Microstructure Evolution and Mechanical Properties of As-Cast and As-Compressed ZM6 Magnesium Alloys during the Two-Stage Aging Treatment Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Initial Properties of As-Cast and As-Compressed ZM6
2.1.1. Casting Process, Chemical Composition, and Hot Compression
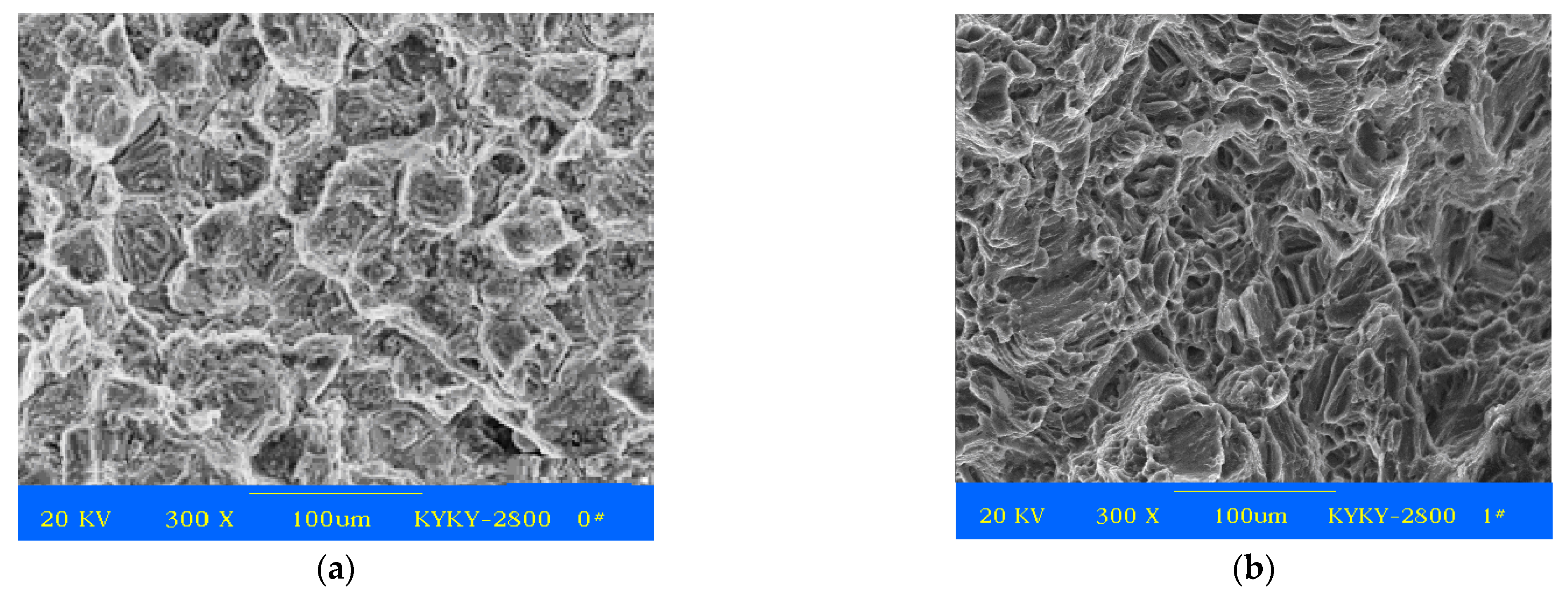
2.1.2. Mechanical Properties of As-Cast and As-Compressed ZM6
2.2. Experimental Heat Treatment Schedules and Property Measurements
2.2.1. Heat Treatment Schedules of As-Cast and As-Compressed ZM6
2.2.2. Microstructure Analysis and Mechanical Properties Measurement
3. Results and Discussion
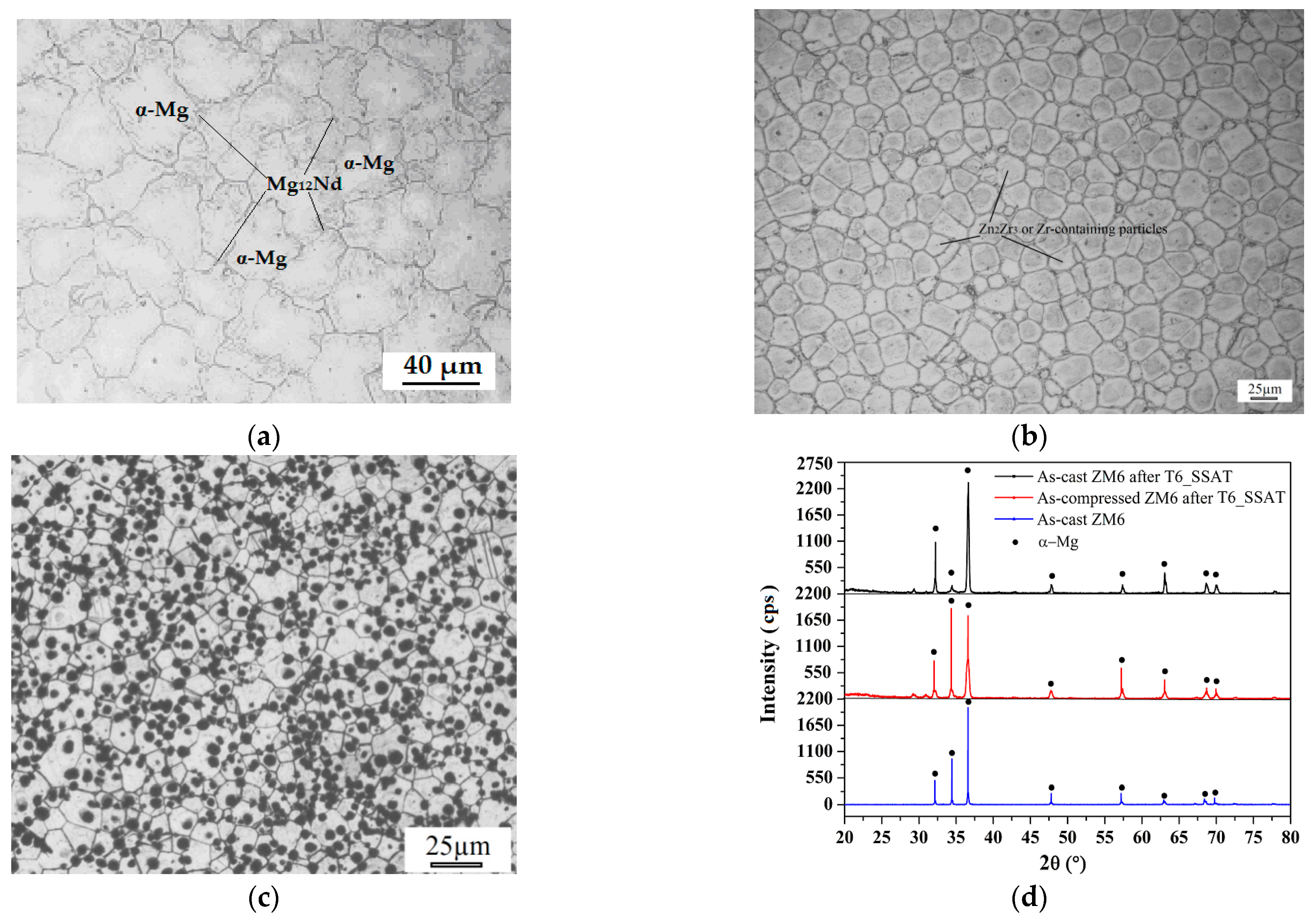
3.1. Analysis of the Alloy Phase Diagram and Initial Microstructures
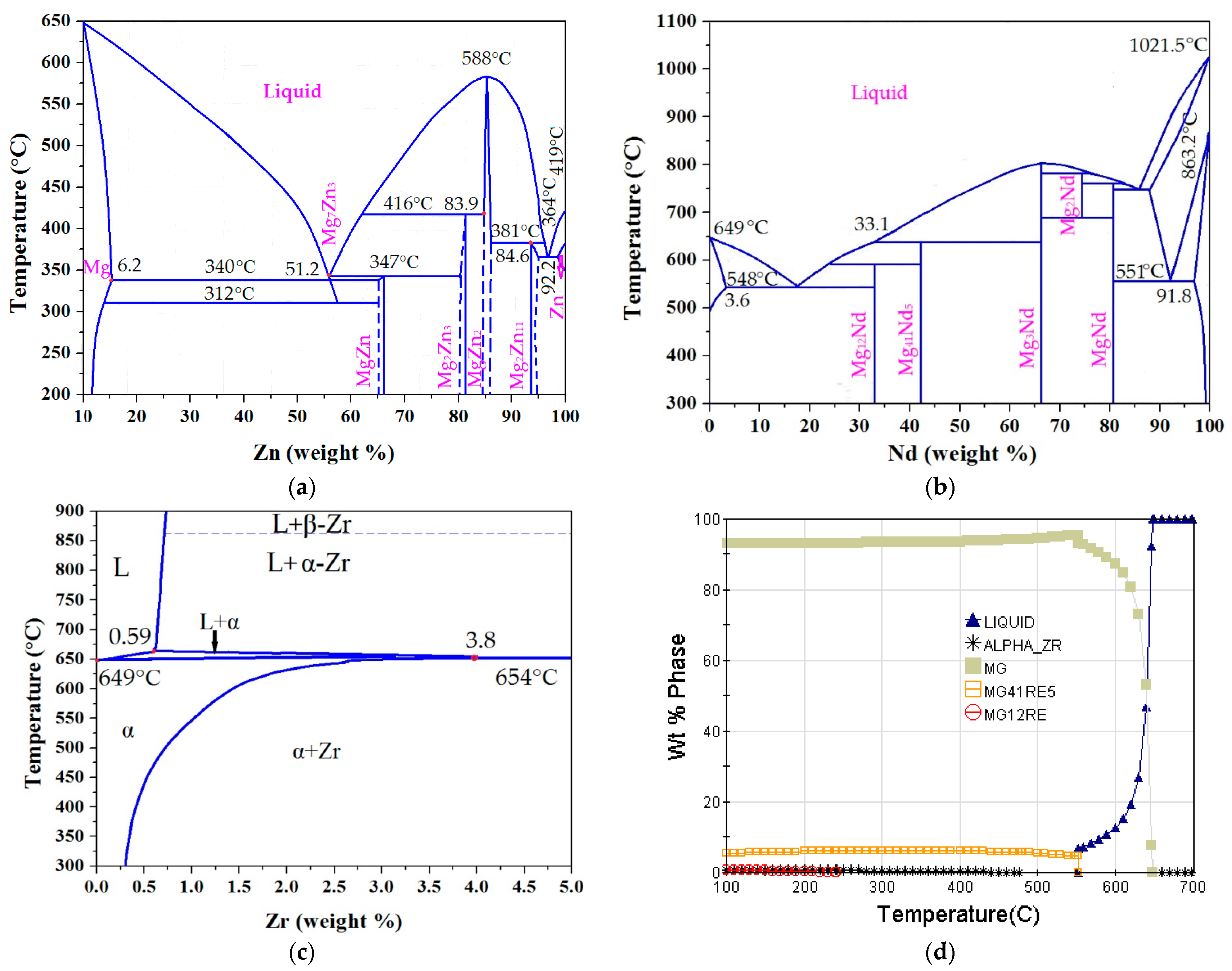
3.1.1. Alloy Phase Diagram Analysis of Mg-Nd-Zn-Zr Alloy
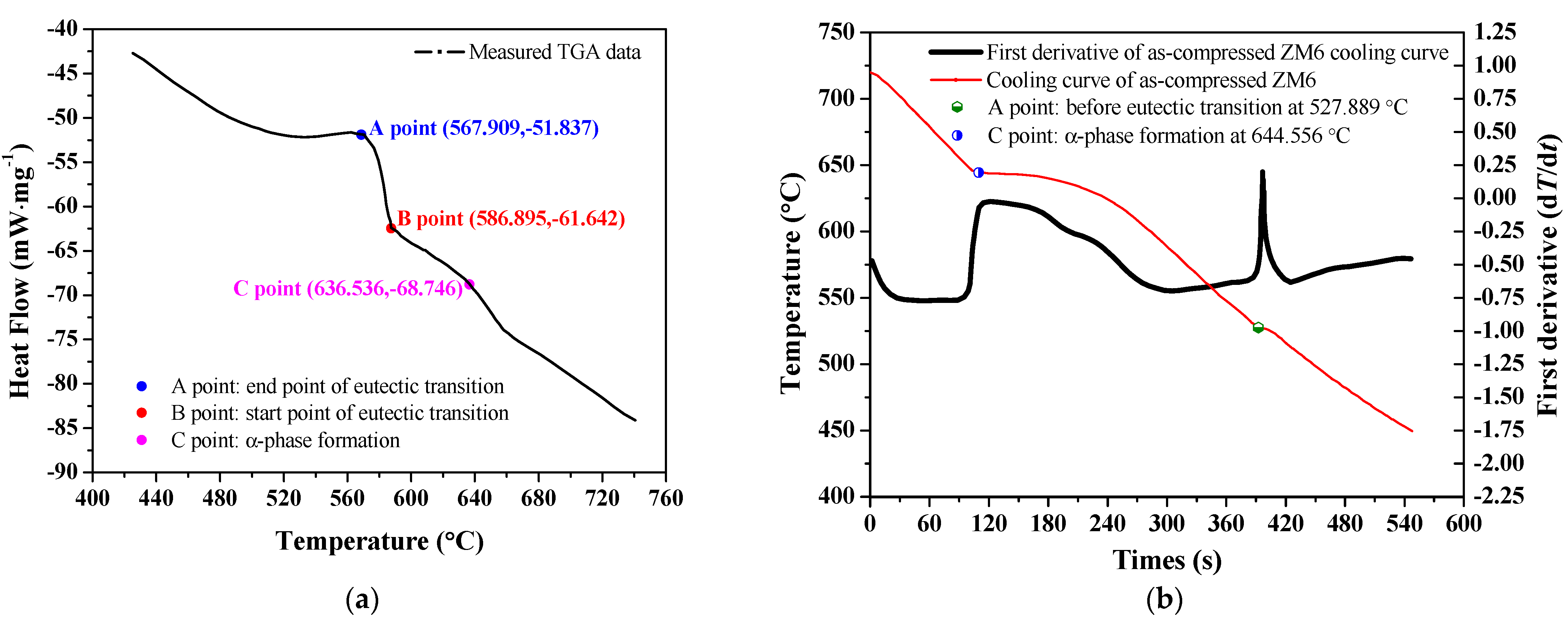
3.1.2. DTA Thermal Analysis Curve and Characteristic Temperatures
3.2. Microstructure and Phase Analysis after Two-Stage Aging Processes
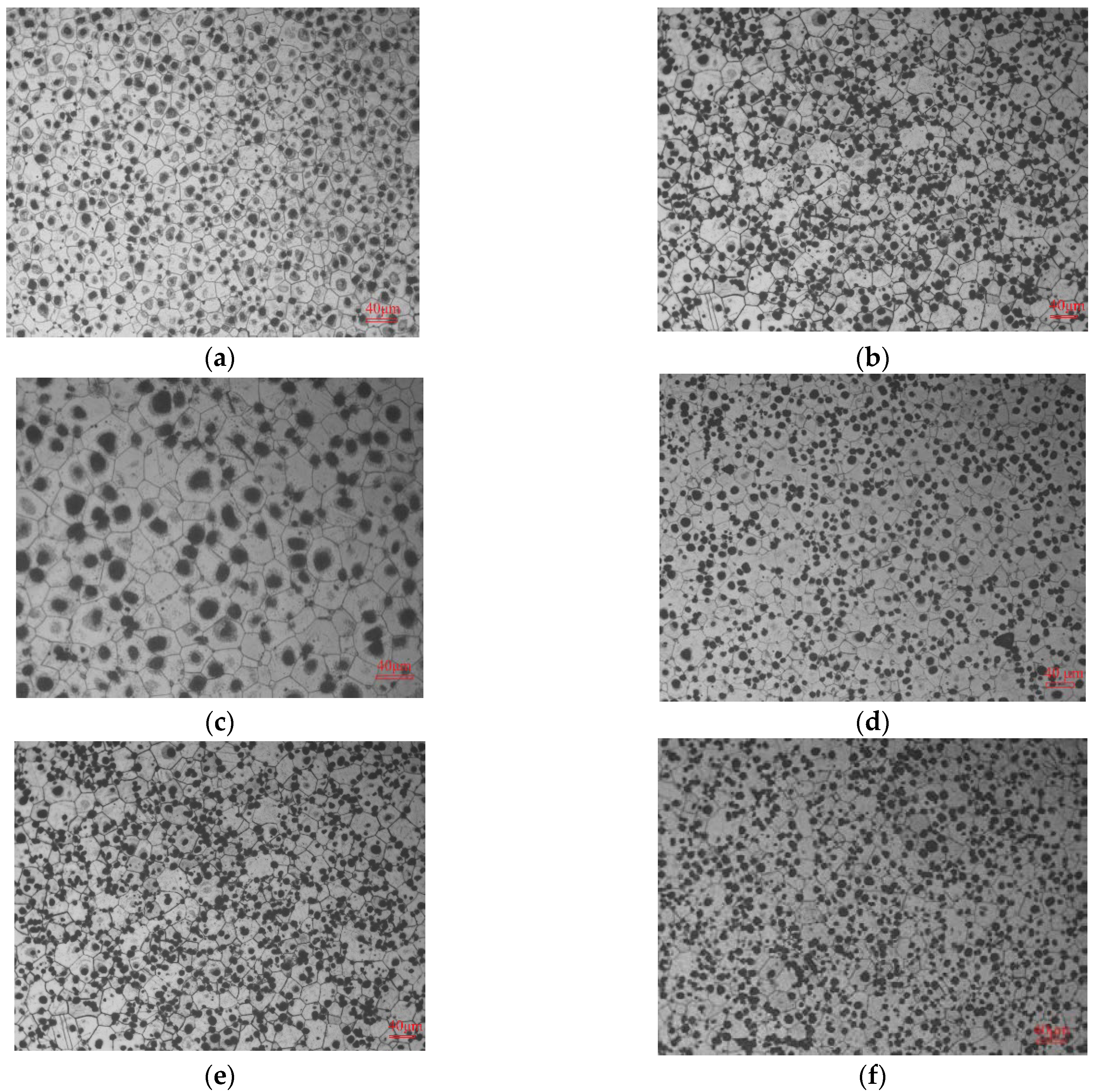
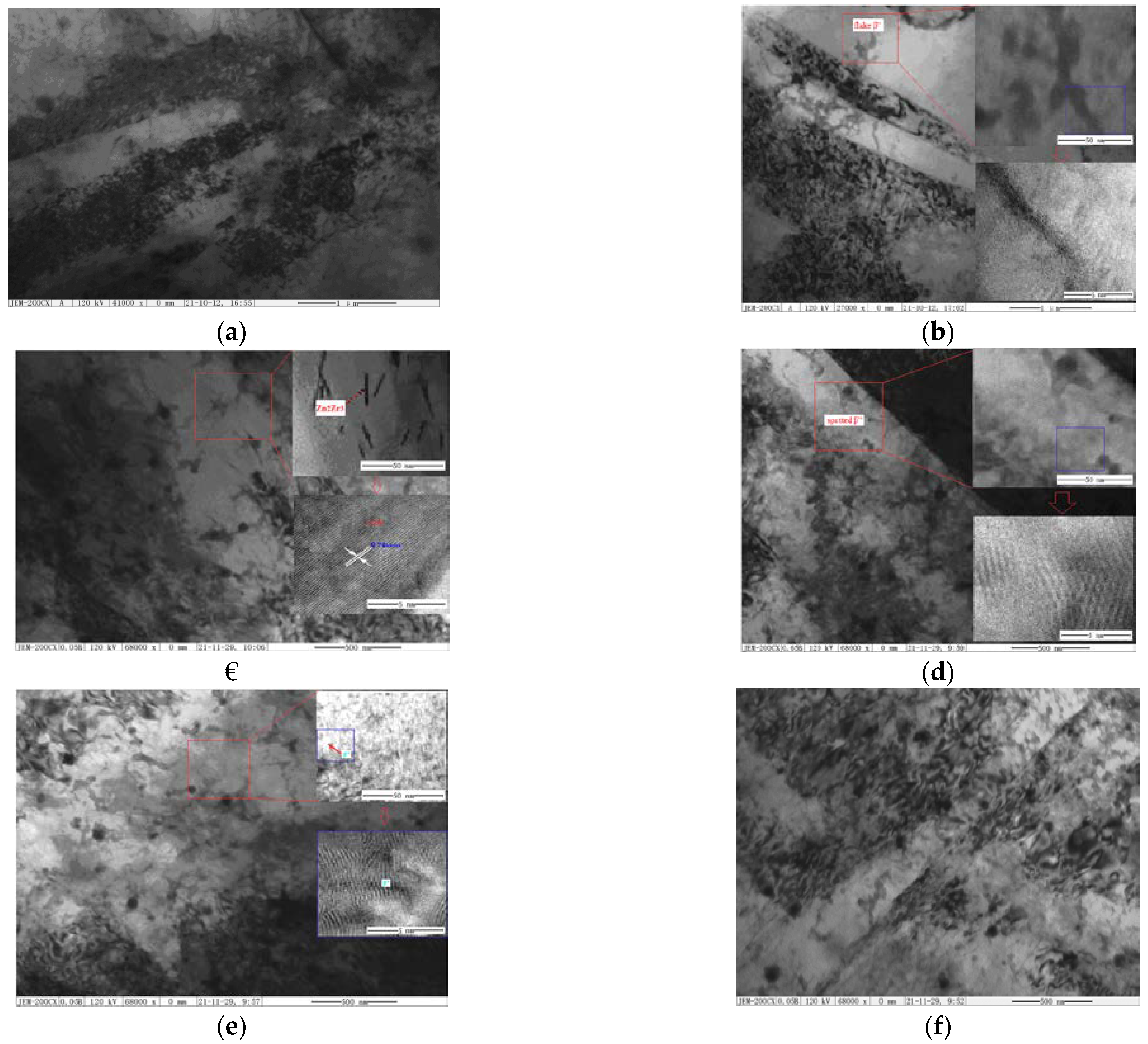
3.2.1. SEM and TEM Analysis after Aging Treatments
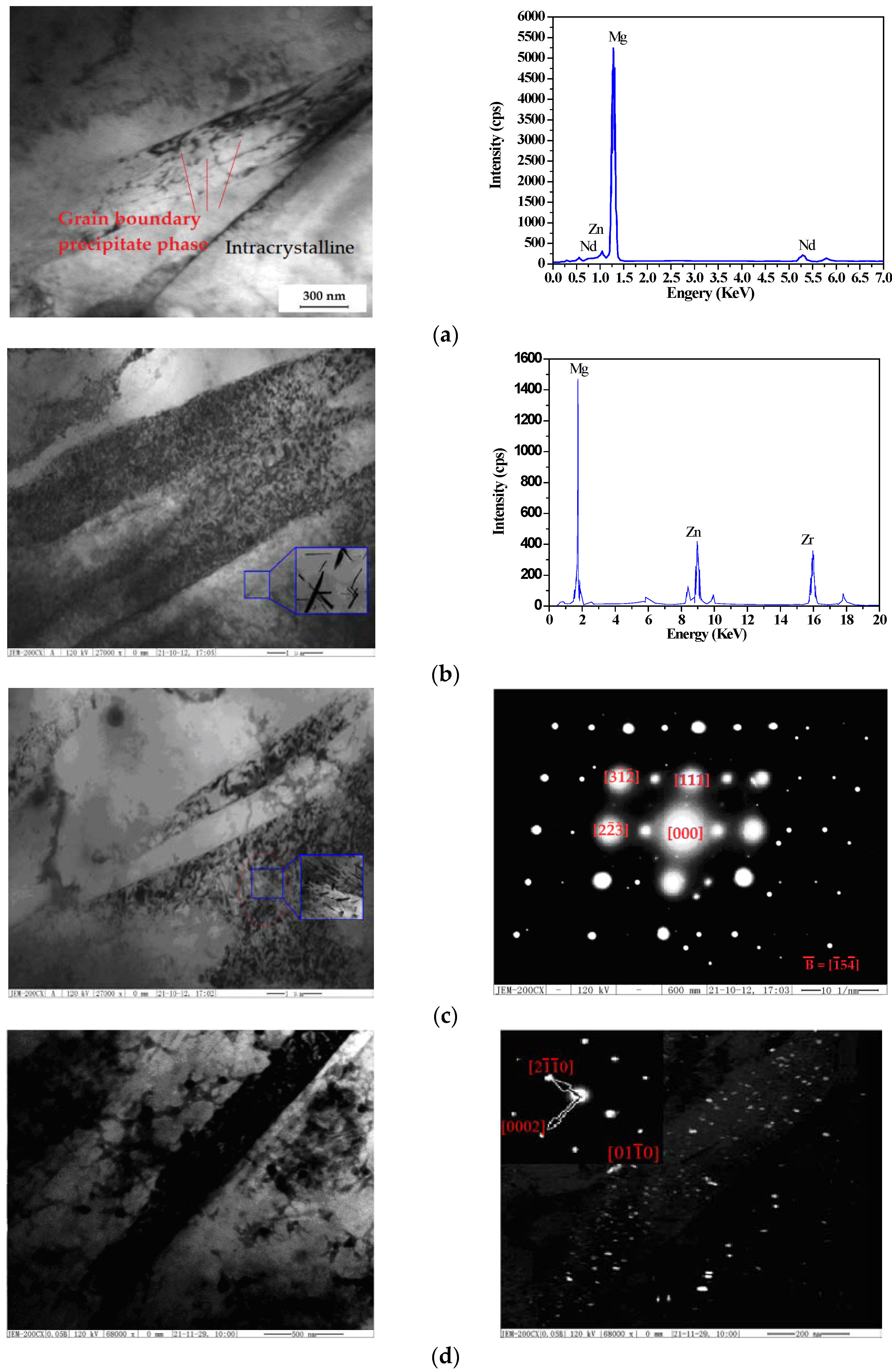
3.2.2. EDS and Phase Analysis after Aging Treatments
3.2.3. Precipitation Mechanism and Strengthening Mechanism
3.3. Experimental Tensile Properties under Various Heat Treatments
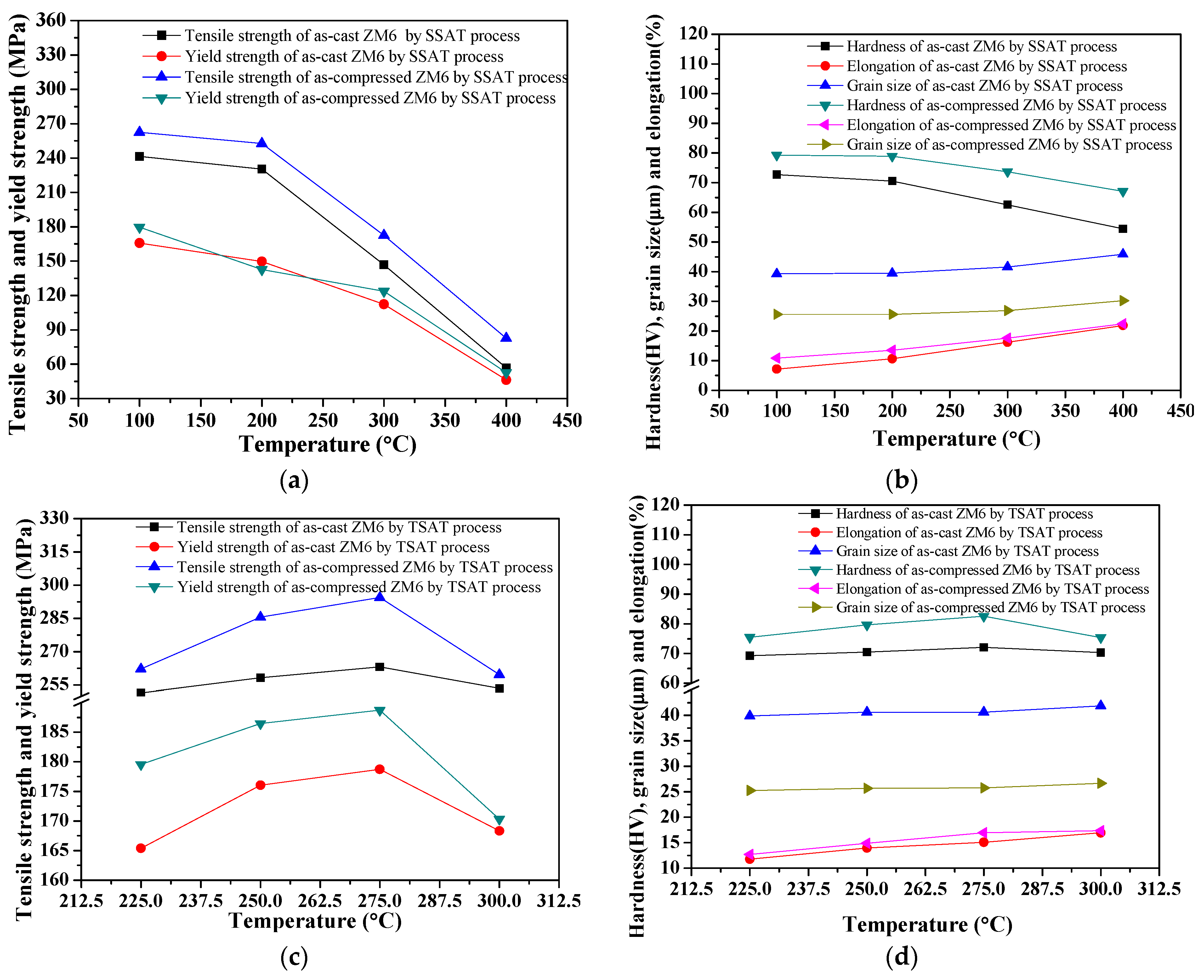
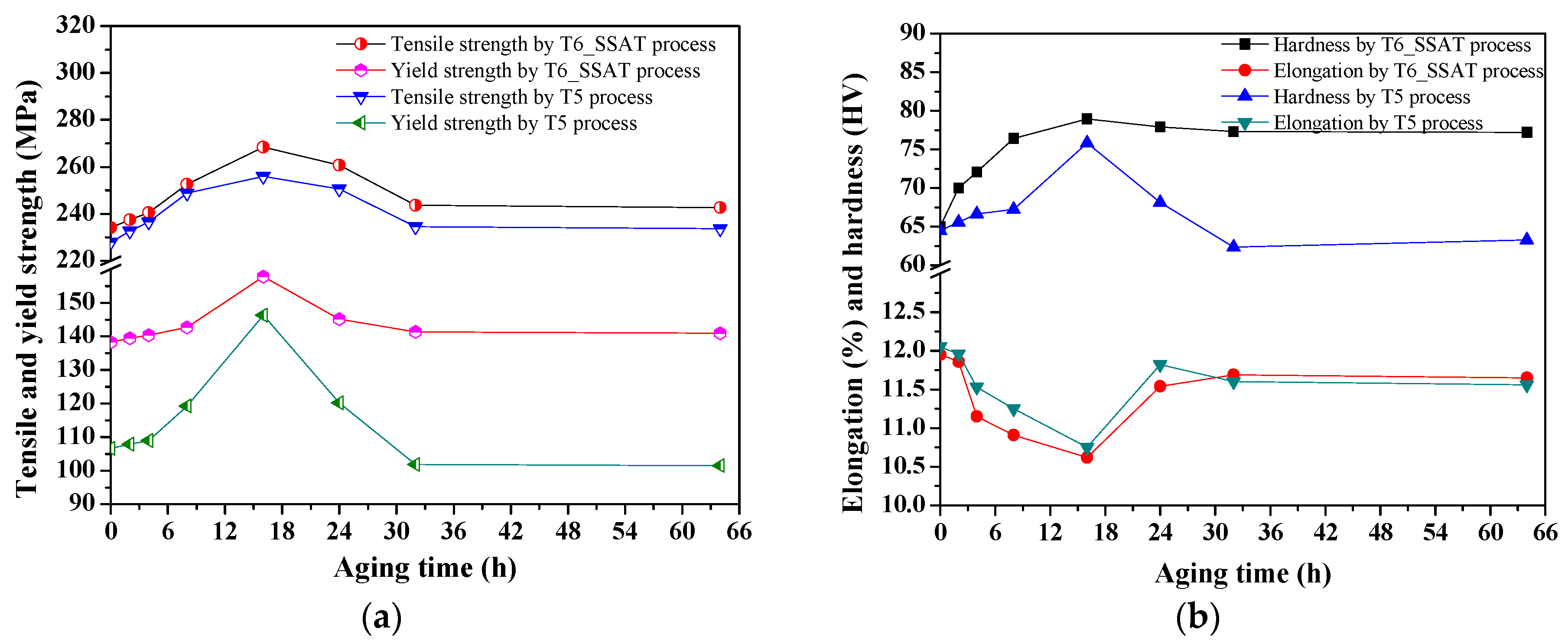
3.3.1. Effect of Aging Temperature on Tensile Properties of As-Cast and As-Compressed ZM6
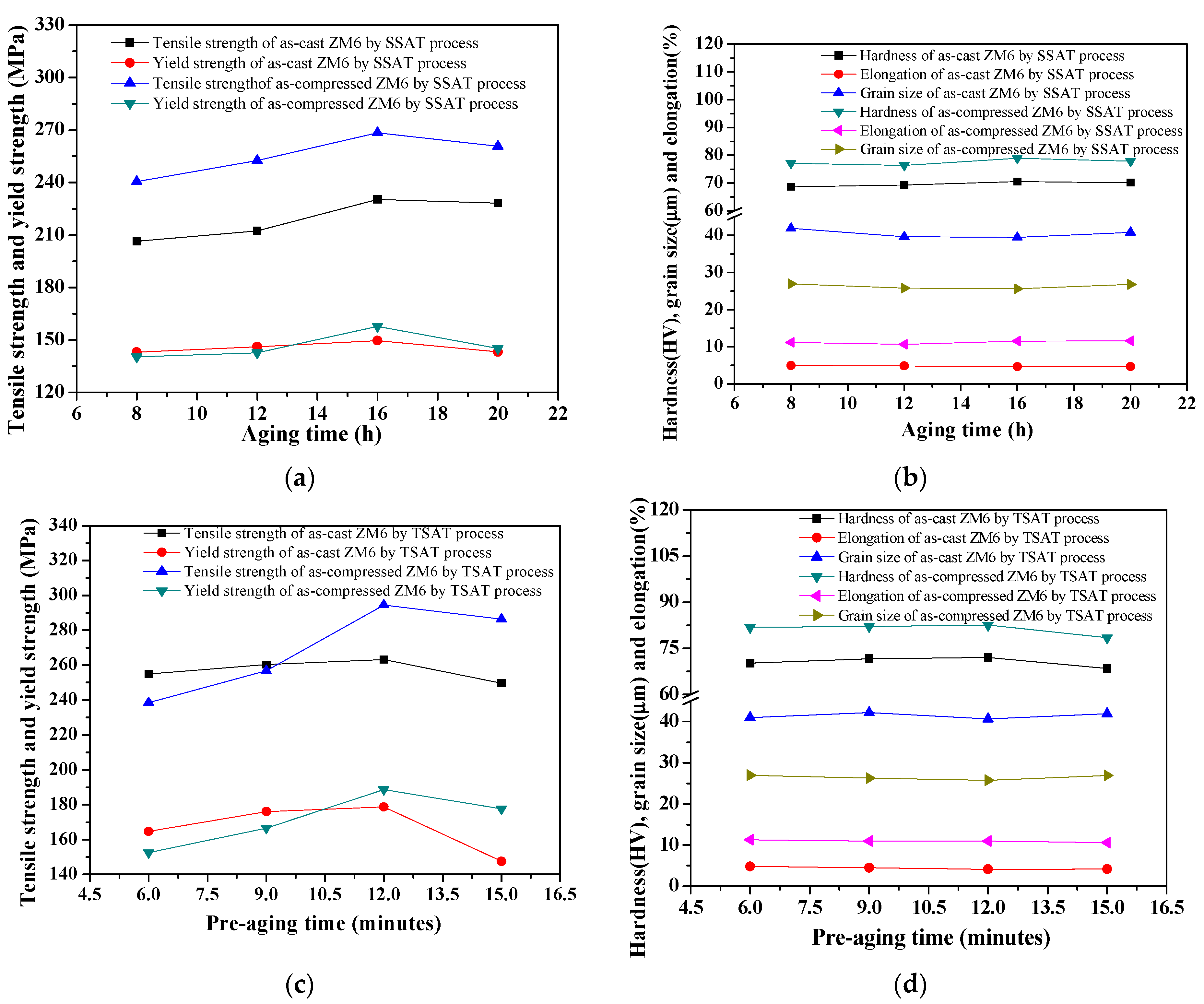
3.3.2. Effect of Aging Time on Tensile Properties of As-Cast and As-Compressed ZM6
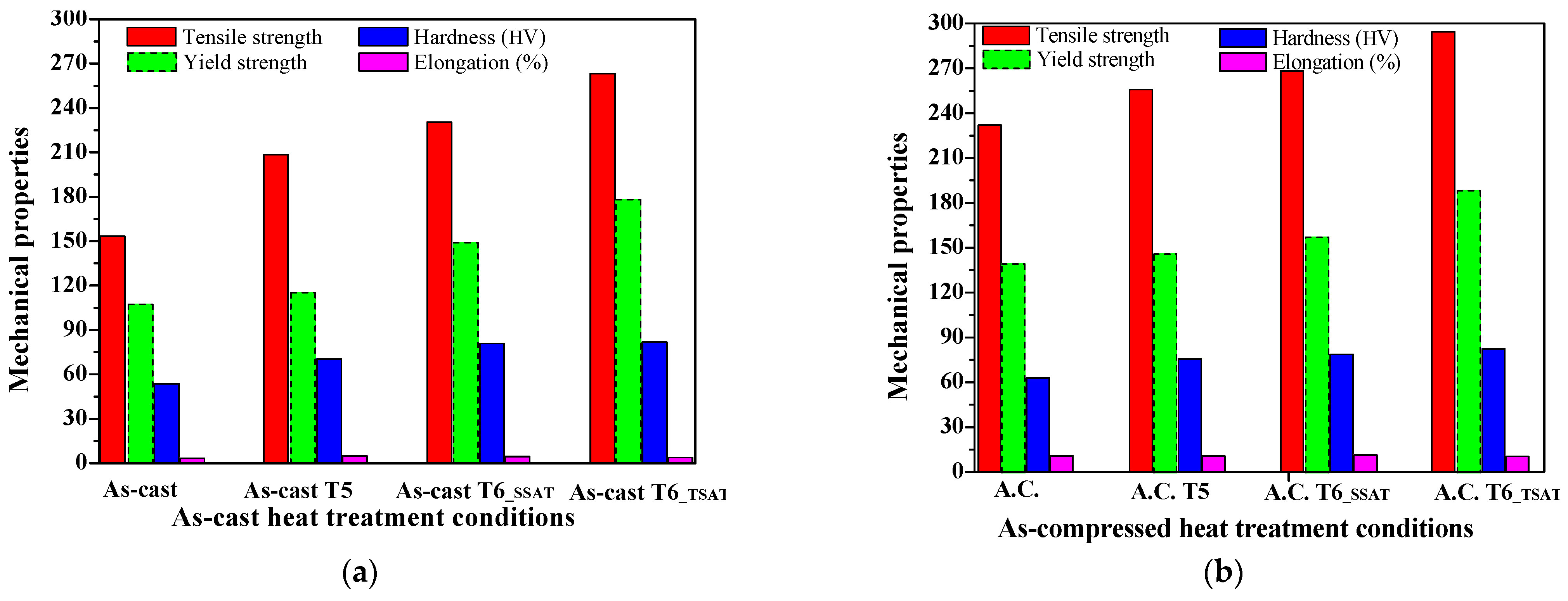
3.3.3. Comparison of Mechanical Properties under Various Heat Treatment Conditions
4. Conclusions
- (1)
- As-cast ZM6 alloys consist of α-Mg and Mg12Nd phases. The Mg12Nd phase is mainly distributed along the grain boundaries. Mg12Nd is distributed in the crystal and the grain boundary in the form of intersecting sheets, which are formed by the aggregation of the point-precipitated phases. The order of precipitation that occurs during the SSAT and TSAT processes was verified to be: supersaturated solution→G.P. zones→α(hcp)→β″(D019)→β′(fcc)→β(bct).
