2007, 85, 1740–1756. [Google Scholar] [CrossRef]
- Idiart, A.E.; López, C.M.; Carol, I. Chemo-mechanical analysis of concrete cracking and degradation due to external sulfate attack: A meso-scale model. Cem. Concr. Compos. 2011, 33, 411–423. [Google Scholar] [CrossRef]
- Yin, G.-J.; Zuo, X.-B.; Li, X.-N.; Zou, Y.-X. An integrated macro-microscopic model for concrete deterioration under external sulfate attack. Eng. Fract. Mech. 2020, 240, 107345. [Google Scholar] [CrossRef]
- Krajcinovic, D.; Basista, M.; Mallick, K.; Sumarac, D. Chemo-micromechanics of brittle solids. J. Mech. Phys. Solids 1992, 40, 965–990. [Google Scholar] [CrossRef]
- Sarkar, S.; Mahadevan, S.; Meeussen, J.C.L.; van der Sloot, H.; Kosson, D.S. Numerical simulation of cementitious materials degradation under external sulfate attack. Cem. Concr. Compos. 2010, 32, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Zhang, Y.X.; Khennane, A. Numerical modelling of degradation of cement-based materials under leaching and external sulfate attack. Comput. Struct. 2015, 158, 1–14. [Google Scholar] [CrossRef]
- Xie, F.; Li, J.; Li, L.; Zhao, G.; Yao, M. Numerical solution and damage evaluation for cast-in-situ piles exposed to external sulfate attack. Constr. Build. Mater. 2019, 214, 269–279. [Google Scholar] [CrossRef]
- Liu, P.; Chen, Y.; Yu, Z.; Lu, Z.; Shi, W. Evolution of the dynamic properties of concrete in a sulfate environment. Constr. Build. Mater. 2020, 245, 118468. [Google Scholar] [CrossRef]
- Ren, J.; Lai, Y.; Bai, R.; Qin, Y. The damage mechanism and failure prediction of concrete under wetting–drying cycles with sodium sulfate solution. Constr. Build. Mater. 2020, 264, 120525. [Google Scholar] [CrossRef]
- Li, J.; Xie, F.; Zhao, G.; Li, L. Experimental and numerical investigation of cast-in-situ concrete under external sulfate attack and drying-wetting cycles. Constr. Build. Mater. 2020, 249, 118789. [Google Scholar] [CrossRef]
- Zuo, X.-B.; Sun, W.; Li, H.; Zhao, Y.-K. Modeling of diffusion-reaction behavior of sulfate ion in concrete under sulfate environments. Comput. Concr. 2012, 10, 79–93. [Google Scholar] [CrossRef]
- Yin, G.-J.; Zuo, X.-B.; Tang, Y.-J.; Ayinde, O.; Wang, J.-L. Numerical simulation on time-dependent mechanical behavior of concrete under coupled axial loading and sulfate attack. Ocean Eng. 2017, 142, 115–124. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Mu, S.; Šavija, B.; Liu, Q. Numerical investigation of external sulfate attack and its effect on chloride binding and diffusion in concrete. Constr. Build. Mater. 2021, 285, 122806. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, W.; Feng, Y.; Castel, A.; Chen, X.; Liu, A. On the competitive antagonism effect in combined chloride-sulfate attack: A numerical exploration. Cem. Concr. Res. 2021, 144, 106406. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, L.; Bindiganavile, V.; Yi, C. Coupled models to describe the combined diffusion-reaction behaviour of chloride and sulphate ions in cement-based systems. Constr. Build. Mater. 2020, 243, 118232. [Google Scholar] [CrossRef]
- Zou, D.; Qin, S.; Liu, T.; Jivkov, A. Experimental and numerical study of the effects of solution concentration and temperature on concrete under external sulfate attack. Cem. Concr. Res. 2021, 139, 106284. [Google Scholar] [CrossRef]
