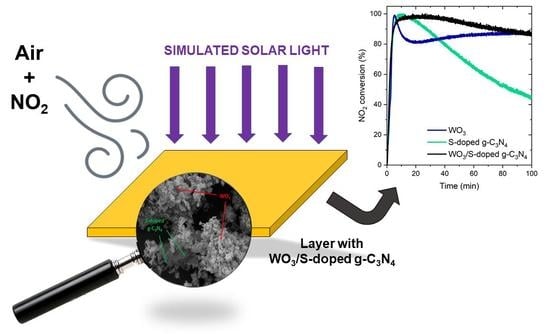
Pilot-Scale Studies of WO3/S-Doped g-C3N4 Heterojunction toward Photocatalytic NOx Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Photocatalysts
2.2.1. Synthesis of WO3
2.2.2. Synthesis of S-Doped-g-C3N4
2.2.3. Synthesis of WO3/S-Doped-g-C3N4 Composite
2.3. Material Characterization
2.4. Coating Methodology
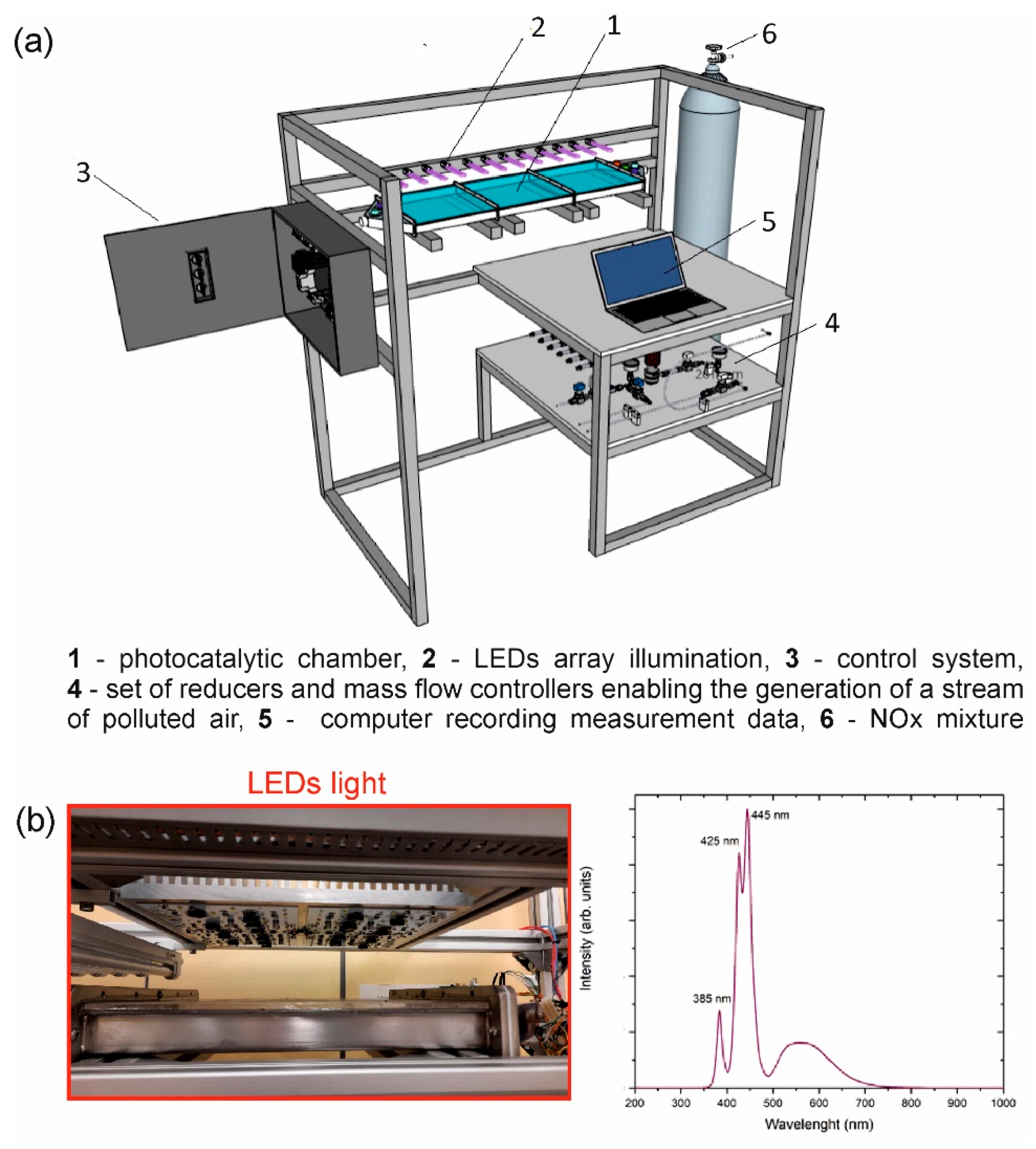
2.5. Photocatalytic Setup
3. Results and Discussion
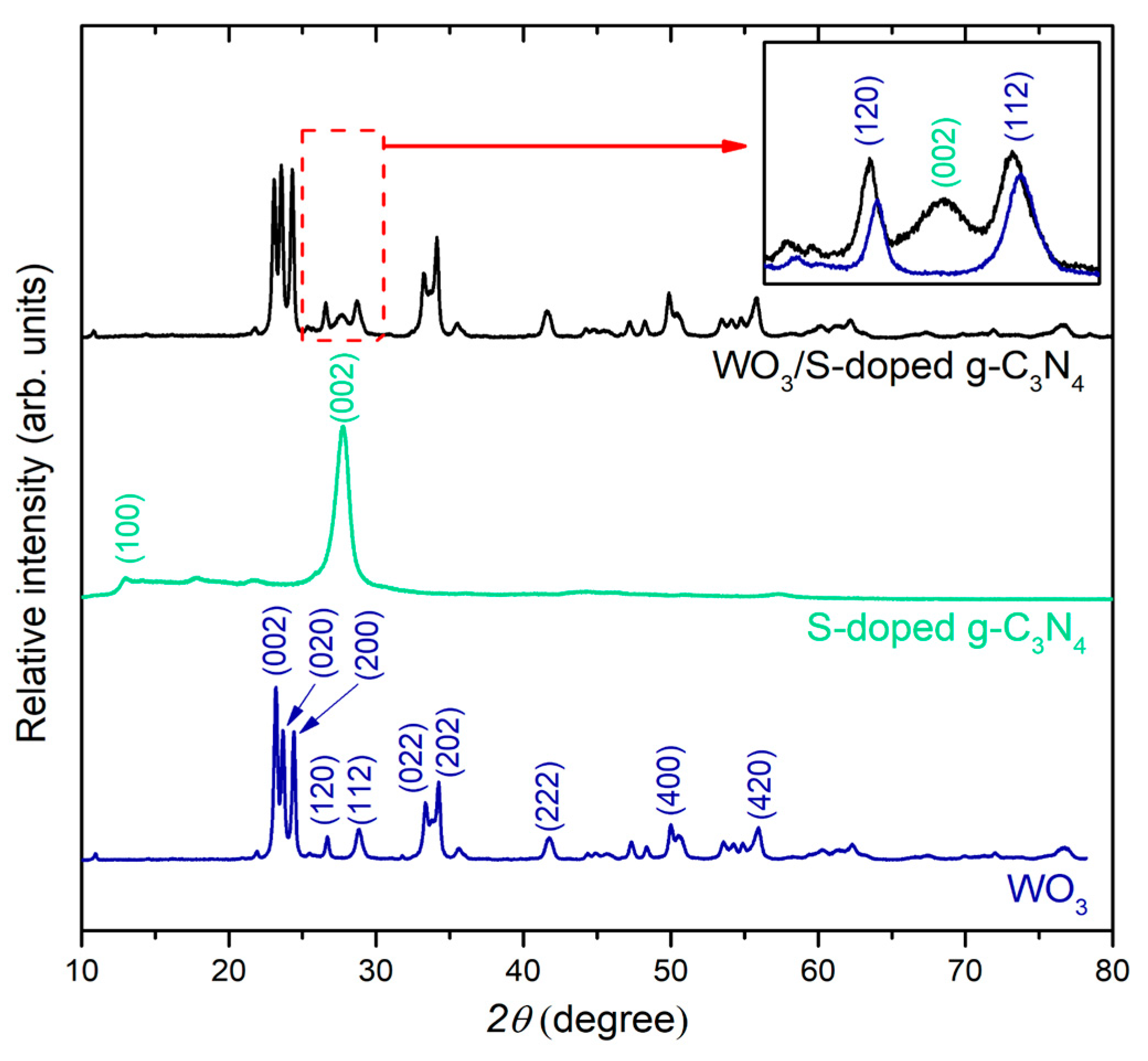
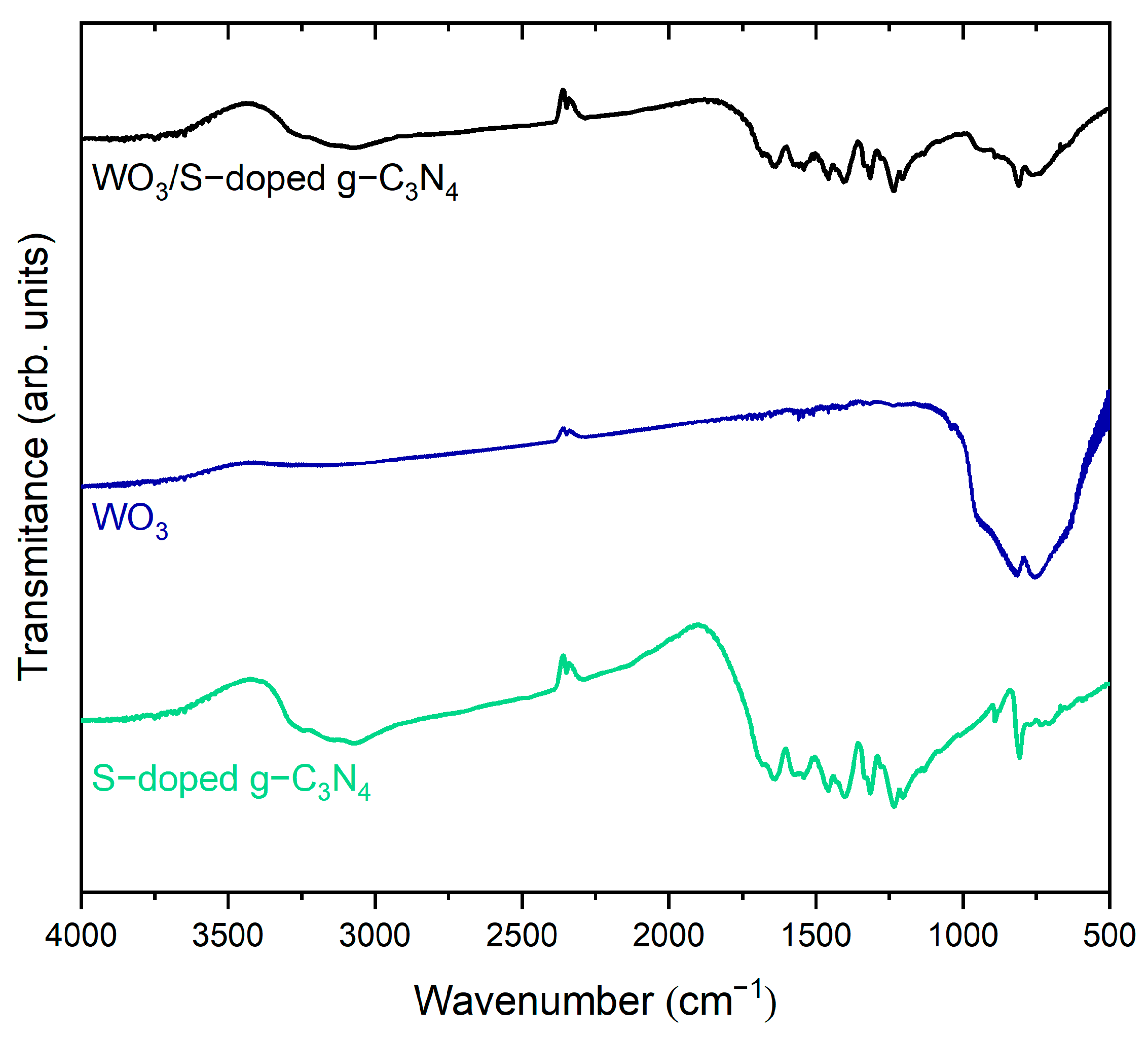
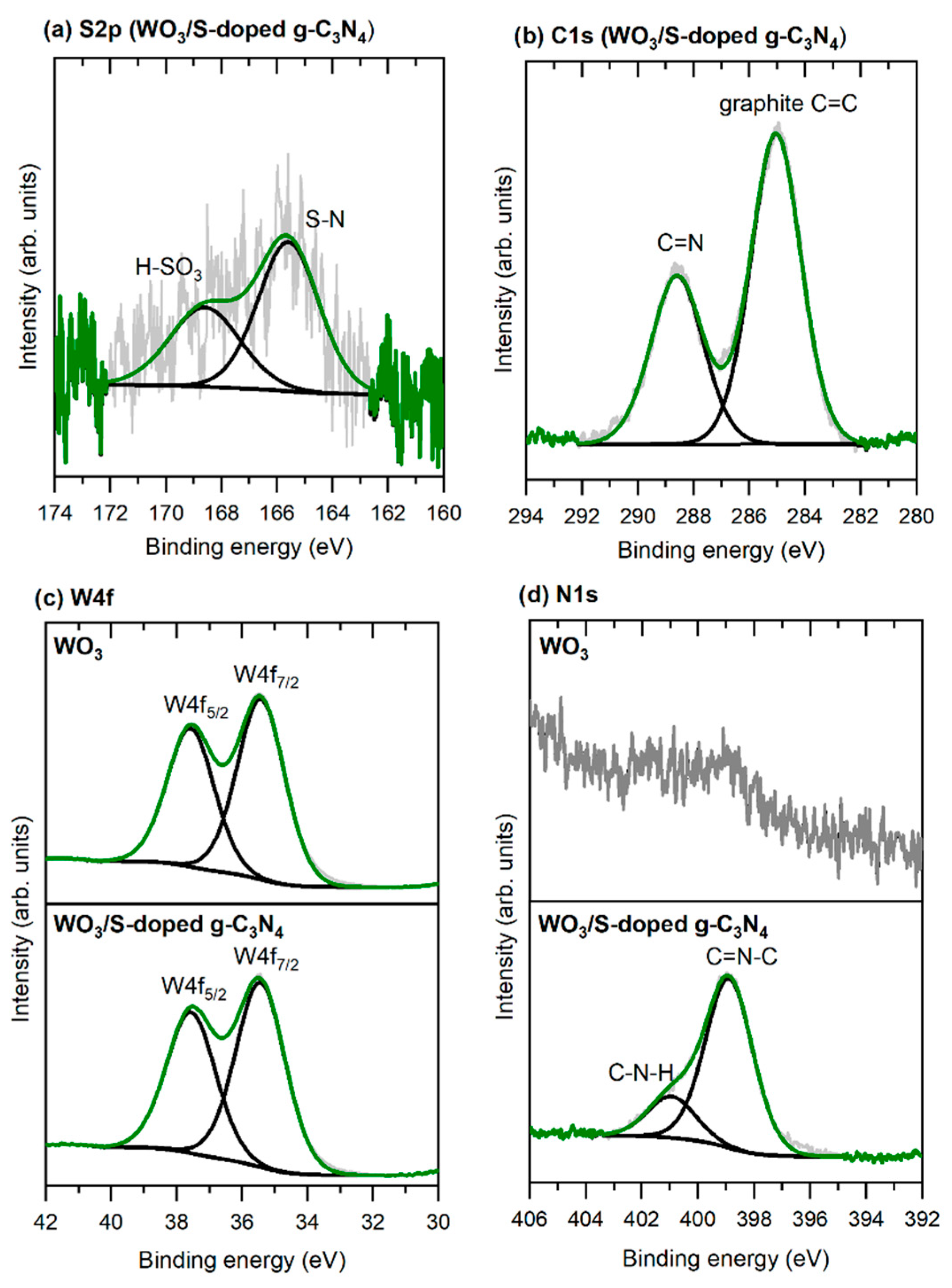
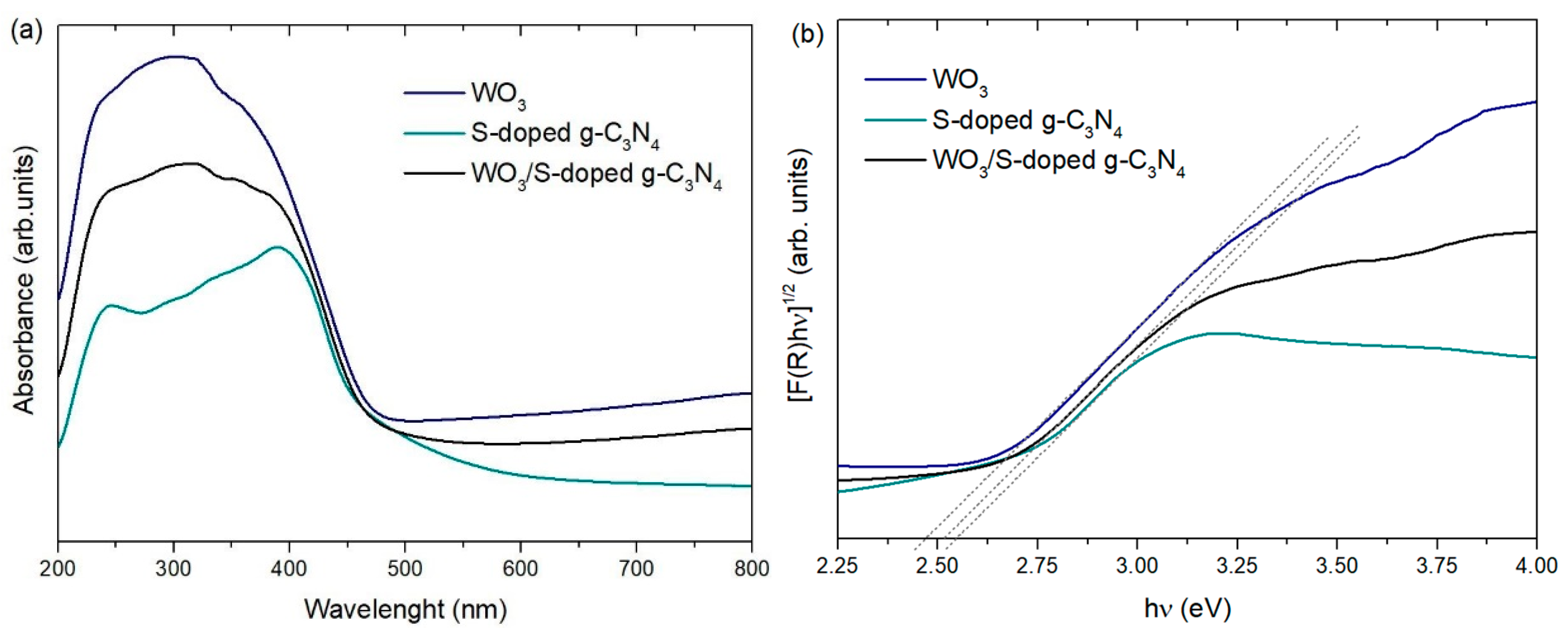
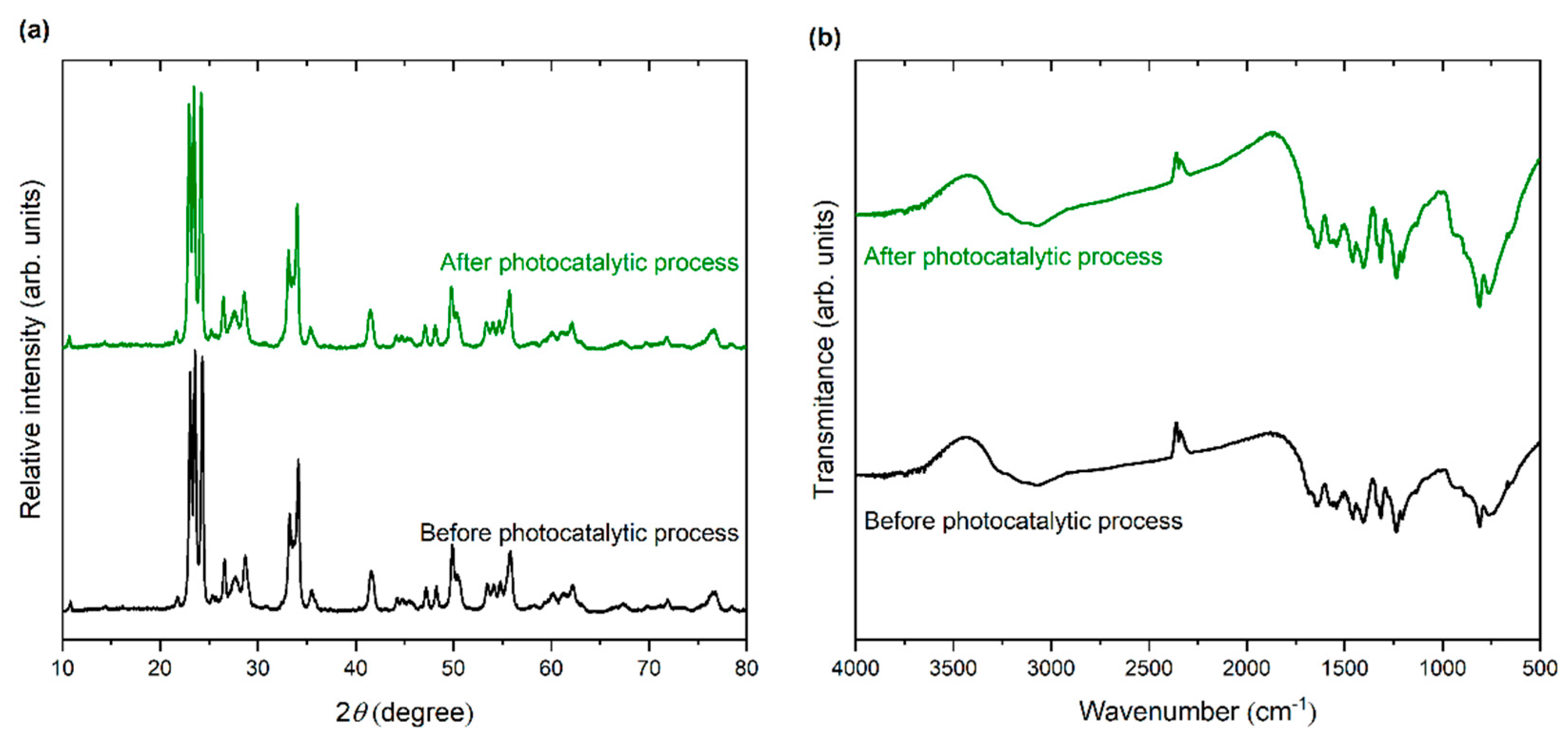
3.1. Characterization of Photocatalysts
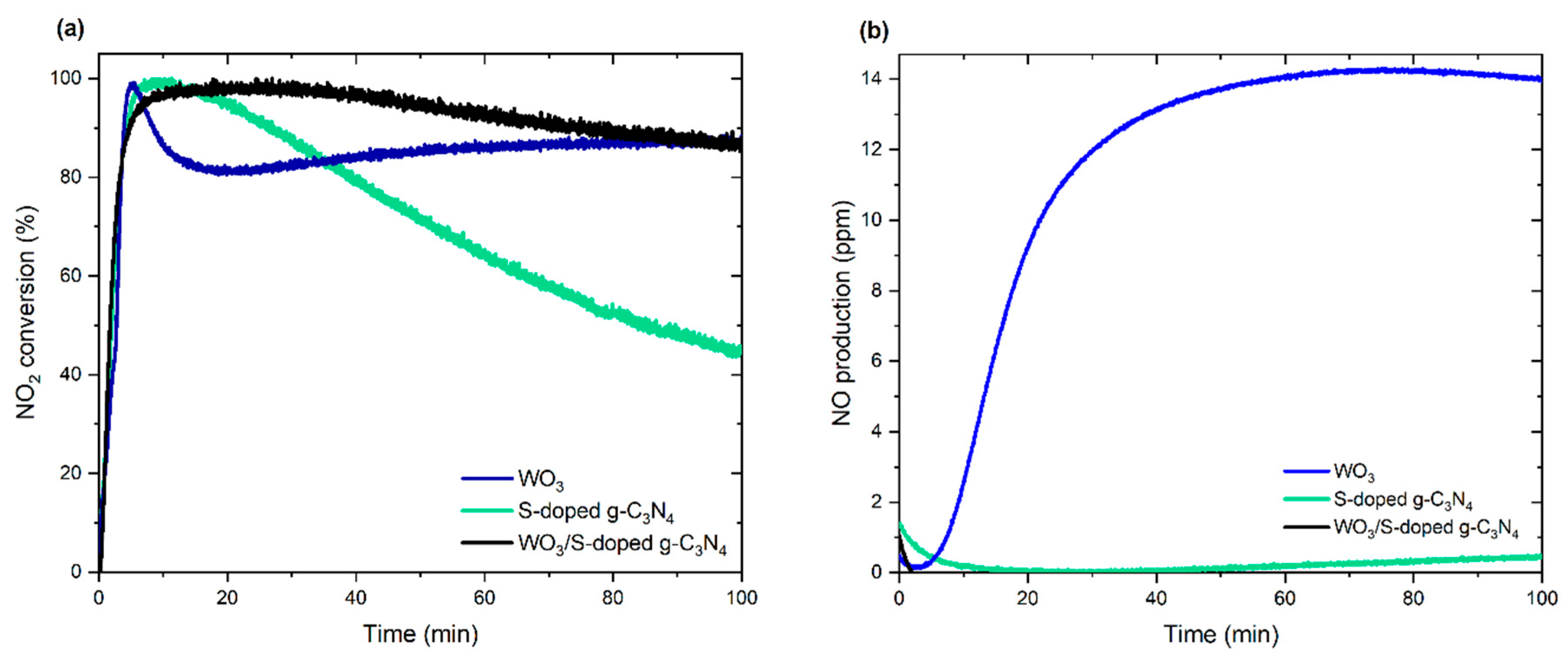
3.2. Photocatalytic Activity and Degradation Mechanism
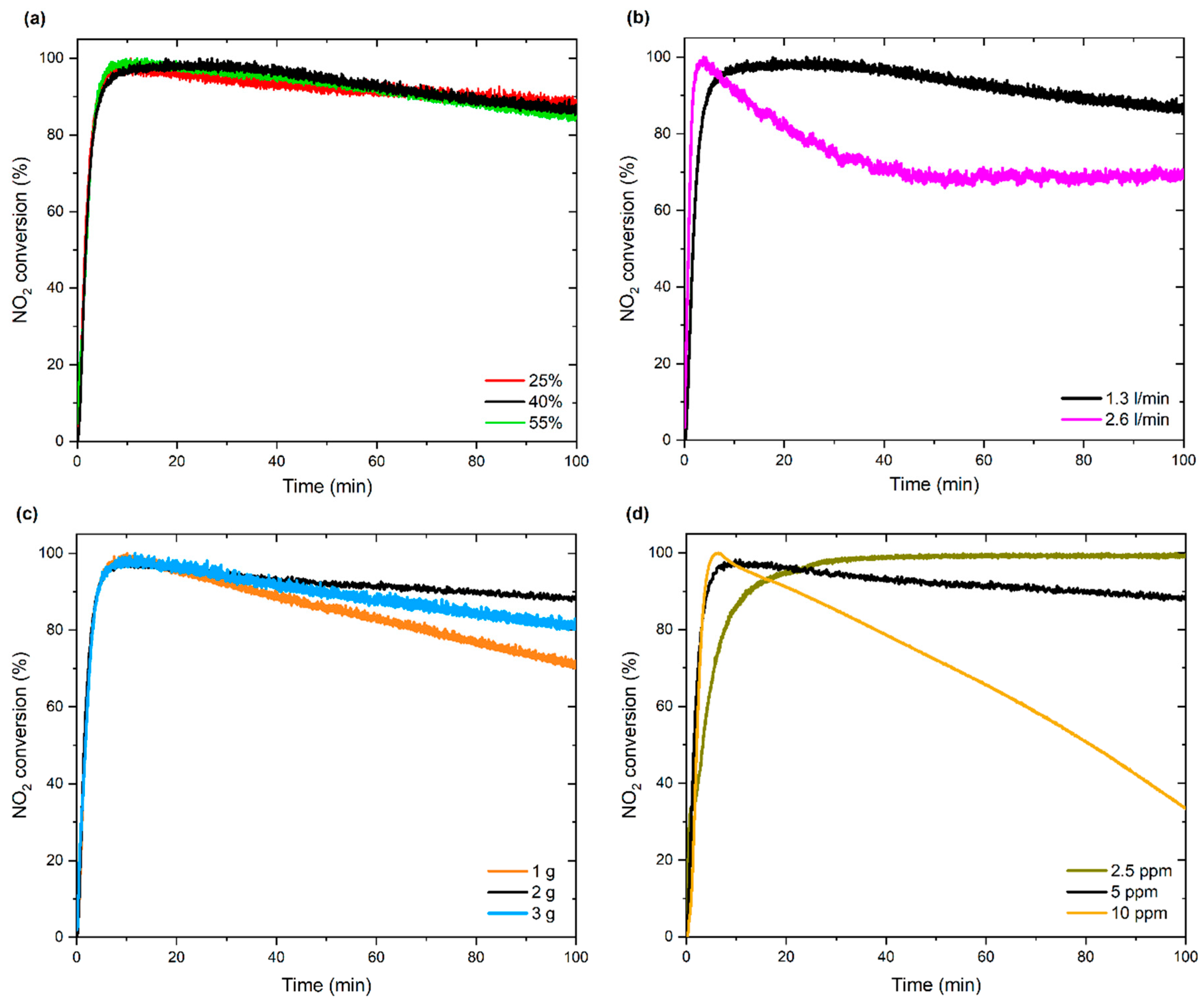
3.2.1. Effect of Process Parameters
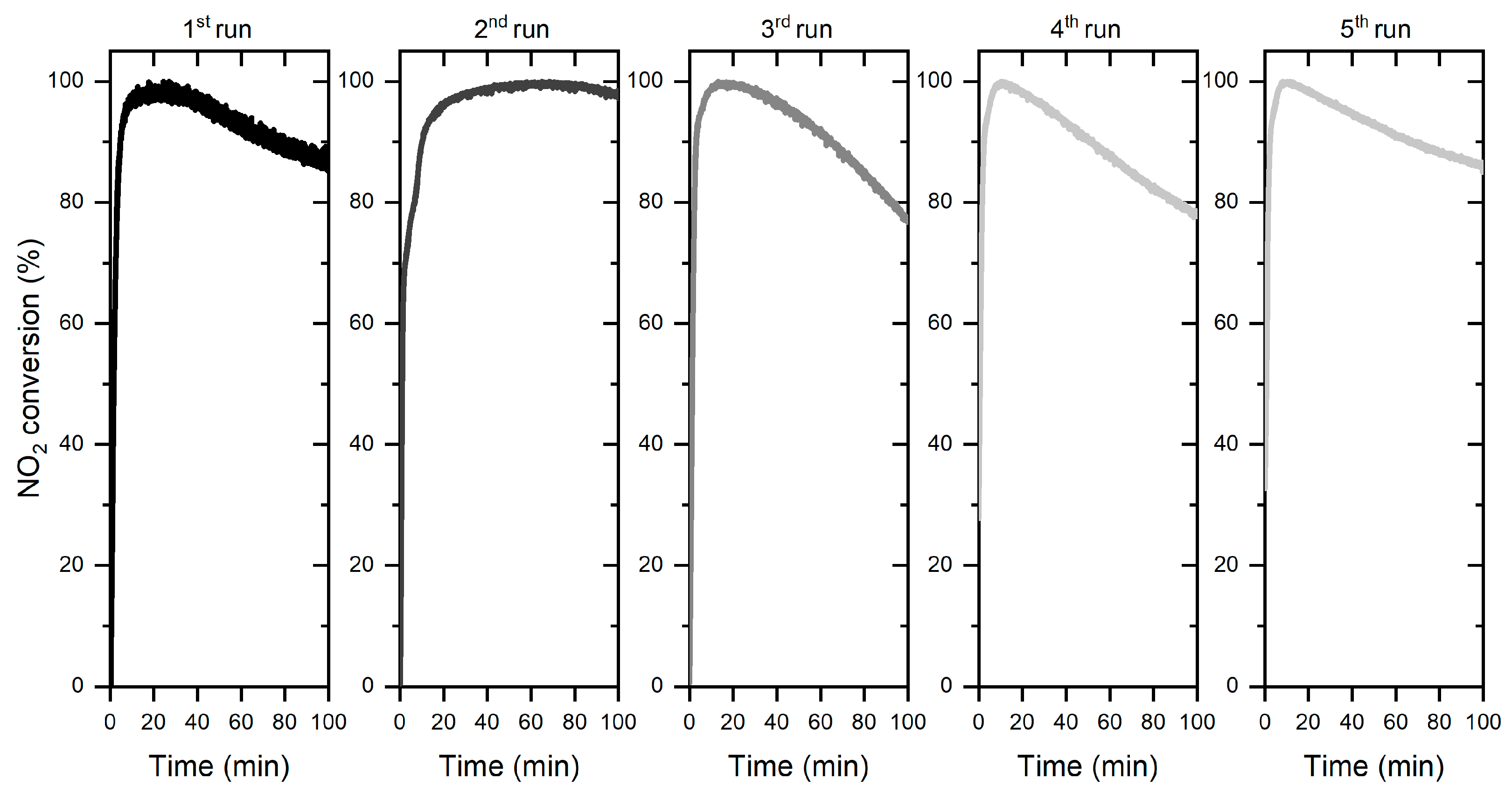
3.2.2. Stability Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, V.H.; Nguyen, B.S.; Huang, C.W.; Le, T.T.; Nguyen, C.C.; Nhi Le, T.T.; Heo, D.; Ly, Q.V.; Trinh, Q.T.; Shokouhimehr, M.; et al. Photocatalytic NOx abatement: Recent advances and emerging trends in the development of photocatalysts. J. Clean. Prod. 2020, 270, 121912. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Geng, J.; Jing, D. Photodecomposition of NOx on Ag/TiO2 composite catalysts in a gas phase reactor. Chem. Eng. J. 2017, 307, 181–188. [Google Scholar] [CrossRef]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxidants Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, P.; Li, H.; Carabineiro, S.A.C. Graphitic