- (2)
- As-compressed ZM6 alloys formed an α-Mg solid solution at 636.54 °C, and when it was cooled to 586.90 °C, it produced a Mg12Nd phase until the end of the eutectic reaction at 567.91 °C. There were no obvious differences in the microstructures of the as-cast and as-compressed ZM6 alloys; however, there was an obvious increase in the number of twins. Moreover, the twins that were inside and outside of the grain boundary were different in the as-compressed samples that did not undergo heat treatment.
- (3)
- After pre-aging at 275 °C for 12 min, a large number of small and dispersed G.P. zones were formed in the α-Mg matrix. After the two-stage aging treatment, the homogeneous lamellar Mg3Nd phase formed along the grain boundary, and the continuous precipitated fine Mg12Nd phase formed in the grains. During the second aging process at 200 °C for 8 h, the G.P. zone was used as the core of the β″ phase; thus, the size of the β″ phase was refined, and the density and dispersion of the β phase were increased at the end of the process.
- (4)
- When the pre-aging time increased, the elongation decreased slightly, and the tensile strength demonstrated an obvious increase. When the pre-aging process was at 275 °C for 12 min and when the secondary aging process was at 200 °C for 8 h, the tensile strength reached 294.46 MPa, which was 26.11 MPa higher than that obtained for the single-stage aging process at 200 °C for 8.25 h.
- (5)
- Compared to the T6 treatment, the tensile strength after the two-stage aging treatment improved great, showing values of up to 294.46 MPa. For the two-stage aging process, the best mechanical properties were obtained at 275 °C for 12 min + 200 °C for 8 h, where hardness values of 82.56 HV and tensile strength of 294.46 MPa were obtained compared to the values of 78.9 HV and 268.35 MPa that were achieved during the T6_SSAT process (aging at 200 °C for 8.25 h).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esgandari, B.A.; Mehrjoo, H.; Nami, B.; Miresmaeili, S.M. The effect of Ca and RE elements on the precipitation kinetics of Mg 17Al12 phase during artificial aging of magnesium alloy AZ91. Mater. Sci. Eng. A 2011, 528, 5018–5024. [Google Scholar] [CrossRef]
