Photocurable Coatings Based on Bio-Renewable Oligomers and Monomers
Abstract
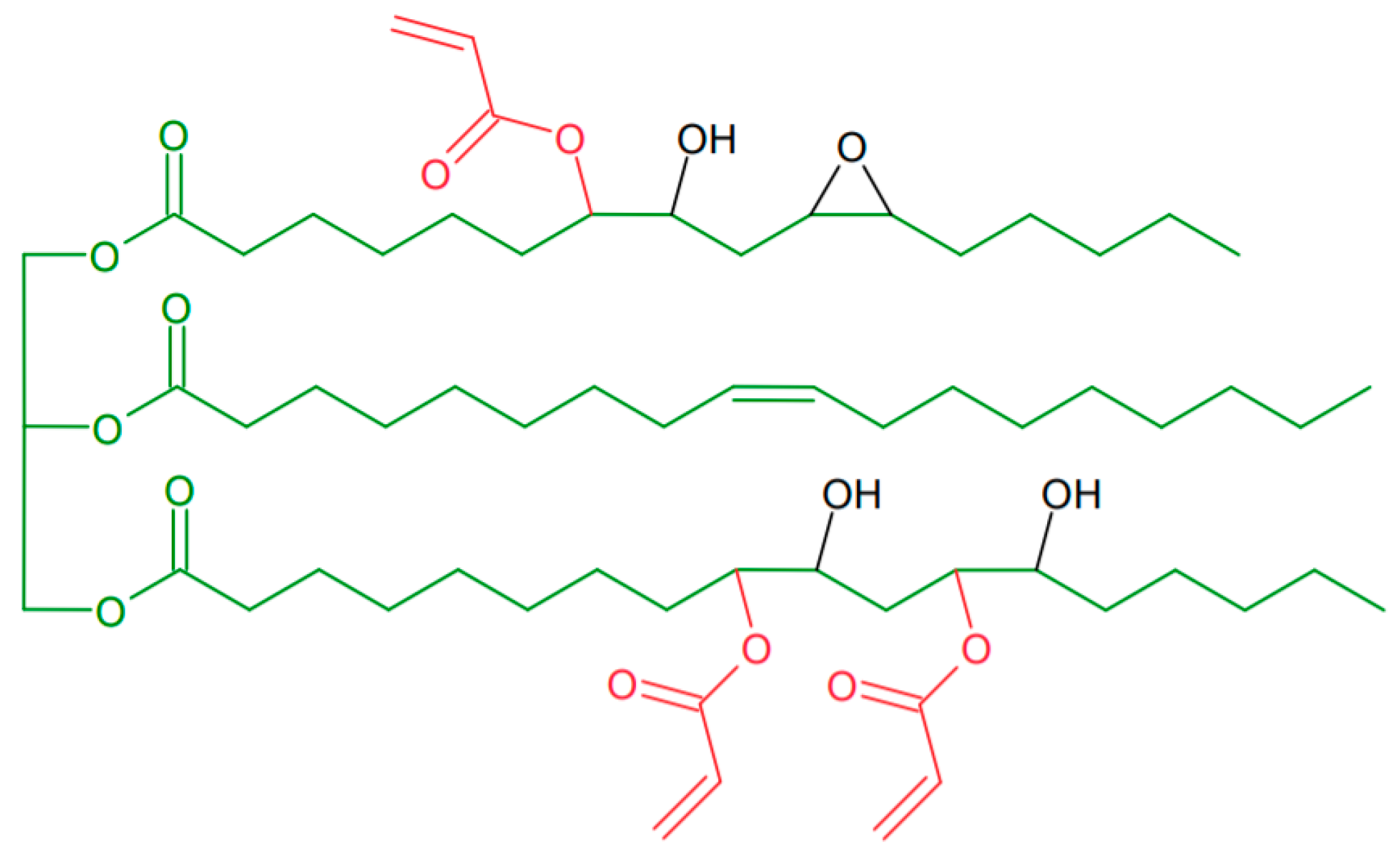
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coatings
2.3. Characteristics of the Photopolymerization Process
2.4. Properties of Cured Coatings
3. Results and Discussion
3.1. Modification of AESO-Based Coatings
3.2. Photocuring Process of Modified AESO-Based Coatings
3.3. Properties of Cured Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, T.Y.; Roper, T.M.; Jonsson, E.S.; Guymon, C.A.; Hoyle, C.E. Thiol−Ene Photopolymerization Kinetics of Vinyl Acrylate/Multifunctional Thiol Mixtures. Macromolecules 2004, 37, 3606–3613. [Google Scholar] [CrossRef]
- Senyurt, A.F.; Wei, H.; Phillips, B.; Cole, M.; Nazarenko, S.; Hoyle, C.E.; Piland, S.G.; Gould, T.E. Physical and Mechanical Properties of Photopolymerized Thiol−Ene/Acrylates. Macromolecules 2006, 39, 6315–6317. [Google Scholar] [CrossRef]
- Chen, Z.; Chisholm, B.J.; Patani, R.; Wu, J.F.; Fernando, S.; Jogodzinski, K.; Webster, D.C. Soy-based UV-curable thiol-ene coatings. J. Coat. Technol. Res. 2010, 7, 603–613. [Google Scholar] [CrossRef]
- Liu, R.; Luo, J.; Ariyasivam, S.; Liu, X.; Chen, Z. High biocontent natural plant oil based UV-curable branched oligomers. Prog. Org. Coat. 2017, 105, 143–148. [Google Scholar] [CrossRef]
- Gandini, A. The irruption of polymers from renewable resources on the scene of macromolecular science and technology. Green Chem. 2011, 13, 1061–1083. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, J.F.; Fernando, S.; Jagodzinski, K. Soy-based, high biorenewable content UV curable coatings. Prog. Org. Coat. 2011, 71, 98–109. [Google Scholar] [CrossRef]
- Raquez, J.M.; Deléglise, M.; Lacrampe, M.F.; Krawczak, P. Thermosetting (bio)materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 4, 487–509. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.P. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Noè, C.; Malburet, S.; Bouvet-Marchand, A.; Graillot, A.; Loubat, C.; Sangermano, M. Cationic photopolymerization of bio-renewable epoxidized monomers. Prog. Org. 2019, 133, 131–138. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Talacka, V.; Ostrauskaite, J. High biorenewable content acrylate photocurable resins for DLP 3D printing. J. Appl. Polym. Sci. 2021, 138, 50233. [Google Scholar] [CrossRef]