- Phung, Q.T.; Maes, N.; Jacques, D.; Perko, J.; De Schutter, G.; Ye, G. Modelling the evolution of microstructure and transport properties of cement pastes under conditions of accelerated leaching. Constr. Build. Mater. 2016, 115, 179–192. [Google Scholar] [CrossRef]
- Kurumisawa, K.; Haga, K.; Hayashi, D.; Owada, H. Effects of calcium leaching on diffusion properties of hardened and altered cement pastes. Phys. Chem. Earth Parts A/B/C 2017, 99, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Mahadevan, S.; Meeussen, J.C.L.; van der Sloot, H.; Kosson, D.S. Sensitivity Analysis of Damage in Cement Materials under Sulfate Attack and Calcium Leaching. J. Mater. Civ. Eng. 2012, 24, 430–440. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Tian, Z.; Ma, J.; Jing, P.; Song, Z. Study on leaching damage mechanism of calcium ions of reactive powder concrete (RPC) under ion corrosion. Constr. Build. Mater. 2021, 269, 121303. [Google Scholar] [CrossRef]
- Jain, J.; Neithalath, N. Analysis of calcium leaching behavior of plain and modified cement pastes in pure water. Cem. Concr. Compos. 2009, 31, 176–185. [Google Scholar] [CrossRef]
- Mainguy, M.; Tognazzi, C.; Torrenti, J.-M.; Adenot, F. Modelling of leaching in pure cement paste and mortar. Cem. Concr. Res. 2000, 30, 83–90. [Google Scholar] [CrossRef]
- Wan, K.; Li, Y.; Sun, W. Experimental and modelling research of the accelerated calcium leaching of cement paste in ammonium nitrate solution. Constr. Build. Mater. 2013, 40, 832–846. [Google Scholar] [CrossRef]
- Geng, G.; Myers, R.J.; Yu, Y.-S.; Shapiro, D.A.; Winarski, R.; Levitz, P.E.; Kilcoyne, D.A.L.; Monteiro, P.J.M. Synchrotron X-ray nanotomographic and spectromicroscopic study of the tricalcium aluminate hydration in the presence of gypsum. Cem. Concr. Res. 2018, 111, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Feng, P.; Liu, Y.; Liu, J.; Bullard, J.W. In situ nano-scale observation of C3A dissolution in water. Cem. Concr. Res. 2020, 132, 106044. [Google Scholar] [CrossRef]
- Brand, A.S.; Feldman, S.B.; Stutzman, P.E.; Ievlev, A.V.; Lorenz, M.; Pagan, D.C.; Nair, S.; Gorham, J.M.; Bullard, J.W. Dissolution and initial hydration behavior of tricalcium aluminate in low activity sulfate solutions. Cem. Concr. Res. 2020, 130, 105989. [Google Scholar] [CrossRef]
- Ye, S.; Feng, P.; Liu, Y.; Liu, J.; Bullard, J.W. Dissolution and early hydration of tricalcium aluminate in aqueous sulfate solutions. Cem. Concr. Res. 2020, 137, 106191. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X.; Qian, J.; Shen, X. Effect of protogenetic alkali sulfates on the hydration and hardening of cement with different tricalcium aluminate content. Constr. Build. Mater. 2020, 256, 119475. [Google Scholar] [CrossRef]
- Li, X.; Li, O.X.; Rao, F.; Song, S.; Ortiz-Lara, N.; Aguilar-Reyes, E.A. Microstructural evolution in sulfate solutions of alkali-activated binders synthesized at various calcium contents. J. Mater. Res. Technol. 2020, 9, 10377–10385. [Google Scholar] [CrossRef]
- Basista, M.; Weglewski, W. Chemically Assisted Damage of Concrete: A Model of Expansion Under External Sulfate Attack. Int. J. Damage Mech. 2009, 18, 155–175. [Google Scholar] [CrossRef]
- Zuo, X.-B.; Sun, W.; Yu, C. Numerical investigation on expansive volume strain in concrete subjected to sulfate attack. Constr. Build. Mater. 2012, 36, 404–410. [Google Scholar] [CrossRef]
- Cefis, N.; Comi, C. Chemo-mechanical modelling of the external sulfate attack in concrete. Cem. Concr. Res. 2017, 93, 57–70. [Google Scholar] [CrossRef]