- Yan, B.; Dong, X.; Ma, R.; Chen, S.; Pan, Z.; Ling, H. Effects of heat treatment on microstructure, mechanical properties and damping capacity of Mg–Zn–Y–Zr alloy. Mater. Sci. Eng. A 2014, 594, 168–177. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, S.; Wu, R.; Hou, L.; Zhang, M. Recent developments in high–strength Mg–RE–based alloys: Focusing on Mg–Gd and Mg–Y systems. J. Magnes. Alloys 2018, 6, 277–291. [Google Scholar] [CrossRef]
- Wang, B.J.; Luan, J.Y.; Xu, D.K.; Sun, J.; Li, C.Q.; Han, E.H. Research progress on the corrosion behavior of magnesium lithium–based alloys: A review. Acta Metall. Sin. 2019, 32, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Wang, L.P.; Feng, Y.C.; Wang, L.; Guo, E.J. Effect of Al content on hot tearing behaviour of Mg–xAl2Ca–2Sm alloys. Mater. Sci. Technol. 2019, 35, 116–126. [Google Scholar] [CrossRef]
- Fu, J.; Lin, W.H.; Chen, Z.R.; Wang, K.Q. Electronic and elastic properties of CaMg2 alloy phase under various pressures by Density Functional Theory. In MATEC Web of Conferences-2016CBNCM; EDP Sciences: Les Ulis, France, 2017; Volume 88, p. 03003. [Google Scholar]
- Fu, J.; Guo, J.K.; Bai, H.; Lin, W.H. Structural and elastic properties of CaMg2 Laves phase by Y-parameter and Reuss-Voigt-Hill methods. IOP Conf. Ser. Mater. Sci. Eng. 2018, 423, 012052. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Dong, J.; Zeng, X.Q. Effect of pre-deformation on aging characteristics and mechanical properties of a Mg-Gd-Nd-Zr alloy. Mater. Sci. Eng. A 2008, 491, 103–109. [Google Scholar] [CrossRef]
- Lin, D.; Wang, L.; Liu, Y.; Cui, J.Z.; Le, Q.C. Effects of plastic deformation on precipitation behavior and tensile fracture behavior of Mg-Gd-Y-Zr alloy. Trans. Nonferrous Metals Soc. 2011, 21, 2160–2167. [Google Scholar] [CrossRef]