carbon nitride: Synthesis, properties, and applications in catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, J.; Li, X.; Li, Y.; Lv, K. Enhanced visible photocatalytic oxidation of NO by repeated calcination of g-C3N4. Appl. Surf. Sci. 2019, 465, 1037–1046. [Google Scholar] [CrossRef]
- Todorova, N.; Papailias, I.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Dallas, P.; Edelmannová, M.; Reli, M.; Koci, K.; Trapalis, C. Photocatalytic H2 Evolution, CO2 Reduction, and NOx Oxidation by Highly Exfoliated g-C3N4. Catalysts 2020, 10, 1147. [Google Scholar] [CrossRef]
- Zhu, Y.; Cui, Y.; Xiao, B.; Ou-yang, J.; Li, H.; Chen, Z. Z-scheme 2D/2D g-C3N4/Sn3O4 heterojunction for enhanced visible-light photocatalytic H2 evolution and degradation of ciprofloxacin. Mater. Sci. Semicond. Process. 2021, 129, 105767. [Google Scholar] [CrossRef]
- Cai, Z.; Huang, Y.; Ji, H.; Liu, W.; Fu, J.; Sun, X. Type-II surface heterojunction of bismuth-rich Bi4O5Br2 on nitrogen-rich g-C3N5 nanosheets for efficient photocatalytic degradation of antibiotics. Sep. Purif. Technol. 2022, 280, 119772. [Google Scholar] [CrossRef]
- Ling, F.; Li, W.; Ye, L. The synergistic effect of non-metal doping or defect engineering and interface coupling on the photocatalytic property of g-C3N4: First-principle investigations. Appl. Surf. Sci. 2019, 473, 386–392. [Google Scholar] [CrossRef]