- Beddoe, R.E.; Dorner, H.W. Modelling acid attack on concrete: Part I. The essential mechanisms. Cem. Concr. Res. 2005, 35, 2333–2339. [Google Scholar] [CrossRef]
- Oliveira, I.; Cavalaro, S.H.P.; Aguado, A. New kinetic model to quantify the internal sulfate attack in concrete. Cem. Concr. Res. 2013, 43, 95–104. [Google Scholar] [CrossRef]
- Campos, A.; López, C.M.; Aguado, A. Diffusion–reaction model for the internal sulfate attack in concrete. Constr. Build. Mater. 2016, 102, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Zou, D.; Liu, T.; Jivkov, A. A chemo-transport-damage model for concrete under external sulfate attack. Cem. Concr. Res. 2020, 132, 106048. [Google Scholar] [CrossRef]
- Kuhl, D.; Bangert, F.; Meschke, G. Coupled chemo-mechanical deterioration of cementitious materials. Part I: Modeling. Int. J. Solids Struct. 2004, 41, 15–40. [Google Scholar] [CrossRef]
- Beddoe, R.E. Modelling acid attack on concrete: Part II. A computer model. Cem. Concr. Res. 2016, 88, 20–35. [Google Scholar] [CrossRef]
- Oey, T.; La Plante, E.C.; Falzone, G.; Hsiao, Y.-H.; Wada, A.; Monfardini, L.; Bauchy, M.; Bullard, J.W.; Sant, G. Calcium nitrate: A chemical admixture to inhibit aggregate dissolution and mitigate expansion caused by alkali-silica reaction. Cem. Concr. Compos. 2020, 110, 103592. [Google Scholar] [CrossRef]
- Li, C.; Song, X.; Jiang, L. A time-dependent chloride diffusion model for predicting initial corrosion time of reinforced concrete with slag addition. Cem. Concr. Res. 2021, 145, 106455. [Google Scholar] [CrossRef]
- Min, H.; Sui, L.; Xing, F.; Tian, H.; Zhou, Y. An effective transport model of sulfate attack in concrete. Constr. Build. Mater. 2019, 216, 365–378. [Google Scholar] [CrossRef]
- Sun, D.; Wu, K.; Shi, H.; Zhang, L.; Zhang, L. Effect of interfacial transition zone on the transport of sulfate ions in concrete. Constr. Build. Mater. 2018, 192, 28–37. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Z.; Zhang, J.; Zhao, J. Study on bond-slip degradation model of CFRP and concrete interface under sulfate erosion environment. Compos. Struct. 2021, 267, 113877. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Munir, M.J.; Wu, Y.-F.; Patnaikuni, I.; Zhou, Y.; Xing, F. Effect of different aggregate treatment techniques on the freeze-thaw and sulfate resistance of recycled aggregate concrete. Cold Reg. Sci. Technol. 2020, 178, 103126. [Google Scholar] [CrossRef]
- Bary, B.; Leterrier, N.; Deville, E.; Le Bescop, P. Coupled chemo-transport-mechanical modelling and numerical simulation of external sulfate attack in mortar. Cem. Concr. Compos. 2014, 49, 70–83. [Google Scholar] [CrossRef]
- Tixier, R.; Mobasher, B. Modeling of Damage in Cement-Based Materials Subjected to External Sulfate Attack. I: Formulation. J. Mater. Civ. Eng. 2003, 15, 305–313. [Google Scholar] [CrossRef]
- Nie, Q.; Zhou, C.; Li, H.; Shu, X.; Gong, H.; Huang, B. Numerical simulation of fly ash concrete under sulfate attack. Constr. Build. Mater. 2015, 84, 261–268. [Google Scholar] [CrossRef]
- Kunther, W.; Lothenbach, B.; Scrivener, K.L. On the relevance of volume increase for the length changes of mortar bars in sulfate solutions. Cem. Concr. Res. 2013, 46, 23–29. [Google Scholar] [CrossRef]