- Fu, P.H.; Peng, L.M.; Jiang, H.Y.; Chang, J.W.; Zhai, C.Q. Effects of heat treatments on the microstructures and mechanical properties of Mg–3Nd–0.2Zn–0.4Zr (wt.%) alloy. Mater. Sci. Eng. A 2008, 486, 183–192. [Google Scholar]
- Ohishi, K.; Hono, K.; Shin, K.S. Effect of pre-aging and Al addition on age-hardening and microstructure in Mg–6wt%Zn alloys. Mater. Sci. Eng. A 2008, 496, 425–433. [Google Scholar] [CrossRef]
- Xu, S.W.; Mastumoto, N.; Kamado, S.; Honma, T.; Kojima, Y. Effect of pre-aging treatment on microstructure and mechanical properties of hot compressed Mg-9Al-1Zn alloy. Mater. Sci. Eng. A 2009, 517, 354–360. [Google Scholar] [CrossRef]
- Paliwal, M.; Das, S.K.; Kim, J.; Jung, I.H. Diffusion of Nd in hcp Mg and interdiffusion coefficients in Mg–Nd system. Scr. Mater. 2015, 108, 11–14. [Google Scholar] [CrossRef]
- Young, L. Grain Refinement of Magnesium. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2002. [Google Scholar]
- Polmear, I.J. Magnesium alloys and applications. Mater. Sci. Technol. 1994, 10, 1–14. [Google Scholar] [CrossRef]
- Fu, P.H.; Peng, L.M.; Jiang, H.Y.; Zhai, C.Q.; Gao, X.; Nie, J.F. Zr-Containing Precipitates in Mg-3wt%Nd-0.2wt%Zn-0.4wt%Zr Alloy during Solution Treatment at 540 °C. Mater. Sci. Forum 2007, 546–549, 97–100. [Google Scholar] [CrossRef]
- Guo, J.K.; Li, Y.X.; Fu, J. Researchon Design and improvement of the investment casting process for the ring–shaped magnesium alloy based on Procast software. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 042006. [Google Scholar] [CrossRef]
- Lan, M.; Mishrak, R.; Balogh, M.P.; Peng, L.; Luo, A.A.; Sachdev, A.K.; Ding, W. Effect of Zn on the microstructure evolution of compressed Mg-3Nd(-Zn)-Zr (wt.%) alloys. Mater. Sci. Eng. A 2012, 543, 12–21. [Google Scholar]
- Hao, Z.; Du, Z.; Yin, Z.; Zhang, X. Effect of hot compression and aging treatment on microstructure and mechanical properties of Mg–6Zn–1Zr–1.5Y alloy. Rare Metal Mater. Eng. 2013, 42, 84–88. [Google Scholar]
- Nie, F.J.; Muddle, C.B. Characterisation of strengthening precipitate phases in a Mg–Y–Nd alloy. Acta Mater. 2000, 48, 1691–1703. [Google Scholar] [CrossRef]