- Skliutas, E.; Lebedevaite, M.; Kasetaite, S.; Rekštytė, S.; Lileikis, S.; Ostrauskaite, J.; Malinauskas, M. A Bio-Based Resin for a Multi-Scale Optical 3D Printing. Sci. Rep. 2020, 10, 9758. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Moxon, S.; Morris, G.A. Biopolymers as wound healing materials. Wound Heal. Biomater. 2016, 2, 261–287. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Bednarczyk, P.; Nowak, M.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Rokicka, J.; Klimowicz, A.; Czech, Z. Sustainable UV-Crosslinkable Acrylic Pressure-Sensitive Adhesives for Medical Application. Int. J. Mol. Sci. 2021, 22, 11840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liang, H.; Bu, J.; Xiong, L.; Huang, S.; Zhang, D.D.; Liang, H.B.; Bu, J.; Xiong, L.; Huang, S.M. UV curable soybean-oil hybrid systems based on thiol-acrylate and thiol-ene-acrylate chemistry. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Noè, C.; Hakkarainen, M.; Sangermano, M. Cationic UV-Curing of Epoxidized Biobased Resins. Polymer 2020, 13, 89. [Google Scholar] [CrossRef]
- Sharma, V.; Kundu, P.P. Addition polymers from natural oils—A review. Prog. Polym. Sci. 2006, 31, 983–1008. [Google Scholar] [CrossRef]
- Zubair, M.; Pradhan, R.A.; Arshad, M.; Ullah, A. Recent Advances in Lipid Derived Bio-Based Materials for Food Packaging Applications. Macromol. Mater. Eng. 2021, 306, 2000799. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.J. The use of renewable feedstock in UV-curable materials—A new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef]
- Pelletier, H.; Belgacem, N.; Gandini, A. Acrylated vegetable oils as photocrosslinkable materials. J. Appl. Polym. Sci. 2006, 99, 3218–3221. [Google Scholar] [CrossRef]
- Lu, J.; Khot, S.; Wool, R.P. New sheet molding compound resins from soybean oil. I. Synthesis and characterization. Polymer 2005, 46, 71–80. [Google Scholar] [CrossRef]
- De Espinosa, L.M.; Ronda, J.C.; Galià, M.; Cádiz, V. A new route to acrylate oils: Crosslinking and properties of acrylate triglycerides from high oleic sunflower oil. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1159–1167. [Google Scholar] [CrossRef]
- Fu, C.; Yang, Z.; Zheng, Z.; Shen, L. Properties of alkoxysilane castor oil synthesized via thiol-ene and its polyurethane/siloxane hybrid coating films. Prog. Org. Coat. 2014, 77, 1241–1248. [Google Scholar] [CrossRef]
- Luo, A.; Jiang, X.; Lin, H.; Yin, J. “Thiol-ene” photo-cured hybrid materials based on POSS and renewable vegetable oil. J. Mater. Chem. 2011, 21, 12753–12760. [Google Scholar] [CrossRef]
- He, M.; Jiang, S.; Xu, R.; Yang, J.; Zeng, Z.; Chen, G. Facile functionalization of soybean oil by thiol-ene photo-click reaction for the synthesis of polyfunctional acrylate. Prog. Org. Coat. 2014, 77, 868–871. [Google Scholar] [CrossRef]
- Chen, J.; Soucek, M.D.; Simonsick, W.J.; Celikay, R.W. Synthesis and photopolymerization of norbornyl epoxidized linseed oil. Polymer 2002, 43, 5379–5389. [Google Scholar] [CrossRef]
