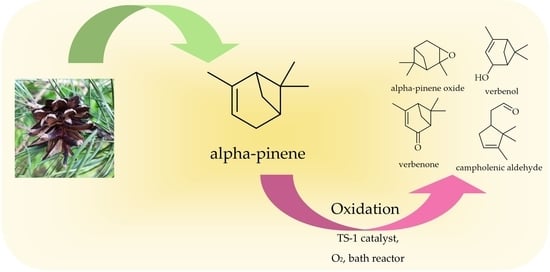
The Studies on α-Pinene Oxidation over the TS-1. The Influence of the Temperature, Reaction Time, Titanium and Catalyst Content
Abstract
:1. Introduction
2. Results and Discussion
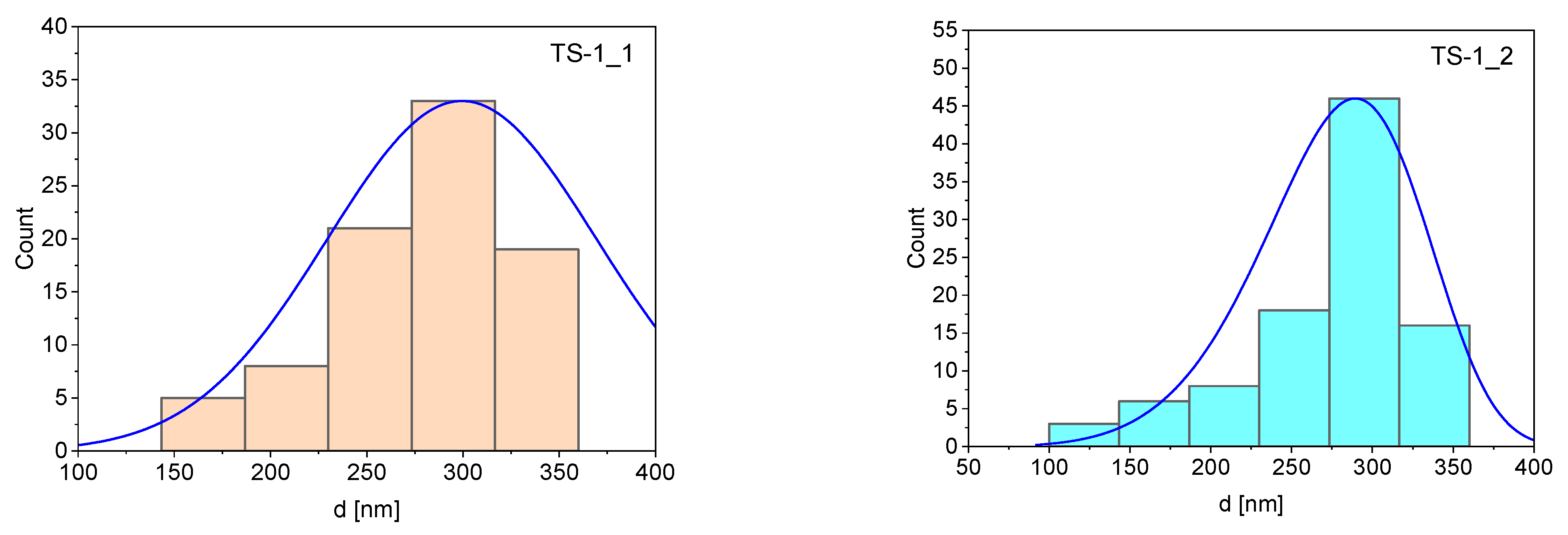
2.1. Characteristics of the TS-1 Materials
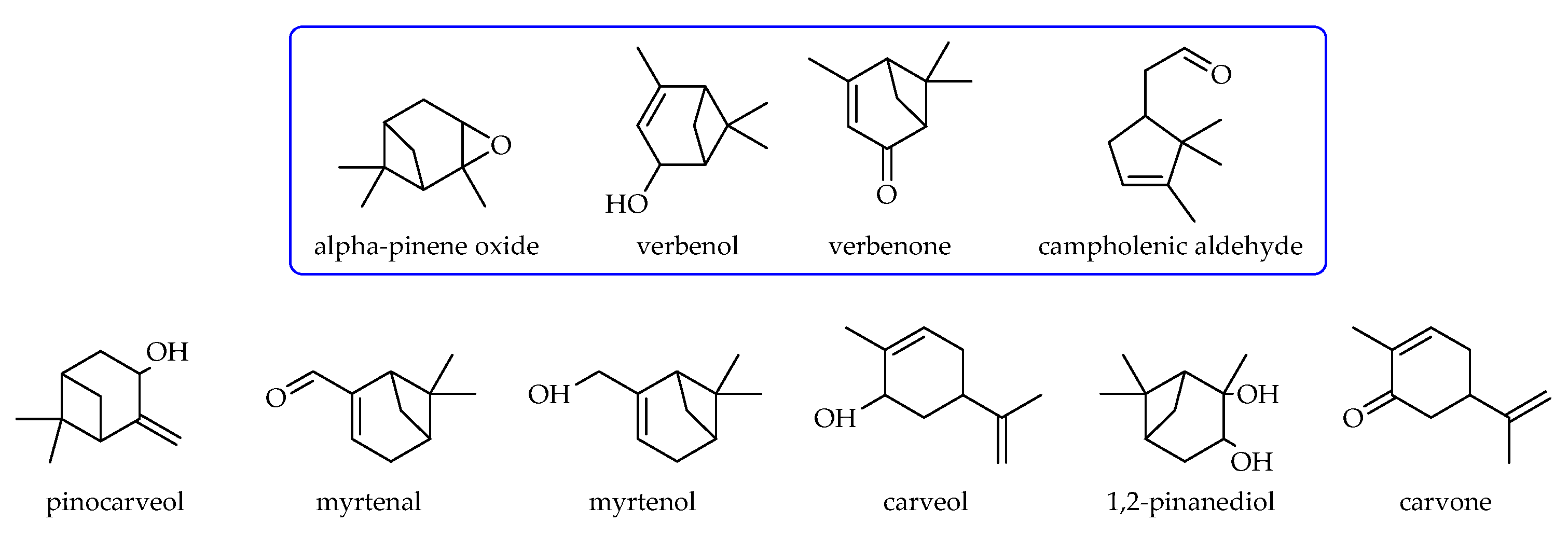
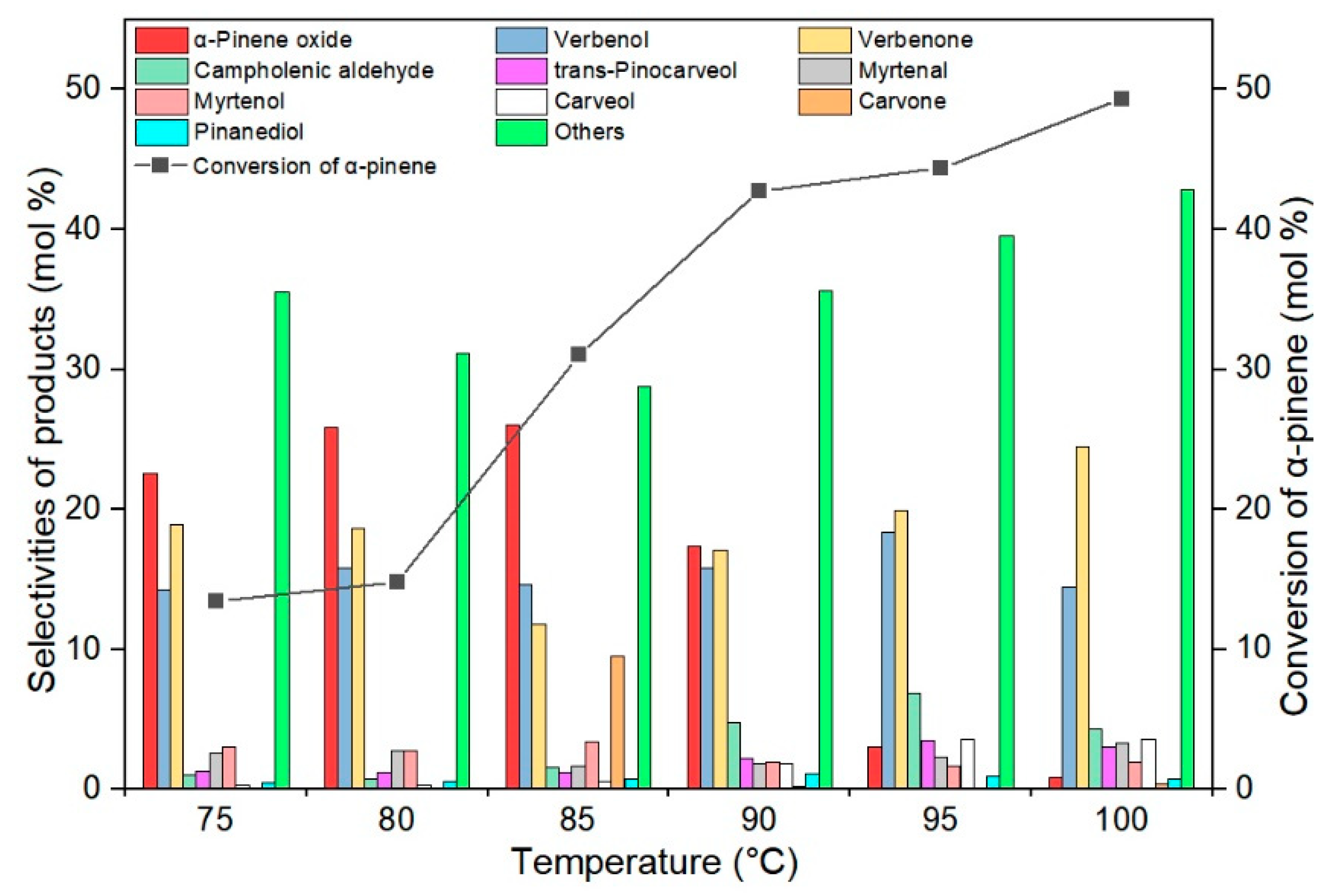
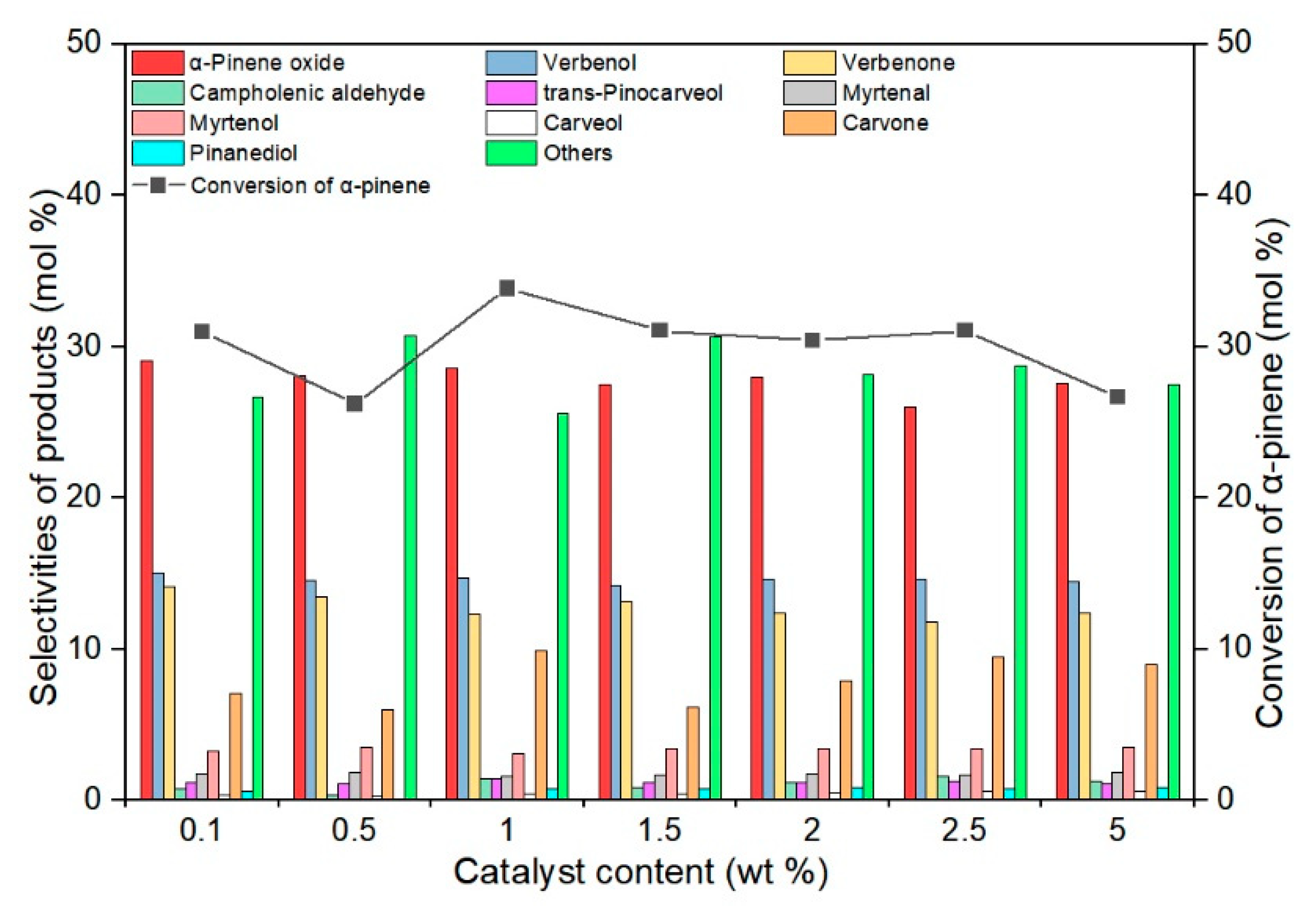
2.2. Studies on the Catalytic Activity of TS-1 Catalysts in the α-Pinene Oxidation Process
3. Materials and Methods
3.1. Raw Materials
3.2. Synthesis of TS-1 Catalysts
3.3. Characteristics of the TS-1 Catalysts
3.4. Oxidation of α-Pinene
3.5. Identification of the Products of Oxidation by the Gas Chromatography Method
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Lin, M.; Tuel, A. Hollow TS-1 Crystals Formed via a Dissolution–Recrystallization Process. Microporous Mesoporous Mater. 2007, 102, 80–85. [Google Scholar] [CrossRef]
- Min, L.; Xingtian, S.; Xieqing, W.; Bin, Z. Titanium-Silicalite Molecular Sieve and the Method for Its Preparation. U.S. Patent 6,475,465, 5 November 2002. [Google Scholar]
- Liu, M.; Xiao, Z.; Dai, J.; Zhong, W.; Xu, Q.; Mao, L.; Yin, D. Manganese-Containing Hollow TS-1: Description of the Catalytic Sites and Surface Properties for Solvent-Free Oxidation of Ethylbenzene. Chem. Eng. J. 2017, 313, 1382–1395. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, T.; Liu, M.; Ji, Y.; Song, C.; Guo, X. Mesoporous/Microporous Titanium Silicalite with Controllable Pore Diameter for Cyclohexene Epoxidation. Ind. Eng. Chem. Res. 2018, 57, 512–520. [Google Scholar] [CrossRef]
