Determination of the Kinetics and Thermodynamic Parameters of Lignocellulosic Biomass Subjected to the Torrefaction Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
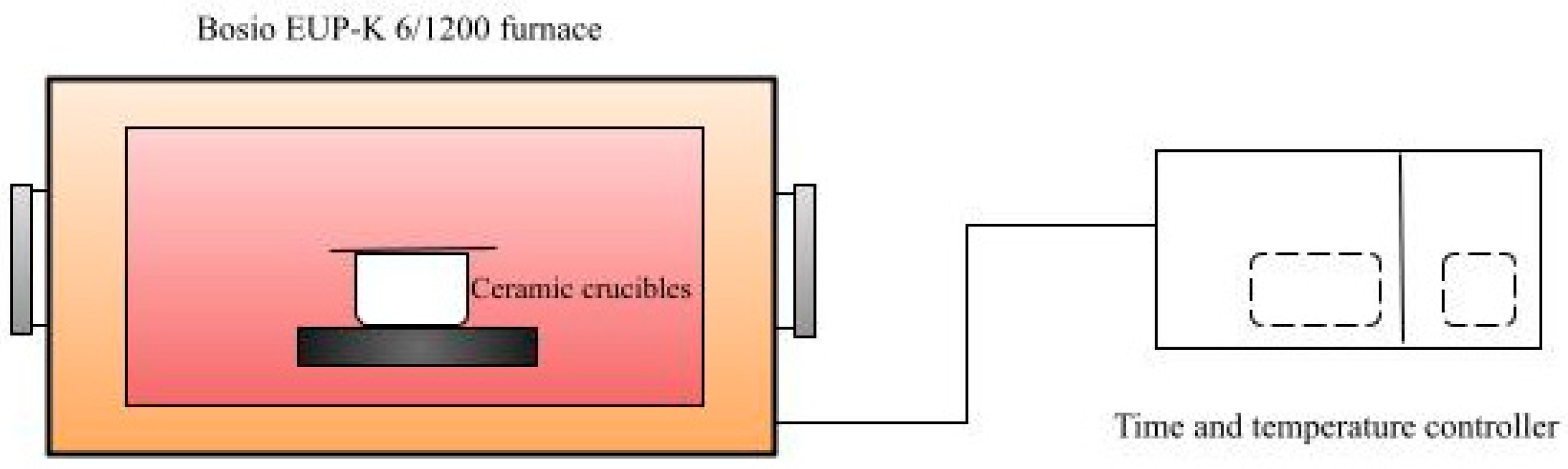
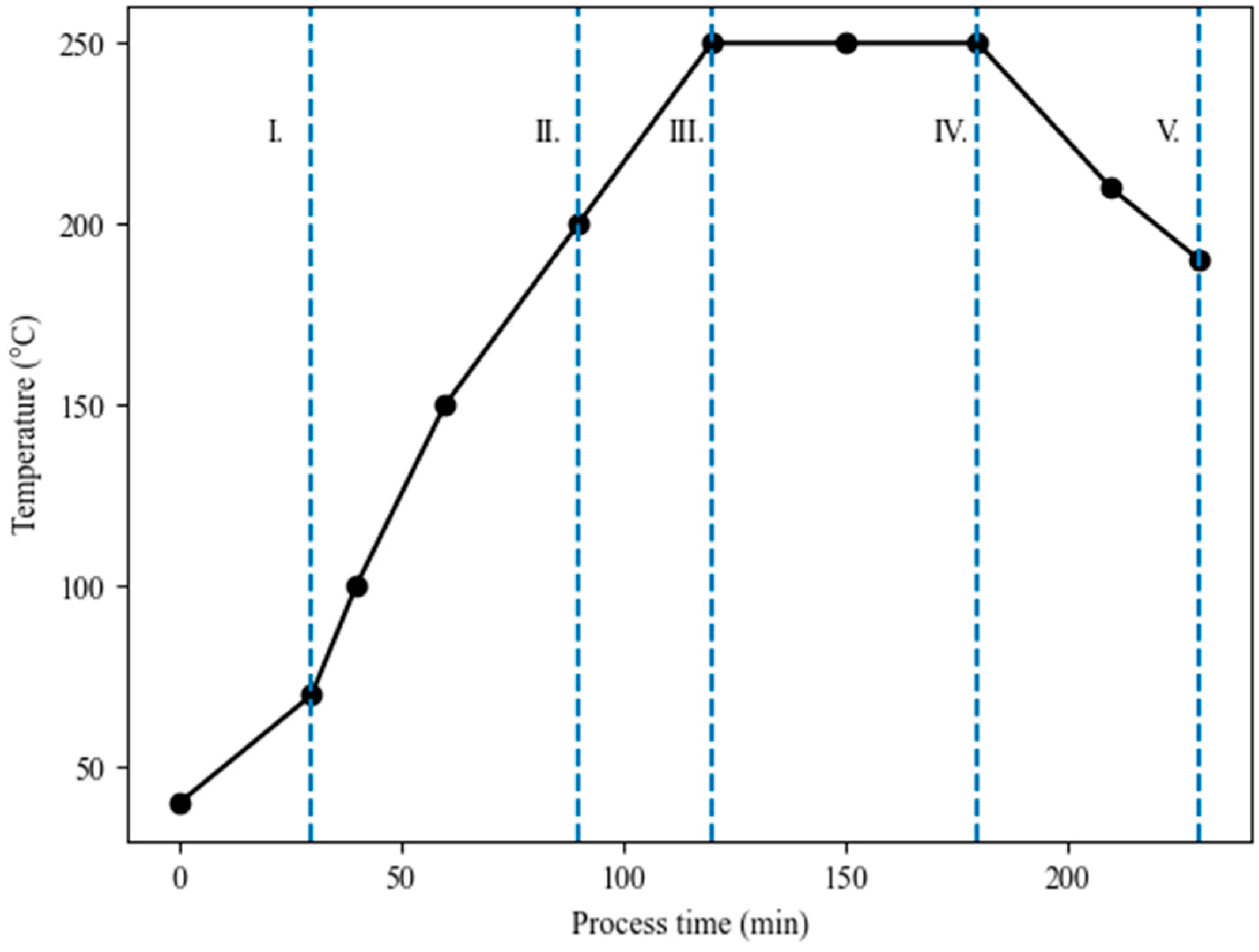
2.2. Torrefaction Process and Biomass Characterization
2.3. Fourier Transform–Infrared Spectroscopy (FTIR)