- Li, F.; Larock, R.C. Synthesis, Structure and Properties of New Tung Oil−Styrene−Divinylbenzene Copolymers Prepared by Thermal Polymerization. Biomacromolecules 2003, 4, 1018–1025. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, J.; Luo, J.; Liu, X. Synthesis and application of novel UV-curable hyperbranched methacrylates from renewable natural tannic acid. Prog. Org. Coat. 2014, 77, 30–37. [Google Scholar] [CrossRef]
- Robertson, M.L.; Chang, K.; Gramlich, W.M.; Hillmyer, M.A. Toughening of polylactide with polymerized soybean oil. Macromolecules 2010, 43, 1807–1814. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Soybean-Oil-Based Waterborne Polyurethane Dispersions: Effects of Polyol Functionality and Hard Segment Content on Properties. Biomacromolecules 2008, 9, 3332–3340. [Google Scholar] [CrossRef]
- Bajpai, M.; Shukla, V.; Singh, D.K.; Singh, M.; Shukla, R. A study of the film properties of pigmented UV-curable epoxidised soybean oil. Pigment. Resin Technol. 2004, 33, 160–164. [Google Scholar] [CrossRef]
- Pfister, D.P.; Baker, J.R.; Henna, P.H.; Lu, Y.; Larock, R.C. Preparation and properties of tung oil-based composites using spent germ as a natural filler. J. Appl. Polym. Sci. 2008, 108, 3618–3625. [Google Scholar] [CrossRef]
- Pan, X.; Webster, D.C. Impact of Structure and Functionality of Core Polyol in Highly Functional Biobased Epoxy Resins. Macromol. Rapid Commun. 2011, 32, 1324–1330. [Google Scholar] [CrossRef]
- Wang, S.J.; Liu, X.; Kong, J.; Tian, W.; Fan, X.D.; Xu, H.; Lu, J.R. Synthesis and UV curing kinetics of rapidly UV-curable hyperbranched polycarbosiloxanes. Polym. Int. 2010, 59, 1323–1330. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Synthesis of high-performance green nanocomposites from renewable natural oils. Polym. Degrad. Stab. 2010, 95, 1399–1405. [Google Scholar] [CrossRef]
- Black, M.; Rawlins, J.W. Thiol–ene UV-curable coatings using vegetable oil macromonomers. Eur. Polym. J. 2009, 45, 1433–1441. [Google Scholar] [CrossRef]
- Khot, S.N.; Lascala, J.J.; Can, E.; Morye, S.S.; Williams, G.I.; Palmese, G.R.; Kusefoglu, S.H.; Wool, R.P. Development and application of triglyceride-based polymers and composites. J. Appl. Polym. Sci. 2001, 82, 703–723. [Google Scholar] [CrossRef]
- Wang, C.; Ding, L.; He, M.; Wei, J.; Li, J.; Lu, R.; Xie, H.; Cheng, R. Facile one-step synthesis of bio-based AESO resins. Eur. J. Lipid Sci. Technol. 2016, 118, 1463–1469. [Google Scholar] [CrossRef]
- Miezinyte, G.; Ostrauskaite, J.; Rainosalo, E.; Skliutas, E.; Malinauskas, M. Photoresins based on acrylated epoxidized soybean oil and benzenedithiols for optical 3D printing. Rapid Prototyp. J. 2019, 25, 378–387. [Google Scholar] [CrossRef]