- Serrano, D.P.; Sanz, R.; Pizarro, P.; Moreno, I.; Shami, S. Narrowing the Mesopore Size Distribution in Hierarchical TS-1 Zeolite by Surfactant-Assisted Reorganization. Microporous Mesoporous Mater. 2014, 189, 71–82. [Google Scholar] [CrossRef]
- Wróblewska, A.; Tołpa, J.; Kłosin, D.; Miądlicki, P.; Koren, Z.C.; Michalkiewicz, B. The Application of TS-1 Materials with Different Titanium Contents as Catalysts for the Autoxidation of α-Pinene. Microporous Mesoporous Mater. 2020, 305, 110384. [Google Scholar] [CrossRef]
- Wróblewska, A.; Milchert, E. Charakterystyka Katalizatorów Tytanowo-Silikalitowych. Przemysł Chem. 2005, 84, 723–728. (In Polish) [Google Scholar]
- Taramasso, M.; Perego, G.; Notari, B. Preparation of Porous Crystalline Synthetic Material Comprosed of Slicon and Titanium Oxides. U.S. Patent 4,410,501, 18 October 1983. [Google Scholar]
- Dusi, M.; Mallat, T.; Baiker, A. Epoxidation of Functionalized Olefins over Solid Catalysts. Catal. Rev. 2000, 42, 213–278. [Google Scholar] [CrossRef]
- Wróblewska, A.; Fajdek, A. Epoxidation of Allyl Alcohol to Glycidol over the Microporous TS-1 Catalyst. J. Hazard. Mater. 2010, 179, 258–265. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Lee, D.F.; Minihan, A.R. Epoxidation of Allyl Alcohol to Glycidol Using Titanium Silicalite TS-1: Effect of the Method of Preparation. Catal. Lett. 1995, 33, 369–385. [Google Scholar] [CrossRef]
- Gao, H.; Lu, G.; Suo, J.; Li, S. Epoxidation of Allyl Chloride with Hydrogen Peroxide Catalyzed by Titanium Silicalite 1. Appl. Catal. A Gen. 1996, 138, 27–38. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Xie, W.; Zhang, H.; Wu, H.; Jiang, Y.; He, M.; Wu, P. Highly Efficient and Selective Production 494 of Epichlorohydrin through Epoxidation of Allyl Chloride with Hydrogen Peroxide over Ti-MWW Catalysts. J. Catal. 