- Kadam, A.N.; Kim, H.; Lee, S.W. Low-temperature in situ fabrication of porous S-doped g-C3N4 nanosheets using gaseous-bubble template for enhanced visible-light photocatalysis. Ceram. Int. 2020, 46, 28481–28489. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.W. Porous nanoplate-like tungsten trioxide/reduced graphene oxide catalyst for sonocatalytic degradation and photocatalytic hydrogen production. Surf. Interfaces 2021, 24, 101075. [Google Scholar] [CrossRef]
- Xiao, T.; Tang, Z.; Yang, Y.; Tang, L.; Zhou, Y.; Zou, Z. In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics. Appl. Catal. B Environ. 2018, 220, 417–428. [Google Scholar] [CrossRef]
- Singh, J.; Arora, A.; Basu, S. Synthesis of coral like WO3/g-C3N4 nanocomposites for the removal of hazardous dyes under visible light. J. Alloys Compd. 2019, 808, 151734. [Google Scholar] [CrossRef]
- Katsumata, H.; Tachi, Y.; Suzuki, T.; Kaneco, S. Z-scheme photocatalytic hydrogen production over WO3/g-C3N4 composite photocatalysts. RSC Adv. 2014, 4, 21405–21409. [Google Scholar] [CrossRef]
- Huang, M.; Wen, X. Experimental study on photocatalytic effect of nano TiO2 epoxy emulsified asphalt mixture. Appl. Sci. 2019, 9, 2464. [Google Scholar] [CrossRef] [Green Version]
- Segundo, I.R.; Freitas, E.; Landi, S.; Costa, M.F.M.; Carneiro, J.O. Smart, photocatalytic and self-cleaning asphalt mixtures: A literature review. Coatings 2019, 9, 696. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Wang, S.; Zhang, J.; Fan, J. Photocatalytic degradation of vehicle exhausts on asphalt pavement by TiO2/rubber composite structure. Constr. Build. Mater. 2015, 81, 224–232. [Google Scholar] [CrossRef]