- Zhang, C.C.; Wang, H.Y.; Min, Z.; Wang, C.; Li, J.H.; Yang, Z.Z.; Jiang, Q.C. Microstructure and tensile properties of AZ61 alloy sheets processed by high–ratio extrusion with subsequent direct aging treatment. Materials 2018, 11, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, P.; Yu, B.; Liu, Z.; Wang, F.; Ju, Y. First–principles calculations of structural, elastic and electronic properties of AB2 type intermetallics in Mg–Zn–Ca–Cu alloy. J. Magnes. Alloy 2013, 1, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Wang, C.; Guo, N.; Pan, H.; Xin, R. Improving tensile and compressive properties of an extruded AZ91 rod by the combined use of torsion deformation and aging treatment. Materials 2017, 10, 280. [Google Scholar] [CrossRef]
- Mao, P.; Yu, B.; Liu, Z.; Wang, F.; Ju, Y. Mechanical, electronic and thermodynamic properties of Mg2Ca laves phase under high pressure: A first–principles calculation. Comput. Mater. Sci. 2014, 88, 61–70. [Google Scholar] [CrossRef]
- Spigarelli, S.; Cabibbo, M.; Evangelista, E.; Talianker, M.; Ezersky, V. Analysis of the creep behaviour of a thixoformed AZ91 magnesium alloy. Mater. Sci. Eng. A 2000, 289, 172–181. [Google Scholar] [CrossRef]
- Cui, Y.; Shi, Y.S.; Zheng, J.; Yan, Z.M.; Zhang, J.S.; Zhang, Z.M.; Wang, Q.; Xue, Y. Influence of heat treatment on the tensile properties and fatigue properties of Mg–8.8Gd–3.5Y–1.5Zn–0.5Zr alloy. Mater. Res. Express 2021, 8, 056518. [Google Scholar] [CrossRef]
- Barylski, A.; Aniołek, K.; Dercz, G.; Kupka, M.; Matuła, I.; Kaptacz, S. The sclerometrical, mechanical, and wear behavior of Mg–Y–Nd magnesium alloy after deep cryogenic treatment combined with heat treatment. Materials 2021, 14, 1218. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.W.; Li, T.; Zhang, K.; Li, X.G.; Li, Y.J.; Ma, M.L.; Shi, G.L.; Du, Z.W.; Liu, W.; Peng, Y.G. Precipitation behavior of Mg–7Gd–3Y–2Zn–0.5Zr alloy during isothermal aging. Materials 2021, 14, 1737. [Google Scholar] [CrossRef]