- Minard, H.; Garrault, S.; Regnaud, L.; Nonat, A. Mechanisms and parameters controlling the tricalcium aluminate reactivity in the presence of gypsum. Cem. Concr. Res. 2007, 37, 1418–1426. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, M.; Hou, D.; Li, Z. External sulfate attack to reinforced concrete under drying-wetting cycles and loading condition: Numerical simulation and experimental validation by ultrasonic array method. Constr. Build. Mater. 2017, 139, 365–373. [Google Scholar] [CrossRef]
- Quennoz, A.; Scrivener, K.L. Hydration of C 3A-gypsum systems. Cem. Concr. Res. 2012, 42, 1032–1041. [Google Scholar] [CrossRef]
- Choi, Y.S.; Yang, E.I. Effect of calcium leaching on the pore structure, strength, and chloride penetration resistance in concrete specimens. Nucl. Eng. Des. 2013, 259, 126–136. [Google Scholar] [CrossRef]
- Zhang, T.; Samson, E. Effect of Temperature on Ionic Transport Properties of Concrete. In Proceedings of the ConMAT Conference, Vancouver, BC, Canada, 22 August 2006. [Google Scholar]
- Peng, J.; Hu, S.; Zhang, J.; Cai, C.S.; Li, L. Influence of cracks on chloride diffusivity in concrete: A five-phase mesoscale model approach. Constr. Build. Mater. 2019, 197, 587–596. [Google Scholar] [CrossRef]
- Maltais, Y.; Samson, E.; Marchand, J. Predicting the durability of Portland cement systems in aggressive environments—Laboratory validation. Cem. Concr. Res. 2004, 34, 1579–1589. [Google Scholar] [CrossRef]
- Charlaix, E. Percolation threshold of a random array of discs: A numerical simulation. J. Phys. A Math. Gen. 1986, 19, L533–L536. [Google Scholar] [CrossRef]
- Somette, D. Critical transport and failure in continuum crack percolation. J. Phys. 1988, 49, 1365–1377. [Google Scholar] [CrossRef]
- Tzevelekou, T.; Lampropoulou, P.; Giannakopoulou, P.P.; Rogkala, A.; Koutsovitis, P.; Koukouzas, N.; Petrounias, P. Valorization of Slags Produced by Smelting of Metallurgical Dusts and Lateritic Ore Fines in Manufacturing of Slag Cements. Appl. Sci. 2020, 10, 4670. [Google Scholar] [CrossRef]
- Petrounias, P.; Giannakopoulou, P.P.; Rogkala, A.; Lampropoulou, P.; Tsikouras, B.; Rigopoulos, I.; Hatzipanagiotou, K. Petrographic and Mechanical Characteristics of Concrete Produced by Different Type of Recycled Materials. Geosciences 2019, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Fu, F.; Wang, H.; Liang, Q.; Yu, A.; Qian, K.; Chen, P. A multi-phase mesoscopic simulation model for the long-term chloride ingress and electrochemical chloride extraction. Constr. Build. Mater. 2021, 270, 121826. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Yamada, K.; Johannesson, B.; Nilsson, L.-O. Development of a multi-species mass transport model for concrete with account to thermodynamic phase equilibriums. Mater. Struct. 2011, 44, 1577–1592. [Google Scholar] [CrossRef]
- Huang, Q.Z.W.; Zhou, C.; Gu, X. Experimental study on moisture transport property of interfacial transition zone in concrete. Jianzhu Jiegou Xuebao/J. Build 2019, 40, 174–180. [Google Scholar]
- Baji, H.; Yang, W.; Li, C.-Q.; Shi, W. Analytical models for effective hydraulic sorptivity, diffusivity and conductivity of concrete with interfacial transition zone. Constr. Build. Mater. 2019, 225, 555–568. [Google Scholar] [CrossRef]
- Liao, K.-X.; Zhang, Y.-P.; Zhang, W.-P.; Wang, Y.; Zhang, R.-L. Modeling constitutive relationship of sulfate-attacked concrete. Constr. Build. Mater. 2020, 260, 119902. [Google Scholar] [CrossRef]