2.4. Thermogravimetric Analysis
2.5. Kinetic Theory
2.5.1. Friedman Isoconversional Kinetic Model
2.5.2. The Kissinger–Akahira–Sunose Isoconversional Kinetic Model
2.6. Thermodynamic Parameters
3. Results
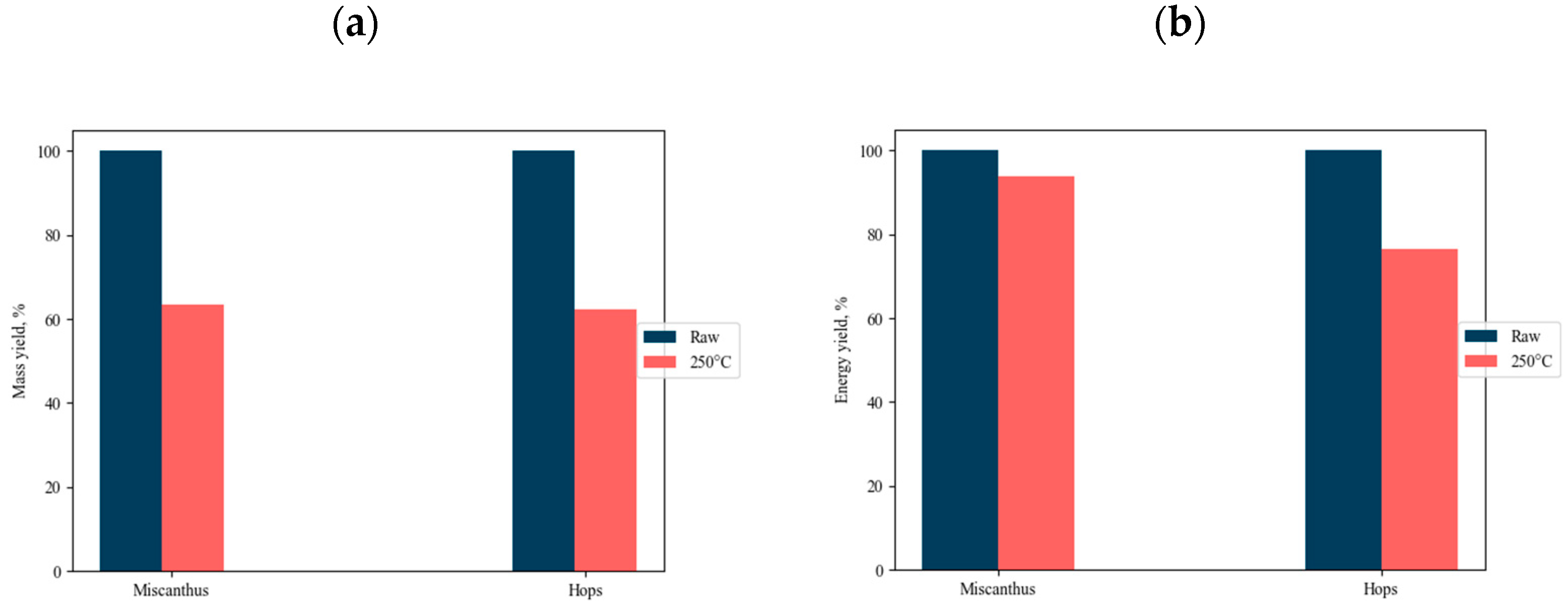
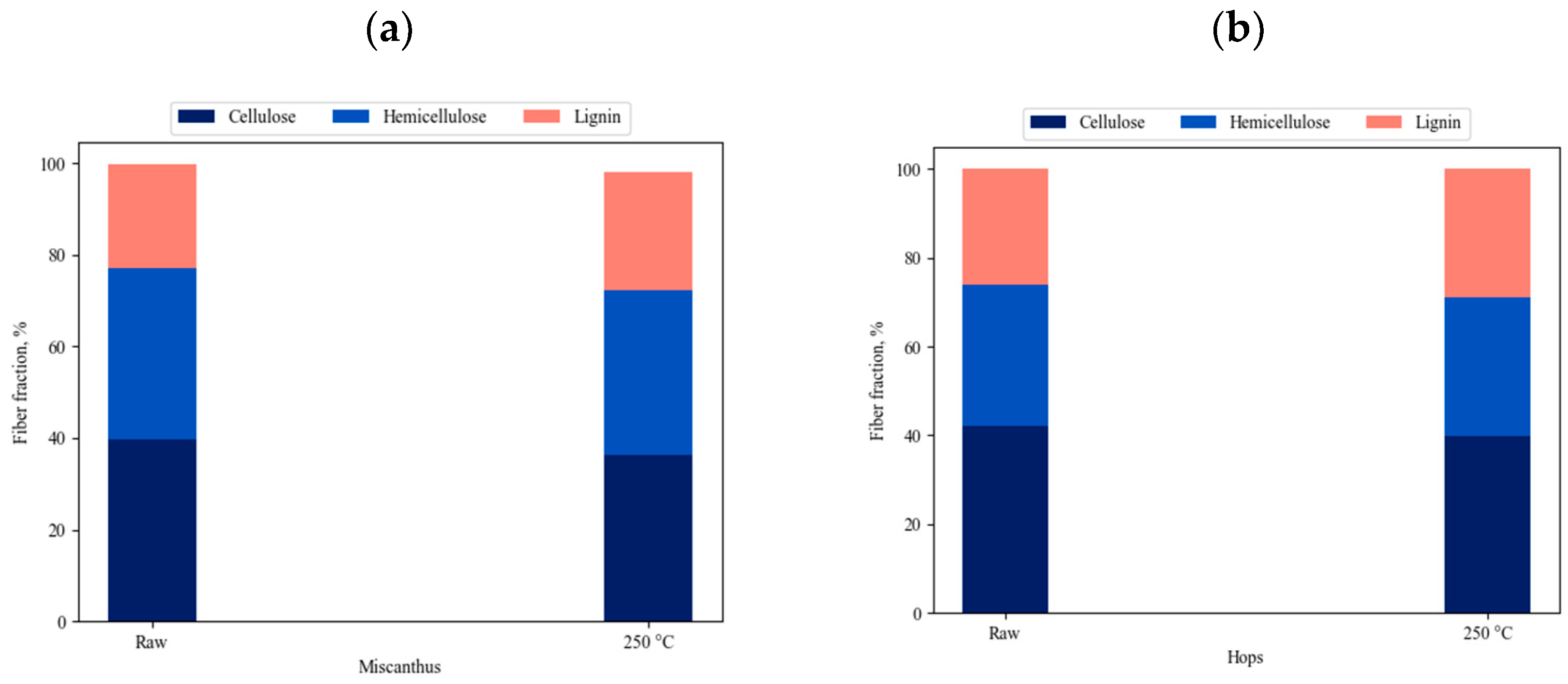
3.1. Characterization of the Raw and Torrefied Material