- Rong, W.; Wu, Y.; Zhang, Y.; Sun, M.; Chen, J.; Peng, L.; Ding, W. Characterization and strengthening effects of γ′ precipitates in a high–strength casting Mg–15Gd–1Zn–0.4Zr (wt.%) alloy. Mater. Charact. 2017, 126, 1–9. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, C.M.; Gao, Y.H.; Jiang, S.N.; Yu, S.L.; Chen, Z.Y. Effects of T5 treatment on microstructure and mechanical properties at elevated temperature of AZ80–Ag alloy. Materials 2019, 12, 3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Li, T.; Zhang, K.; Li, M.; Li, X.; Li, Y.; Ma, M.; Shi, G. Effect of Zn content on the microstructures, mechanical properties, and damping capacities of Mg–7Gd–3Y–1Nd–0.5Zr based alloys. J. Alloys Compd. 2019, 773, 919–926. [Google Scholar] [CrossRef]
- Ullmann, M.; Kittner, K.; Prahl, U. Hot deformation and dynamic recrystallisation behaviour of twin–roll cast Mg–6.8Y–2.5Zn–0.4Zr magnesium alloy. Materials 2021, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Sivapragash, M.; Lakshminarayanan, P.R.; Karthikeyan, R.; Hanumantha, M.; Bhatt, R.R. Hot deformation behavior of ZE41A magnesium alloy. Mater. Des. 2008, 29, 860–866. [Google Scholar] [CrossRef]
- Luo, A.A.; Powell, B.R.; Balogh, M.P. Creep and microstructure of magnesium–aluminum–calcium based alloys. Metall. Mater. Trans. A 2002, 33, 567–574. [Google Scholar] [CrossRef]
- Suzuki, M.; Sato, H.; Maruyama, K.; Oikawa, H. Creep deformation behavior and dislocation substructures of Mg–Y binary alloys. Mater. Sci. Eng. A 2001, 319, 751–755. [Google Scholar] [CrossRef]
- Unigovski, Y.; Keren, Z.; Eliezer, A.; Gutman, E.M. Creep behavior of pure magnesium and Mg–Al alloys in active environments. Mater. Sci. Eng. A 2005, 398, 188–197. [Google Scholar] [CrossRef]
- Milicka, K.; Dobe, F. Creep behaviour of a magnesium alloy AS21 and its fibre–strengthened composite. J. Alloys Compd. 2004, 378, 167–171. [Google Scholar] [CrossRef]
- Mordike, B.L. Creep resistant magnesium alloys. Mater. Sci. Eng. A 2002, 324, 103–112. [Google Scholar] [CrossRef]
- Regev, M.; Aghion, E.; Rosen, A.; Bamberger, M. Creep studies of coarse–grained AZ91D magnesium castings. Mater. Sci. Eng. A 1998, 252, 6–16. [Google Scholar] [CrossRef]
- Lü, Y.; Wang, Q.; Zeng, X.; Ding, W.; Zhai, C.; Zhu, Y. Effects of rare earths on the microstructure properties and fracture behavior of Mg–Al alloys. Mater. Sci. Eng. A 2000, 278, 66–76. [Google Scholar] [CrossRef]