- Nevshupa, R.; Jimenez-Relinque, E.; Grande, M.; Martinez, E.; Castellote, M. Assessment of urban air pollution related to potential nanoparticle emission from photocatalytic pavements. J. Environ. Manag. 2020, 272, 111059. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A. On-farm pilot-scale testing of black ultraviolet light and photocatalytic coating for mitigation of odor, odorous VOCs, and greenhouse gases. Chemosphere 2019, 221, 778–784. [Google Scholar] [CrossRef]
- Hu, J.; Xia, H.; Hu, H.; Zhang, Y.; Jiang, H.; Chen, B. Synthesis and efficient near-infrared quantum cutting of Pr 3+/Yb 3+ co-doped LiYF 4 single crystals. J. Appl. Phys. 2012, 112, 073518. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Li, W.; Liu, Q.; Yang, Y.; Li, Y.; Chen, Q. Enhanced photoelectrochemical performance of WO3 film with HfO2 passivation layer. Int. J. Hydrogen Energy 2015, 40, 8856–8863. [Google Scholar] [CrossRef]
- Najafi-Ashtiani, H.; Bahari, A.; Gholipour, S.; Hoseinzadeh, S. Structural, optical and electrical properties of WO3–Ag nanocomposites for the electro-optical devices. Appl. Phys. A Mater. Sci. Process. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Kim, D.; Kim, G.; Bae, H.; Kim, E.; Moon, B.; Cheon, D.; Tarte, N.H. An external energy independent WO3/MoCl5 nano-sized catalyst for the superior degradation of crystal violet and rhodamine B dye. Catalysts 2019, 9, 642. [Google Scholar] [CrossRef] [Green Version]