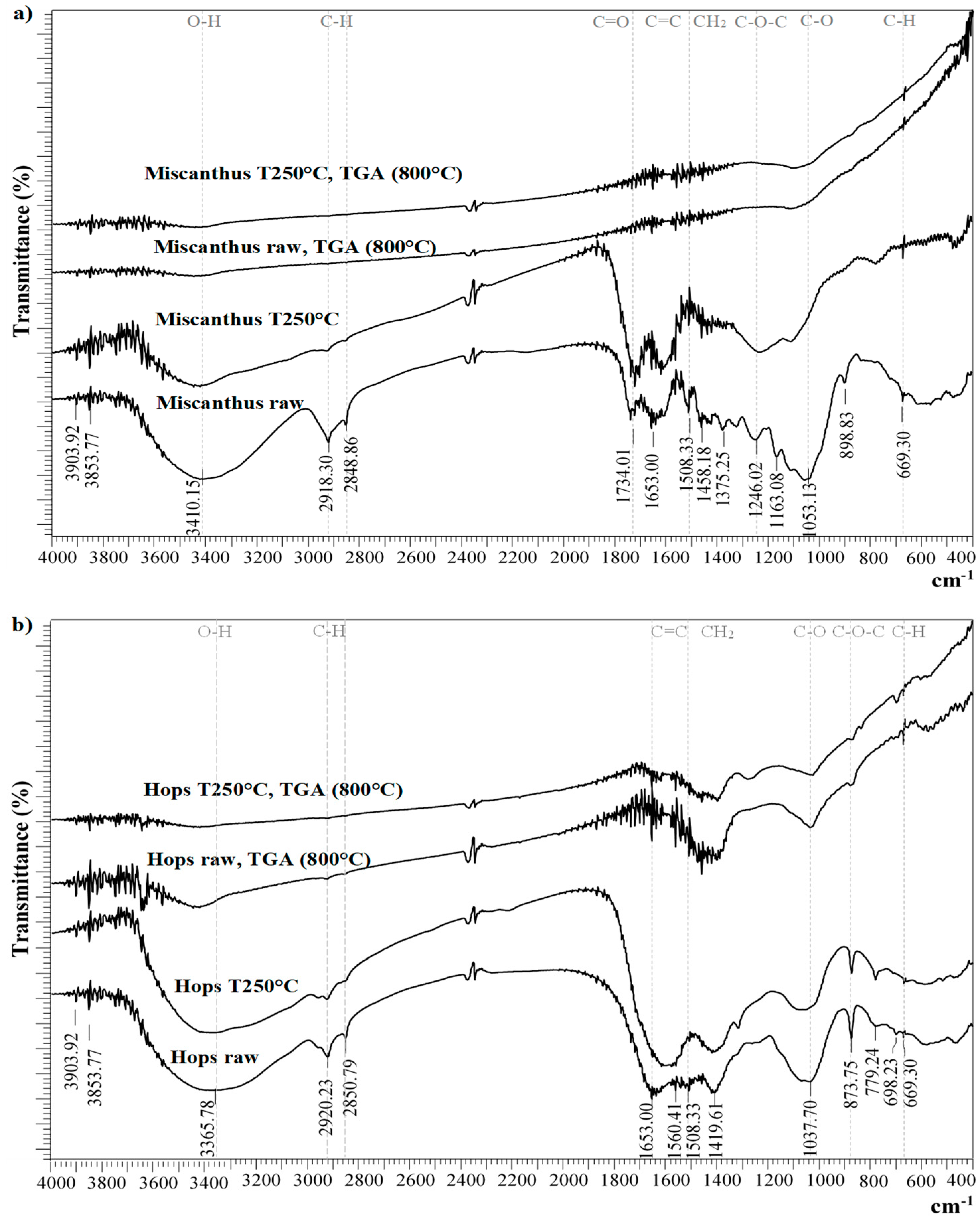
3.2. Fourier Transform–Infrared Spectroscopy (FTIR)
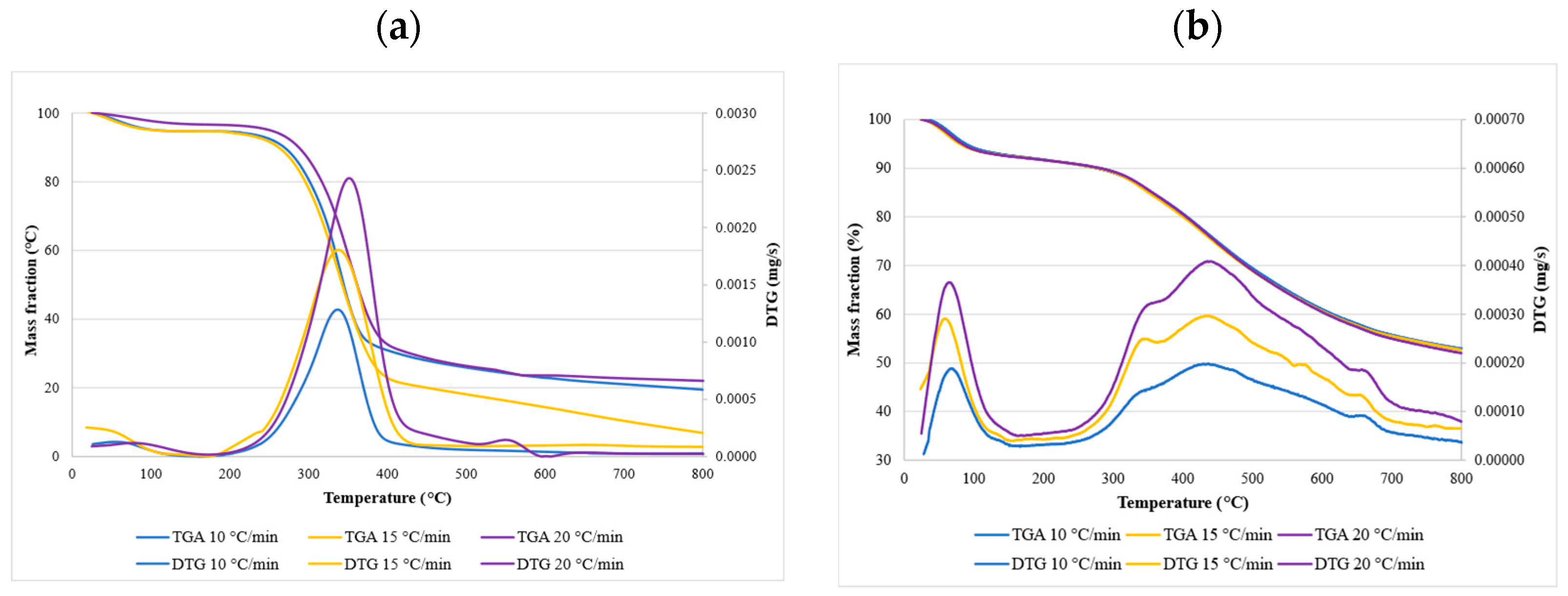
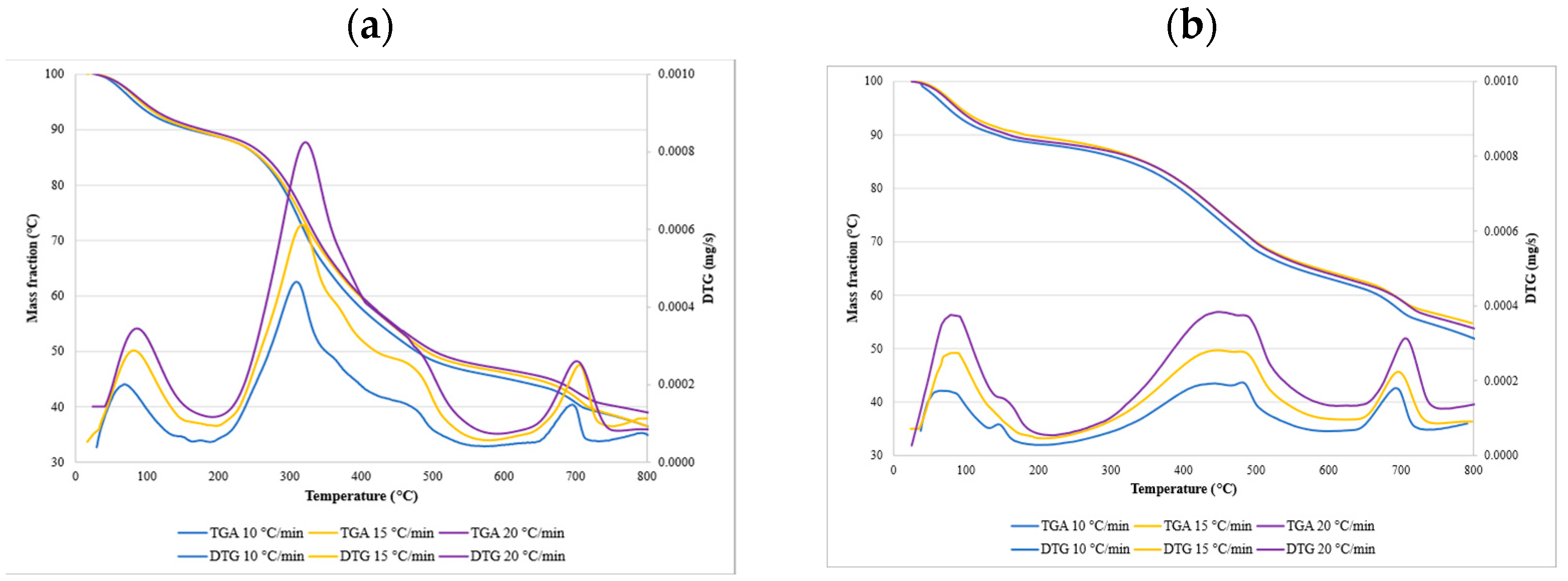