2007, 246, 205–214. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, J.; Yu, S. Gas Phase Safety-Control Conditions for Epoxidation of Allyl Chloride with Hydrogen Peroxide Catalysed by TS-1. J. Loss Prev. Process Ind. 2014, 32, 201–206. [Google Scholar] [CrossRef]
- Wróblewska, A. The Epoxidation of Limonene over the TS-1 and Ti-SBA-15 Catalysts. Molecules 2014, 19, 19907–19922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wróblewska, A.; Drewnowska, E.; Gawarecka, A. The Epoxidation of Diallyl Ether to Allyl-Glycidyl Ether over the TS-1 Catalyst. React. Kinet. Mech. Catal. 2016, 118, 719–731. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Y.; He, M.; Tatsumi, T. A Novel Titanosilicate with MWW Structure: Catalytic Properties in Selective 503 Epoxidation of Diallyl Ether with Hydrogen Peroxide. J. Catal. 2004, 228, 183–191. [Google Scholar] [CrossRef]
- Kwon, S.; Schweitzer, N.M.; Park, S.; Stair, P.C.; Snurr, R.Q. A Kinetic Study of Vapor-Phase Cyclohexene Epoxidation by H2O2 over Mesoporous TS-1. J. Catal. 2015, 326, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Tuel, A.; Moussa-Khouzami, S.; Taarit, Y.B.; Naccache, C. Hydroxylation of Phenol over TS-1: Surface and Solvent Effects. J. Mol. Catal. 1991, 68, 45–52. [Google Scholar] [CrossRef]
- Tuel, A.; Taarit, Y. ben Comparison between TS-1 and TS-2 in the Hydroxylation of Phenol with Hydrogen Peroxide. Appl. Catal. A Gen. 1993, 102, 69–77. [Google Scholar] [CrossRef]
- Machałowski, T.; Idaszek, J.; Chlanda, A.; Heljak, M.; Piasecki, A.; Święszkowski, W.; Jesionowski, T. Naturally Prefabricated 3D Chitinous Skeletal Scaffold of Marine Demosponge Origin, Biomineralized Ex Vivo as a Functional Biomaterial. Carbohydr. Polym. 2022, 275, 118750. [Google Scholar] [CrossRef] [PubMed]