- Fahimirad, B.; Asghari, A.; Rajabi, M. Magnetic graphitic carbon nitride nanoparticles covalently modified with an ethylenediamine for dispersive solid-phase extraction of lead(II) and cadmium(II) prior to their quantitation by FAAS. Microchim. Acta 2017, 184, 3027–3035. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, S.; Yu, J.S. Novel ordered nanoporous graphitic C3N4 as a support for Pt-Ru anode catalyst in direct methanol fuel cell. J. Mater. Chem. 2007, 17, 1656–1659. [Google Scholar] [CrossRef]
- Wang, Z.T.; Xu, J.L.; Zhou, H.; Zhang, X. Facile synthesis of Zn(II)-doped g-C3N4 and their enhanced photocatalytic activity under visible light irradiation. Rare Met. 2019, 38, 459–467. [Google Scholar] [CrossRef]
- Lin, M.Z.; Chen, H.; Chen, W.F.; Nakaruk, A.; Koshy, P.; Sorrell, C.C. Effect of single-cation doping and codoping with Mn and Fe on the photocatalytic performance of TiO2 thin films. Int. J. Hydrogen Energy 2014, 39, 21500–21511. [Google Scholar] [CrossRef]
- Chhabra, T.; Bahuguna, A.; Dhankhar, S.S.; Nagaraja, C.M.; Krishnan, V. Sulfonated graphitic carbon nitride as a highly selective and efficient heterogeneous catalyst for the conversion of biomass-derived saccharides to 5-hydroxymethylfurfural in green solvents. Green Chem. 2019, 21, 6012–6026. [Google Scholar] [CrossRef]
- Gao, D.; Liu, Y.; Liu, P.; Si, M.; Xue, D. Atomically Thin B doped g-C3N4 Nanosheets: High-Temperature Ferromagnetism and calculated Half-Metallicity. Sci. Rep. 2016, 6, 35768. [Google Scholar] [CrossRef]