- Lu, S.H.; Wu, D.; Chen, R.S.; Han, E.H. The effect of twinning on dynamic recrystallization behavior of Mg–Gd–Y alloy during hot compression. J. Alloys Compd. 2019, 803, 277–290. [Google Scholar] [CrossRef]
- Dębski, A.; Terlicka, S.; Gąsior, W.; Gierlotka, W.; Pęska, M.; Dworecka-Wójcik, J.; Polański, M. Calorimetric studies of magnesium-rich Mg-Pd alloys. Materials 2021, 14, 680. [Google Scholar] [CrossRef]
- Chang, J.W.; Fu, P.H.; Guo, X.W.; Peng, L.M.; Ding, W.J. The effects of heat treatment and zirconium on the corrosion behaviour of Mg–3Nd–0.2Zn–0.4Zr (wt.%) alloy. Corros. Sci. 2007, 49, 2612–2627. [Google Scholar] [CrossRef]



| Tensile Properties | Hardness | ||||
|---|---|---|---|---|---|
| σb (MPa) | σ0.2 (MPa) | δ (%) | ø (%) | HV | |
| As-cast ZM6 | 153.36 | 108.13 | 3.14 | 4.5 | 54.12 |
| As-compressed ZM6 | 232.16 | 139.75 | 10.97 | - | 63.26 |
| Single-Stage Aging of ZM6 | Two-Stage Aging of ZM6 | ||||
|---|---|---|---|---|---|
| Condition | Solid Solution | Aging Process | Solid Solution | Pre-Aging Process | Final–Stage Aging Process |
| As-cast T5 | - | 200 °C × 8.25 h | - | 275 °C × 0.25 h | 200 °C × 8 h |
| As-cast T6 | 525 °C × 4 h | 100–400 °C × 8.25 h | 525 °C × 4 h | 200–350 °C × 0.05–0.25 h | 200 °C × 0–64 h |
| As-compressed T4 | 525 °C × 4 h | - | 525 °C × 4 h | - | - |
| As-compressed T5 | - | 200 °C × 8.25 h | - | 275 °C × 0.25 h | 200 °C × 8 h |
| As-compressed T6 | 525 °C × 4 h | 100–400 °C × 8.25 h | 525 °C × 4 h | 200–350 °C × 0.05–0.25 h | 200 °C × 0–64 h |
| Element | Spot Position | Mass % | Atom % | Spot Position | Mass % | Atom % | Spot Position | Mass % | Atom % | Spot Position | Mass % | Atom % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg K | Spot 1 in Figure 8a | 60.46 | 88.26 | Spot 3 in Figure 8a | 83.72 | 96.64 | Spot 1 in Figure 8b | 75.75 | 93.71 | Spot 3 in Figure 8b | 81.70 | 95.70 |
| Nd L | 32.28 | 7.94 | 15.06 | 2.93 | 19.14 | 3.99 | 15.15 | 2.99 | ||||
| Zn K | 6.34 | 3.44 | 0.47 | 0.20 | 4.74 | 2.18 | 2.64 | 1.15 | ||||
| Zr L | 0.92 | 0.36 | 0.75 | 0.23 | 0.36 | 0.12 | 0.51 | 0.16 | ||||