- Bula, K.; Klapiszewski, Ł.; Piasecki, A.; Jesionowski, T. The Role of Inorganic-Organic Bio-Fillers Containing Kraft Lignin in Improvement in Functional Properties of Polyethylene. Materials 2021, 14, 2114. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejczak, P.; Collins, M.N.; Jesionowski, T.; Klapiszewski, Ł. The Role of Lignin and Lignin-Based Materials in Sustainable Construction—A Comprehensive Review. Int. J. Biol. Macromol. 2021, 187, 624–650. [Google Scholar] [CrossRef]
- Norström, E. Terpenes as Renewable Monomers for Biobased Materials. Master’s Thesis, KTH, Stockholm, Sweden, 2011. [Google Scholar]
- Nurzyńska-Widferak, R. Aktywność Biologiczna Olejków Eterycznych Roślin z Rodziny Pinaceae. Ann. Univ. Mariae Curie-Skłodowska EEE Hortic. 2015, 25, 19–31. (In Polish) [Google Scholar]
- Lewicka, L.; Bełdowicz, M.; Kąkol, B.; Kulig-Adamiak, A.; Obukowicz, B. Terpentyna Balsamiczna i Siarczanowa 525 jako Surowiec do Otrzymywania Syntetyków Zapachowych. Przemysł Chem. 2005, 84, 242–246. (In Polish) [Google Scholar]
- Wang, W.; Li, N.; Luo, M.; Zu, Y.; Efferth, T. Antibacterial Activity and Anticancer Activity of Rosmarinus Officinalis L. Essential Oil Compared to That of Its Main Components. Molecules 2012, 17, 2704–2713. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.C.R.; Lopes, P.M.; de Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of A-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-Terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Melgunow, M.S.; Mrowiec-Białoń, J.; Jarzębski, A.B.; Kholdeeva, O.A. H2O2-Based Allylic Oxidation of α-Pinene over Different Single Site Catalysts. J. Catal. 2005, 235, 535. [Google Scholar] [CrossRef]