- Alman, V.; Singh, K.; Bhat, T.; Sheikh, A.; Gokhale, S. Sunlight Assisted improved photocatalytic degradation of rhodamine B using Pd-loaded g-C3N4/WO3 nanocomposite. Appl. Phys. A Mater. Sci. Process. 2020, 126, 724. [Google Scholar] [CrossRef]
- Gong, S.; Jiang, Z.; Zhu, S. Heterojunctions With Enhanced Visible-Light Photocatalytic Activities. J. Nanopart Res. 2018, 20, 310–323. [Google Scholar] [CrossRef]
- Wang, P.; Guan, Z.; Li, Q.; Yang, J. Efficient visible-light-driven photocatalytic hydrogen production from water by using Eosin Y-sensitized novel g-C3N4/Pt/GO composites. J. Mater. Sci. 2018, 53, 774–786. [Google Scholar] [CrossRef]
- Dalton, J.S.; Janes, P.; Jones, N.; Hallam, K.R.; Nicholson, J.A.; Allen, G.C. Photocatalytic oxidation of NOx gases using TiO2: A spectroscopic approach. Environ. Pollut. 2002, 45120, 415–422. [Google Scholar] [CrossRef]
- Hao, Q.; Song, Y.; Ji, H.; Mo, Z.; She, X.; Deng, J.; Muhmood, T.; Wu, X.; Yuan, S.; Xu, H.; et al. Surface N modified 2D g-C3N4 nanosheets derived from DMF for photocatalytic H2 evolution. Appl. Surf. Sci. 2018, 459, 845–852. [Google Scholar] [CrossRef]
- Wang, C.; Fu, M.; Cao, J.; Wu, X.; Hu, X.; Dong, F. BaWO4/g-C3N4 heterostructure with excellent bifunctional photocatalytic performance. Chem. Eng. J. 2020, 385, 123833. [Google Scholar] [CrossRef]
- Wu, Z.; Sheng, Z.; Liu, Y.; Wang, H.; Mo, J. Deactivation mechanism of PtOx/TiO2 photocatalyst towards the oxidation of NO in gas phase. J. Hazard. Mater. 2011, 185, 1053–1058. [Google Scholar] [CrossRef]
- Yu, J.C.C.; Lasek, J.; Nguyen, V.H.; Yu, Y.H.; Wu, J.C.S. Visualizing reaction pathway for the photo-transformation of NO2 and N2 into NO over WO3 photocatalyst. Res. Chem. Intermed. 2017, 43, 7159–7169. [Google Scholar] [CrossRef]