- Grauzeliene, S.; Valaityte, D.; Motiekaityte, G.; Ostrauskaite, J. Bio-Based Crosslinked Polymers Synthesized from Functionalized Soybean Oil and Squalene by Thiol–Ene UV Curing. Material 2021, 14, 2675. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, X.; Liu, R.; Liu, X.; Liu, J. Highly functional bio-based acrylates with a hard core and soft arms: From synthesis to enhancement of an acrylated epoxidized soybean oil-based UV-curable coating. Prog. Org. Coat. 2019, 134, 342–348. [Google Scholar] [CrossRef]
- Wu, J.; Wang, C.; Mu, C.; Lin, W. A waterborne polyurethane coating functionalized by isobornyl with enhanced antibacterial adhesion and hydrophobic property. Eur. Polym. J. 2018, 108, 498–506. [Google Scholar] [CrossRef]
- Droesbeke, M.A.; Simula, A.; Asua, J.M.; Du Prez, F.E. Biosourced terpenoids for the development of sustainable acrylic pressure-sensitive adhesives via emulsion polymerisation. Green Chem. 2020, 22, 4561–4569. [Google Scholar] [CrossRef]
- Kim, S.K.; Guymon, C.A. Effects of polymerizable organoclays on oxygen inhibition of acrylate and thiol-acrylate photopolymerization. Polymer (Guildf) 2012, 53, 1640–1650. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, P.; Liu, Y.H.; Shang, Z.R.; Hu, H.C.; Zhang, Z.H. Magnetically separable graphene oxide anchored sulfonic acid: A novel, highly efficient and recyclable catalyst for one-pot synthesis of 3,6-di(pyridin-3-yl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles in deep eutectic solvent under microwave irradiation. RSC Adv. 2016, 6, 106160–106170. [Google Scholar] [CrossRef]
- Ketonen, M. UV Curing of Hybrid Coatings the Sol-Gel Process; Tampere University of Applied Sciences: Tampere, Finland, 2011. [Google Scholar]
- Srivastava, A.; Agarwal, D.; Mistry, S.; Singh, J. UV curable polyurethane acrylate coatings for metal surfaces. Pigment. Resin Technol. 2008, 37, 217–223. [Google Scholar] [CrossRef]
- Uemura, Y.; Shimasaki, T.; Teramoto, N.; Shibata, M. Thermal and mechanical properties of bio-based polymer networks by thiol-ene photopolymerizations of gallic acid and pyrogallol derivatives. J. Polym. Res. 2016, 23, 1–10. [Google Scholar] [CrossRef]
- Konuray, O.; Fernández-Francos, X.; De la Flor, S.; Ramis, X.; Serra, À. The Use of Click-Type Reactions in the Preparation of Thermosets. Polymer 2020, 12, 1084. [Google Scholar] [CrossRef]
- Grauzeliene, S.; Navaruckiene, A.; Skliutas, E.; Malinauskas, M.; Serra, A.; Ostrauskaite, J. Vegetable Oil-Based Thiol-Ene/Thiol-Epoxy Resins for Laser Direct Writing 3D Micro-/Nano-Lithography. Polymer 2021, 13, 872. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, C.E.; Bowman, C.N. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]