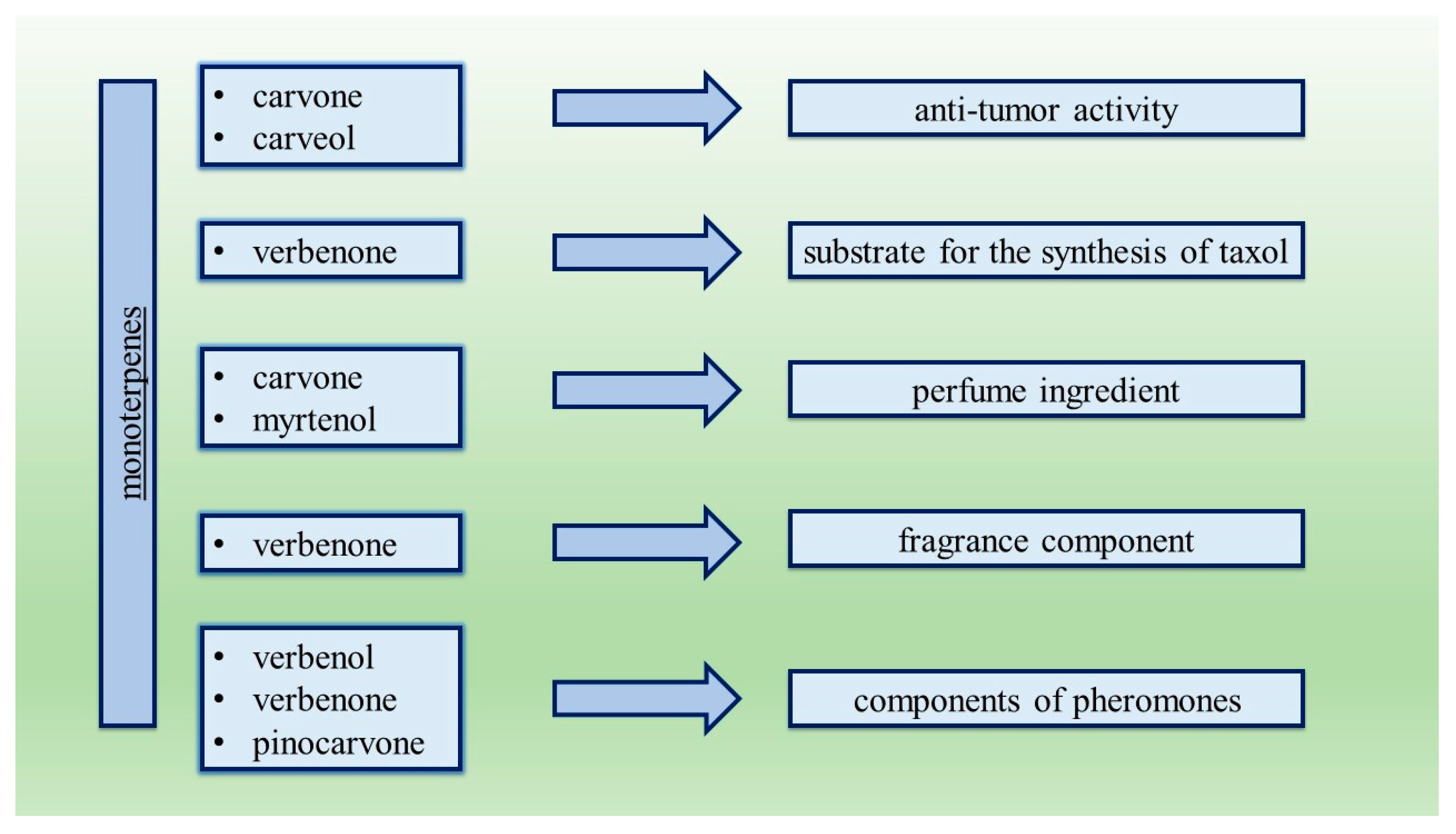
- Crowell, P.L. Monoterpenes in Breast Cancer Chemoprevention. Breast Cancer Res. Treat. 1997, 46, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Wender, P.A.; Mucciaro, T.P. A New and Practical Approach to the Synthesis of Taxol and Taxol Analogs: The Pinene Path. J. Am. Chem. Soc. 1992, 114, 5878–5879. [Google Scholar] [CrossRef]
- Ohloff, G. Scent and Fragrances. The Fascination of Odors and Their Chemical Perspectives; Springer: Berlin/Heidelberg, Germany, 1994; pp. 154–158. [Google Scholar]
- Trytek, M.; Paduch, R.; Fiedurek, J.; Kandefer-Szerszeń, M. Monoterpeny—Stare Związki, Nowe Zastosowania i 539 Biotechnologiczne Metody Ich Otrzymywania. Biotechnologia 2007, 1, 135–155. [Google Scholar]
- De Carvalho, C.C.; Da Fonseca, M.M.R. Carvone: Why and How Should One Bother to Produce This Terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Agrawal, R.; Joseph, R. Bioconversion of Alpha Pinene to Verbenone by Resting Cells of Aspergillus Niger. Appl. Microbiol. Biotechnol. 2000, 53, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Schlyter, F.; Birgersson, G.; Byers, J.A.; Lofqvist, J.; Bergstrom, G. Field response of spruce bark beetle, Ips typographus, to aggregation pheromone candidates. J. Chem. Ecol. 1987, 13, 701–716. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejczyk, A. Naturalne Związki Organiczne (Natural Organic Compounds); PWN: Warsaw, Poland, 2004; pp. 175–228. (In Polish) [Google Scholar]
- Casuscelli, S.G.; Eimer, G.A.; Canepa, A.; Heredia, A.C.; Poncio, C.E.; Crivello, M.E.; Perez, C.F.; Aguilar, A.; Herrero, E.R. Ti-MCM-41 as Catalyst for α-Pinene Oxidation. Catal. Today 2008, 133–135, 678–683. [Google Scholar] [CrossRef]