- Méndez-Román, R.; Cardona-Martínez, N. Relationship between the formation of surface species and catalyst deactivation during the gas-phase photocatalytic oxidation of toluene. Catal. Today 1998, 40, 353–365. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Wu, H.Y.; Bai, H. Photocatalytic reduction of NO2 and CO2 using molybdenum-doped titania nanotubes. Chem. Eng. J. 2015, 269, 60–66. [Google Scholar] [CrossRef]
- Li, Z.; Wu, S.; Zhang, J.; Yuan, Y.; Wang, Z.; Zhu, Z. Improving Photovoltaic Performance Using Perovskite/Surface-Modified Graphitic Carbon Nitride Heterojunction. Sol. RRL 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Yang, L.; Hakki, A.; Zheng, L.; Jones, M.R.; Wang, F.; Macphee, D.E. Photocatalytic concrete for NOx abatement: Supported TiO2 efficiencies and impacts. Cem. Concr. Res. 2019, 116, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Lasek, J.; Yu, Y.H.; Wu, J.C.S. Removal of NOx by photocatalytic processes. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 29–52. [Google Scholar] [CrossRef]
- Maggos, T.; Bartzis, J.G.; Leva, P.; Kotzias, D. Application of photocatalytic technology for NOx removal. Appl. Phys. A Mater. Sci. Process. 2007, 89, 81–84. [Google Scholar] [CrossRef]
- Casagrande, C.A.; Repette, W.L.; Hotza, D. Effect of environmental conditions on degradation of NOx gases by photocatalytic nanotitania-based cement mortars after long-term hydration. J. Clean. Prod. 2020, 274, 123067. [Google Scholar] [CrossRef]