| Mg K | Spot 2 in Figure 8a | 78.24 | 94.27 | Spot 4 in Figure 8a | 79.65 | 94.82 | Spot 2 in Figure 8b | 60.67 | 88.15 | Spot 4 in Figure 8b | 85.98 | 96.98 |
| Nd L | 16.06 | 3.26 | 14.90 | 2.99 | 31.17 | 7.63 | 12.21 | 2.32 | ||||
| Zn K | 5.05 | 2.26 | 3.68 | 1.63 | 6.95 | 3.75 | 1.31 | 0.55 | ||||
| Zr L | 0.65 | 0.21 | 1.77 | 0.56 | 1.21 | 0.47 | 0.50 | 0.15 |
| Condition | σb (MPa) | σ0.2 (MPa) | δ (%) | Hardness (HV) | Strengthening Mechanisms |
|---|---|---|---|---|---|
| As-cast | 153.36 | 108.13 | 3.14 | 54.12 | M.S. + S.S.S. |
| As-cast T6-SSAT | 230.32 | 149.65 | 4.63 | 70.50 | M.S. + S.S.S. + P.S. |
| As-cast T6-TSAT | 263.16 | 178.75 | 4.07 | 72.05 | M.S. + S.S.S. + P.S. |
| As-compressed | 232.16 | 139.75 | 10.67 | 63.26 | M.S. + F.G.S. |
| As-compressed + T4 | 208.42 | 115.81 | 12.96 | 59.63 | M.S. + S.S.S. + F.G.S. |
| As-compressed + T5 | 255.88 | 146.28 | 10.75 | 75.82 | M.S. + F.G.S. + P.S. |
| As-compressed + T6_SSAT | 268.35 | 157.65 | 11.52 | 78.92 | M.S. + S.S.S. + F.G.S.+ P.S. |
| As-compressed + T6_TSAT | 294.46 | 188.72 | 10.93 | 82.56 | M.S. + S.S.S. + F.G.S.+ P.S. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Chen, S. Microstructure Evolution and Mechanical Properties of As-Cast and As-Compressed ZM6 Magnesium Alloys during the Two-Stage Aging Treatment Process. Materials 2021, 14, 7760. https://doi.org/10.3390/ma14247760
Fu J, Chen S. Microstructure Evolution and Mechanical Properties of As-Cast and As-Compressed ZM6 Magnesium Alloys during the Two-Stage Aging Treatment Process. Materials. 2021; 14(24):7760. https://doi.org/10.3390/ma14247760
Chicago/Turabian StyleFu, Jia, and Su Chen. 2021. "Microstructure Evolution and Mechanical Properties of As-Cast and As-Compressed ZM6 Magnesium Alloys during the Two-Stage Aging Treatment Process" Materials 14, no. 24: 7760. https://doi.org/10.3390/ma14247760
APA StyleFu, J., & Chen, S. (2021). Microstructure Evolution and Mechanical Properties of As-Cast and As-Compressed ZM6 Magnesium Alloys during the Two-Stage Aging Treatment Process. Materials, 14(24), 7760. https://doi.org/10.3390/ma14247760