- Kapoor, M.P.; Raj, A. Epoxidation over niobium and titanium grafted MCM-41 and MCM-48 mesoporous molecular sieves. Stud. Surf. Sci. Catal. 2000, 129, 327–334. [Google Scholar]
- Suh, Y.-W.; Kim, N.-K.; Ahn, W.-S.; Rhee, H.-K. One-Pot Synthesis of Campholenic Aldehyde from α-Pinene over Ti-HMS Catalyst II: Effects of Reaction Conditions. J. Mol. Catal. A Chem. 2003, 198, 309–316. [Google Scholar] [CrossRef]
- Suh, Y.-W.; Kim, N.-K.; Ahn, W.-S.; Rhee, H.-K. Redox-Mesoporous Molecular Sieve as a Bifunctional Catalyst for the One-Pot Synthesis of Campholenic Aldehyde from α-Pinene. J. Mol. Catal. A Chem. 2001, 174, 249–254. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Serrano, D.P.; Sanz, R.; Pizarro, P.; Moreno, I. Tailoring the Properties of Hierarchical TS-1 Zeolite Synthesized from Silanized Protozeolitic Units. Appl. Catal. A Gen. 2012, 435–436, 32–42. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Han, Q.; Xu, L.; Zhang, S.; Yuan, Y.; Xu, L. Postsynthesis of Hierarchical TS-1 and Its Unique Catalytic Performance in the Direct Hydroxylation of Toluene. Appl. Catal. A Gen. 2020, 598, 117588. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites, 5th revised ed.; Elsevier: Amsterdam, The Netherlands, 2007; p. 276. [Google Scholar]
- Ko, Y.S.; Ahn, W.S. Characterization and Catalytic Properties of Titanium Silicalite-1 Catalyst. Korean J. Chem. Eng. 1998, 15, 182–191. [Google Scholar] [CrossRef]
- Cundy, C.S.; Forrest, J.O. Some Observations on the Preparation and Properties of Colloidal Silicalites. Microporous Mesoporous Mater. 2004, 72, 67–80. [Google Scholar] [CrossRef]
- Zhang, T.; Zuo, Y.; Liu, M.; Song, C.; Guo, X. Synthesis of Titanium Silicalite-1 with High Catalytic Performance for 1-Butene Epoxidation by Eliminating the Extraframework Ti. ACS Omega 2016, 1, 1034–1040. [Google Scholar] [CrossRef]