- Li, S.; Hao, Z.; Wang, K.; Tong, M.; Yang, Y.; Jiang, H.; Xiao, Y.; Zhang, F. Visible light-enabled selective depolymerization of oxidized lignin by an organic photocatalyst. Chem. Commun. 2020, 56, 11243–11246. [Google Scholar] [CrossRef] [PubMed]


| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Bandgap (eV) | Average Carrier Lifetime (ns) | |
|---|---|---|---|---|---|
| 300 nm | 415 nm | ||||
| WO3 | 14.0 | 0.0069 | 2.48 | - | - |
| S-doped g-C3N4 | 14.5 | 0.0073 | 2.55 | 15.3 | 16.1 |
| WO3/S-doped g-C3N4 | 11.5 | 0.0058 | 2.51 | 17.6 | 14.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalkińska, M.; Fiszka Borzyszkowska, A.; Grzegórska, A.; Karczewski, J.; Głuchowski, P.; Łapiński, M.; Sawczak, M.; Zielińska-Jurek, A. Pilot-Scale Studies of WO3/S-Doped g-C3N4 Heterojunction toward Photocatalytic NOx Removal. Materials 2022, 15, 633. https://doi.org/10.3390/ma15020633
Kowalkińska M, Fiszka Borzyszkowska A, Grzegórska A, Karczewski J, Głuchowski P, Łapiński M, Sawczak M, Zielińska-Jurek A. Pilot-Scale Studies of WO3/S-Doped g-C3N4 Heterojunction toward Photocatalytic NOx Removal. Materials. 2022; 15(2):633. https://doi.org/10.3390/ma15020633
Chicago/Turabian StyleKowalkińska, Marta, Agnieszka Fiszka Borzyszkowska, Anna Grzegórska, Jakub Karczewski, Paweł Głuchowski, Marcin Łapiński, Mirosław Sawczak, and Anna Zielińska-Jurek. 2022. "Pilot-Scale Studies of WO3/S-Doped g-C3N4 Heterojunction toward Photocatalytic NOx Removal" Materials 15, no. 2: 633. https://doi.org/10.3390/ma15020633