| Samples | Weight Ratio (wt%) | Biobased Content (wt%) | ||||
|---|---|---|---|---|---|---|
| AESO | BioDA | BioMA | T | PI | ||
| A-100 | 100 | - | - | - | 3 | 56 |
| A-90 | 90 | - | 10 | - | 3 | 57.4 |
| A-80 | 80 | - | 20 | - | 3 | 58.8 |
| A-70 | 70 | - | 30 | - | 3 | 60.2 |
| A-60 | 60 | - | 40 | - | 3 | 61.6 |
| A-50 | 50 | - | 50 | - | 3 | 63 |
| B-75 | 75 | 25 | - | - | 3 | 57.5 |
| B-50 | 50 | 50 | - | - | 3 | 59 |
| B-25 | 25 | 75 | - | - | 3 | 60.5 |
| B-0 | - | 100 | - | - | 3 | 62 |
| C-95 | 95 | - | - | 5 | 3 | 53.2 |
| C-90 | 90 | - | - | 10 | 3 | 50.4 |
| C-80 | 80 | - | - | 20 | 3 | 44.8 |
| C-70 | 70 | - | - | 30 | 3 | 39.2 |
| C-60 | 60 | - | - | 40 | 3 | 33.6 |
| D-0.5 | 80 | 20 | - | - | 0.5 | 58.8 |
| D-1 | 80 | 20 | - | - | 1 | 58.8 |
| D-3 | 80 | 20 | - | - | 3 | 58.8 |
| D-5 | 80 | 20 | - | - | 5 | 58.8 |
| D-7.5 | 80 | 20 | - | - | 7.5 | 58.8 |
| D-10 | 80 | 20 | - | - | 10 | 58.8 |
| Sample | Tack-Free Time (s) | Pendulum Hardness | Crosscut Adhesion | Gloss (GU) | Yellowing Index |
|---|---|---|---|---|---|
| A-100 | - | 49 | 4 | 122 | 5.00 |
| A-90 | - | 66 | 2 | 132 | 4.66 |
| A-80 | - | 100 | 2 | 134 | 4.47 |
| A-70 | - | 128 | 1 | 135 | 4.35 |
| A-60 | - | 130 | 1 | 138 | 4.12 |
| A-50 | 9 | 133 | 1 | 140 | 4.35 |
| B-75 | - | 97 | 2 | 105 | 4.67 |
| B-50 | - | 134 | 3 | 103 | 4.42 |
| B-25 | - | 205 | 3 | 97 | 422 |
| B-0 | 9 | 260 | 3 | 91 | 3.95 |
| C-95 | - | 52 | 2 | 113 | 4.63 |
| C-90 | - | 52 | 2 | 111 | 455 |
| C-80 | - | 42 | 2 | 106 | 4.73 |
| C-70 | - | 36 | 1 | 103 | 4.61 |
| C-60 | 9 | 36 | 0 | 101 | 4.80 |
| D-0.5 | - | 53 | 1 | 38 | 4.40 |
| D-1 | - | 74 | 2 | 67 | 4.44 |
| D-3 | - | 100 | 2 | 134 | 4.47 |
| D-5 | - | 104 | 2 | 136 | 4.57 |
| D-7.5 | - | 116 | 3 | 138 | 4.87 |
| D-10 | - | 118 | 4 | 140 | 5.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bednarczyk, P.; Nowak, M.; Mozelewska, K.; Czech, Z. Photocurable Coatings Based on Bio-Renewable Oligomers and Monomers. Materials 2021, 14, 7731. https://doi.org/10.3390/ma14247731
Bednarczyk P, Nowak M, Mozelewska K, Czech Z. Photocurable Coatings Based on Bio-Renewable Oligomers and Monomers. Materials. 2021; 14(24):7731. https://doi.org/10.3390/ma14247731
Chicago/Turabian StyleBednarczyk, Paulina, Małgorzata Nowak, Karolina Mozelewska, and Zbigniew Czech. 2021. "Photocurable Coatings Based on Bio-Renewable Oligomers and Monomers" Materials 14, no. 24: 7731. https://doi.org/10.3390/ma14247731
APA StyleBednarczyk, P., Nowak, M., Mozelewska, K., & Czech, Z. (2021). Photocurable Coatings Based on Bio-Renewable Oligomers and Monomers. Materials, 14(24), 7731. https://doi.org/10.3390/ma14247731