- Xiong, G.; Jia, Q.; Cao, Y.; Liu, L.; Guo, Z. The Effect of Acid Treatment on the Active Sites and Reaction Intermediates of the Low-Cost TS-1 in Propylene Epoxidation. RSC Adv. 2017, 7, 24046–24054. [Google Scholar] [CrossRef] [Green Version]
- Gadekar, S.P.; Lande, M.K. TS-1 Zeolite as a Lewis Acid Catalyst for Solvent-Free One-Pot Synthesis of 1,3-Thiazolidin-4-Ones. Res. Chem. Intermed. 2019, 45, 237–247. [Google Scholar] [CrossRef]
- Kuznetsova, L.I.; Kuznetsova, N.I.; Lisitsyn, A.S.; Beck, I.E.; Likholobov, V.A.; Ancel, J.E. Liquid-Phase Oxidation of α-Pinene with Oxygen Catalyzed by Carbon-Supported Platinum Metals. Kinet. Catal. 2007, 48, 38–44. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; Yu, H.; Peng, F.; Wang, H. Aerobic Oxidation of α-Pinene Catalyzed by Carbon Nanotubes. Catal. Sci. Technol. 2015, 5, 3935–3944. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Robles-Dutenhefner, P.; Menini, L.; Gusevskaya, E.V. Cobalt Catalyzed Autoxidation of Monoterpenes in Acetic Acid and Acetonitrile Solutions. J. Mol. Catal. A Chem. 2003, 201, 71–77. [Google Scholar] [CrossRef]
- Ancel, J.E.; Maksimchuk, N.V.; Simakova, I.L.; Semikolenov, V.A. Kinetic Peculiarities of α-Pinene Oxidation by Molecular Oxygen. Appl. Catal. A Gen. 2004, 272, 109–114. [Google Scholar] [CrossRef]
- Emanuel, N.M.; Knopre, D.G. Course of Chemical Kinetics; Vysshaya Shkola: Moscow, Russia, 1984; p. 237. [Google Scholar]


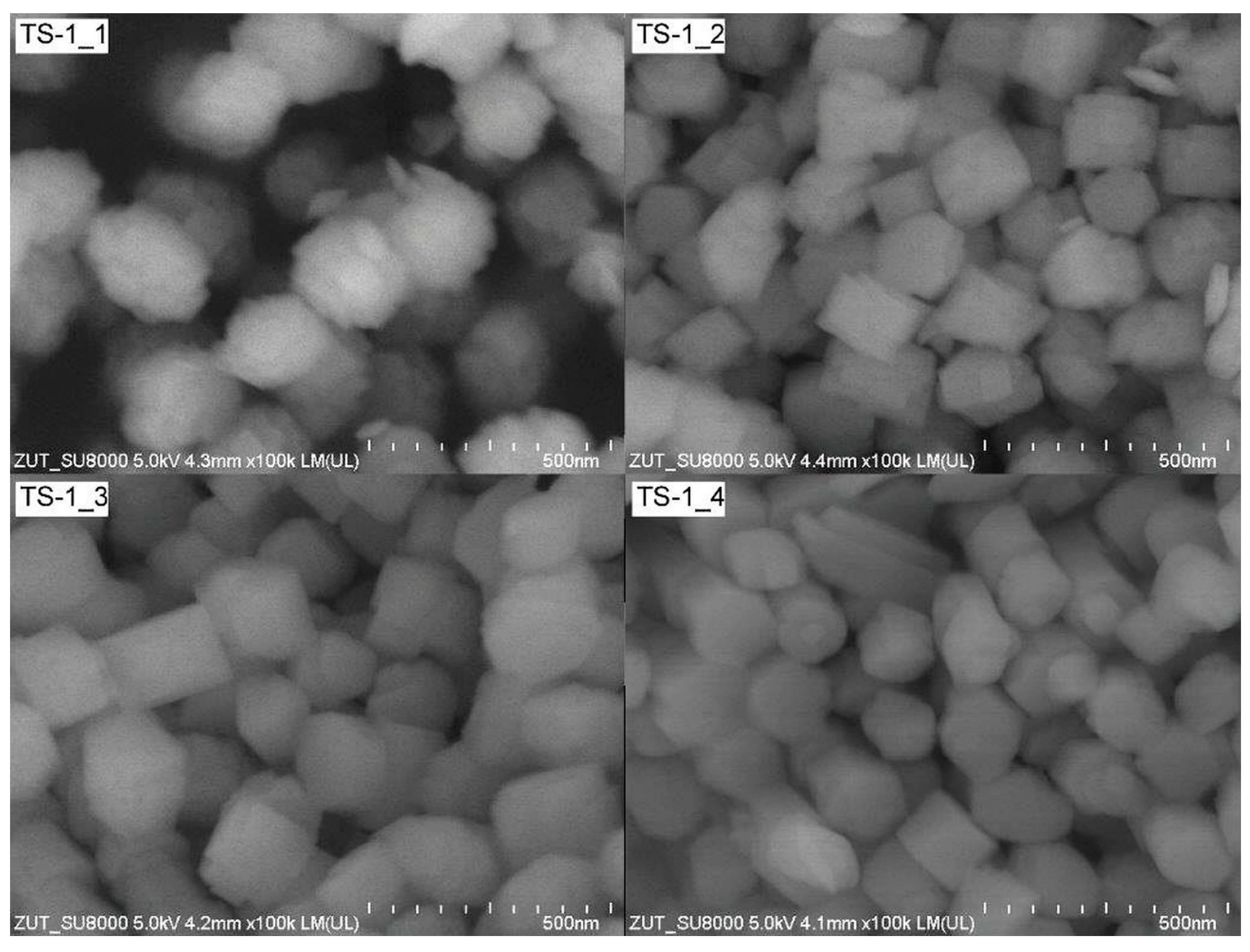
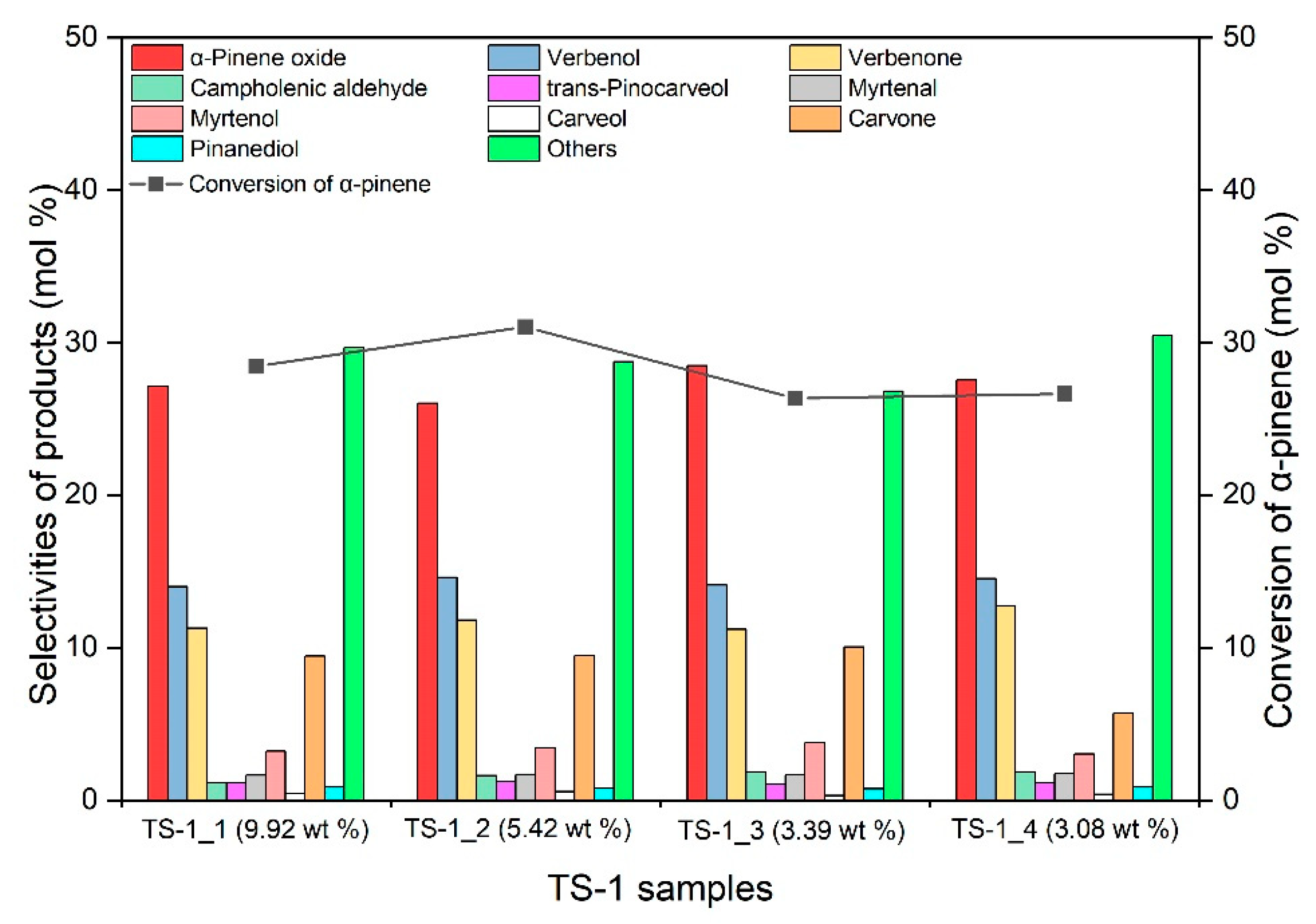


| TS-1 Material | Ti (wt%) | SBET (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) |
|---|---|---|---|---|