- Bary, B.; Béjaoui, S. Assessment of diffusive and mechanical properties of hardened cement pastes using a multi-coated sphere assemblage model. Cem. Concr. Res. 2006, 36, 245–258. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, A.; Ruan, X. Spatial variability of chloride and its influence on thickness of concrete cover: A two-dimensional mesoscopic numerical research. Eng. Struct. 2015, 95, 154–169. [Google Scholar] [CrossRef]
- Mao, L.; Hu, Z.; Xia, J.; Feng, G.; Azim, I.; Yang, J.; Liu, Q. Multi-phase modelling of electrochemical rehabilitation for ASR and chloride affected concrete composites. Compos. Struct. 2019, 207, 176–189. [Google Scholar] [CrossRef]
- Chen, X.; Yu, A.; Liu, G.; Chen, P.; Liang, Q. A multi-phase mesoscopic simulation model for the diffusion of chloride in concrete under freeze–thaw cycles. Constr. Build. Mater. 2020, 265, 120223. [Google Scholar] [CrossRef]
- Wee, T.H.; Suryavanshi, A.K.; Wong, S.F.; Anisur Rahman, A.K.M. Sulfate resistance of concrete containing mineral admixtures. Mater. J. 2000, 97, 536–549. [Google Scholar]
- Qi, B.; Gao, J.; Chen, F.; Shen, D. Evaluation of the damage process of recycled aggregate concrete under sulfate attack and wetting-drying cycles. Constr. Build. Mater. 2017, 138, 254–262. [Google Scholar] [CrossRef]
- Rozière, E.; Loukili, A.; El Hachem, R.; Grondin, F. Durability of concrete exposed to leaching and external sulphate attacks. Cem. Concr. Res. 2009, 39, 1188–1198. [Google Scholar] [CrossRef] [Green Version]




| Parameters | Value | References |
|---|---|---|
| Diffusion coefficient of sulfate ion, | 2.7 × 10−11 [m2/s] | [5] |
| Chemical reaction rate constant in Equation (1), | 3.05 × 10−8 [mol/m3 s] | [61] |
| Calcium ion concentration in the saturated liquid phase, | 22 [mol/m3] | [35] |
| Diffusion coefficient of calcium ion, | 2.7 × 10−11 [m2/s] | [5] |
| Chemical reaction rate constant in Equation (9), | 1.22 × 10−9 [mol/m3 s] | [61] |
| Diffusion coefficient of aluminate, | 2.7 × 10−11 [m2/s] | * |
| Aluminate concentration in the saturated liquid phase, | 22 [mol/m3] | * |
| Model parameters in Equation (12), k | 0.16 [--] | [16] |
| Model parameters in Equation (12), m | 2.3 [--] | [16] |
| Volume fraction of the initial porosity being filled before the expansion0. in Equation (13) | 0.05~0.4 [--] | [40] |
| Volume change rate of aluminate consumed per unit concentration, | 1 × 10−4~1 × 10−3 [m3/mol] | [73] |
| Volume change rate of leaching unit calcium ions, | 3.3 × 10−5 [m3/mol] | [61] |
| Threshold strain at which the microcracks start forming, | 4 × 10−5 [--] | [16] |
| Cs,max [mol/m3] | CCA0 [mol/m3] | CCH0 [mol/m3] | CCSH0 [mol/m3] | q | f | |
|---|---|---|---|---|---|---|
| 110 | 149.6 | 1445 | 4842 | 3 | 0.23 |
| Cs,max [mol/m3] | CCA0 [mol/m3] | CCH0 [mol/m3] | CCSH0 [mol/m3] | as [mol/m3] | q | f |
|---|---|---|---|---|---|---|
| 201 | 141.3 | 1902 | 1807 | 3 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Gu, X.; Xia, X.; Li, X.; Zhang, Q. A Chemical-Transport-Mechanics Numerical Model for Concrete under Sulfate Attack. Materials 2021, 14, 7710. https://doi.org/10.3390/ma14247710
Chen X, Gu X, Xia X, Li X, Zhang Q. A Chemical-Transport-Mechanics Numerical Model for Concrete under Sulfate Attack. Materials. 2021; 14(24):7710. https://doi.org/10.3390/ma14247710
Chicago/Turabian StyleChen, Xuandong, Xin Gu, Xiaozhou Xia, Xing Li, and Qing Zhang. 2021. "A Chemical-Transport-Mechanics Numerical Model for Concrete under Sulfate Attack" Materials 14, no. 24: 7710. https://doi.org/10.3390/ma14247710