| TS-1_1 | 9.92 | 438 | 0.355 | 0.168 |
| TS-1_2 | 5.42 | 463 | 0.397 | 0.180 |
| TS-1_3 | 3.39 | 488 | 0.423 | 0.191 |
| TS-1_4 | 3.08 | 484 | 0.430 | 0.189 |
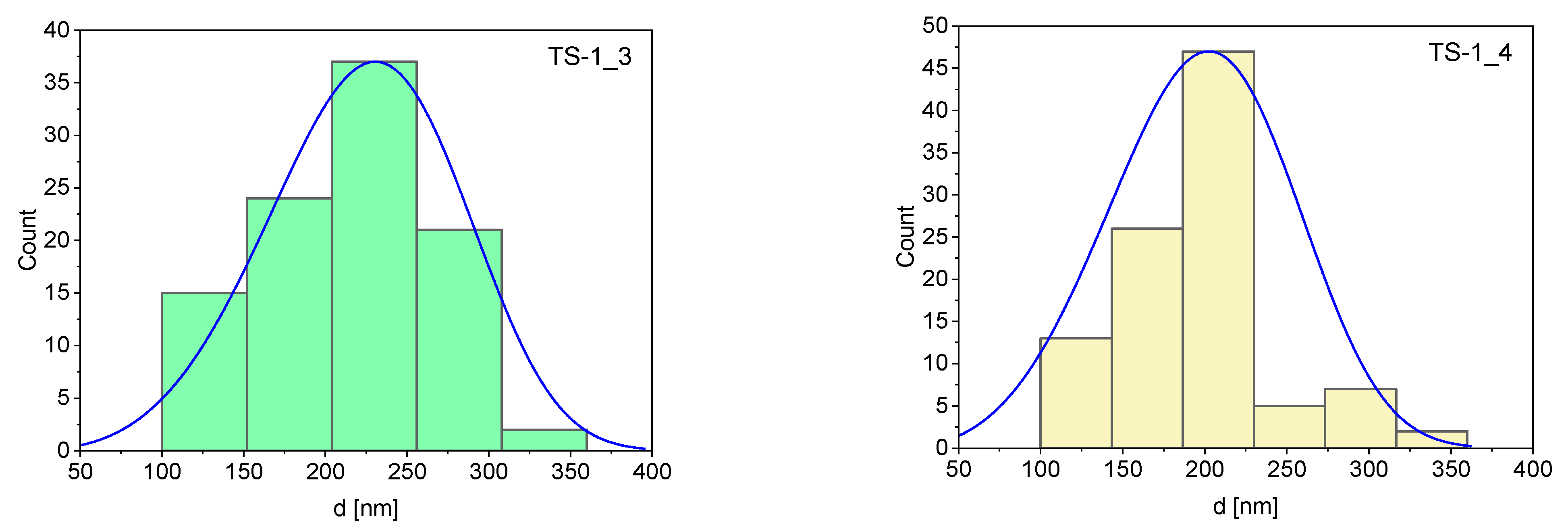
| Sample | Mean (nm) | Standard Deviation | Minimum (nm) | Maximum (nm) |
|---|---|---|---|---|
| TS-1_1 | 299 | 69.8 | 150 | 450 |
| TS-1_2 | 277 | 52.1 | 130 | 380 |
| TS-1_3 | 223 | 58.5 | 110 | 360 |
| TS-1_4 | 198 | 50.3 | 120 | 350 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska, A.; Grzeszczak, J.; Miądlicki, P.; Kiełbasa, K.; Kujbida, M.; Kamińska, A.; Michalkiewicz, B. The Studies on α-Pinene Oxidation over the TS-1. The Influence of the Temperature, Reaction Time, Titanium and Catalyst Content. Materials 2021, 14, 7799. https://doi.org/10.3390/ma14247799
Wróblewska A, Grzeszczak J, Miądlicki P, Kiełbasa K, Kujbida M, Kamińska A, Michalkiewicz B. The Studies on α-Pinene Oxidation over the TS-1. The Influence of the Temperature, Reaction Time, Titanium and Catalyst Content. Materials. 2021; 14(24):7799. https://doi.org/10.3390/ma14247799
Chicago/Turabian StyleWróblewska, Agnieszka, Jadwiga Grzeszczak, Piotr Miądlicki, Karolina Kiełbasa, Marcin Kujbida, Adrianna Kamińska, and Beata Michalkiewicz. 2021. "The Studies on α-Pinene Oxidation over the TS-1. The Influence of the Temperature, Reaction Time, Titanium and Catalyst Content" Materials 14, no. 24: 7799. https://doi.org/10.3390/ma14247799
APA StyleWróblewska, A., Grzeszczak, J., Miądlicki, P., Kiełbasa, K., Kujbida, M., Kamińska, A., & Michalkiewicz, B. (2021). The Studies on α-Pinene Oxidation over the TS-1. The Influence of the Temperature, Reaction Time, Titanium and Catalyst Content. Materials, 14(24), 7799. https://doi.org/10.3390/ma14247799