3.3. The Thermal Degradation Process
3.4. Kinetic Analysis
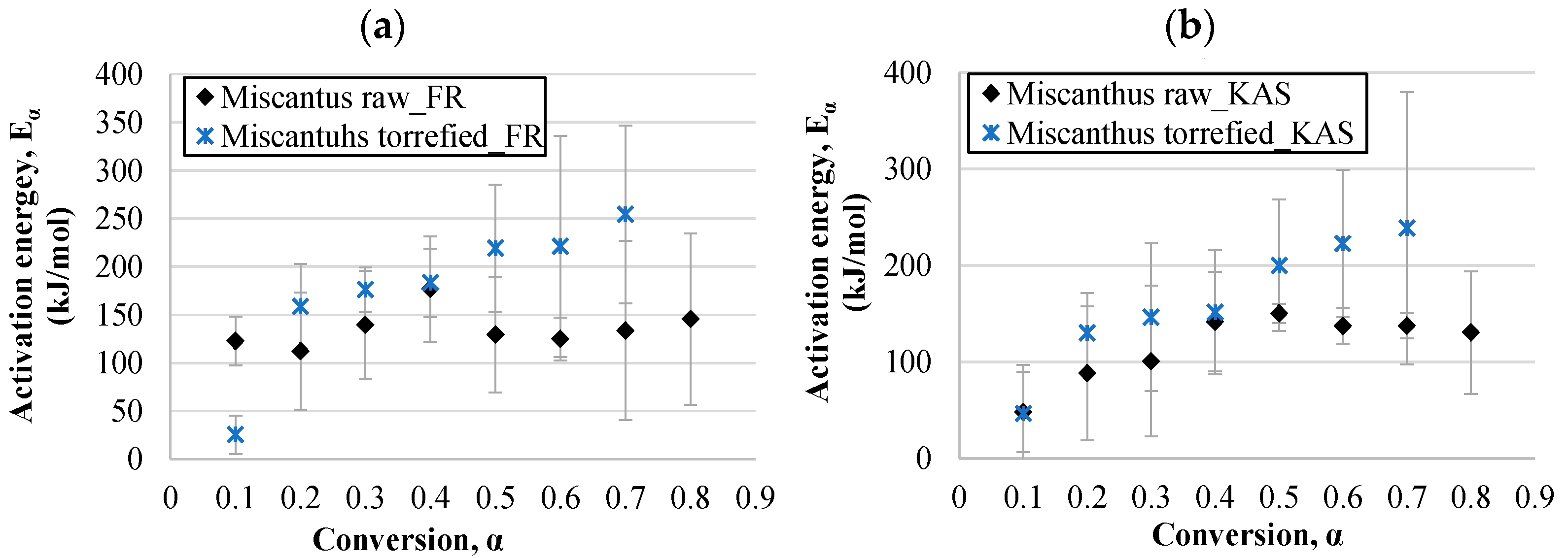
3.4.1. Activation Energy
3.4.2. The Pre-Exponential Factor
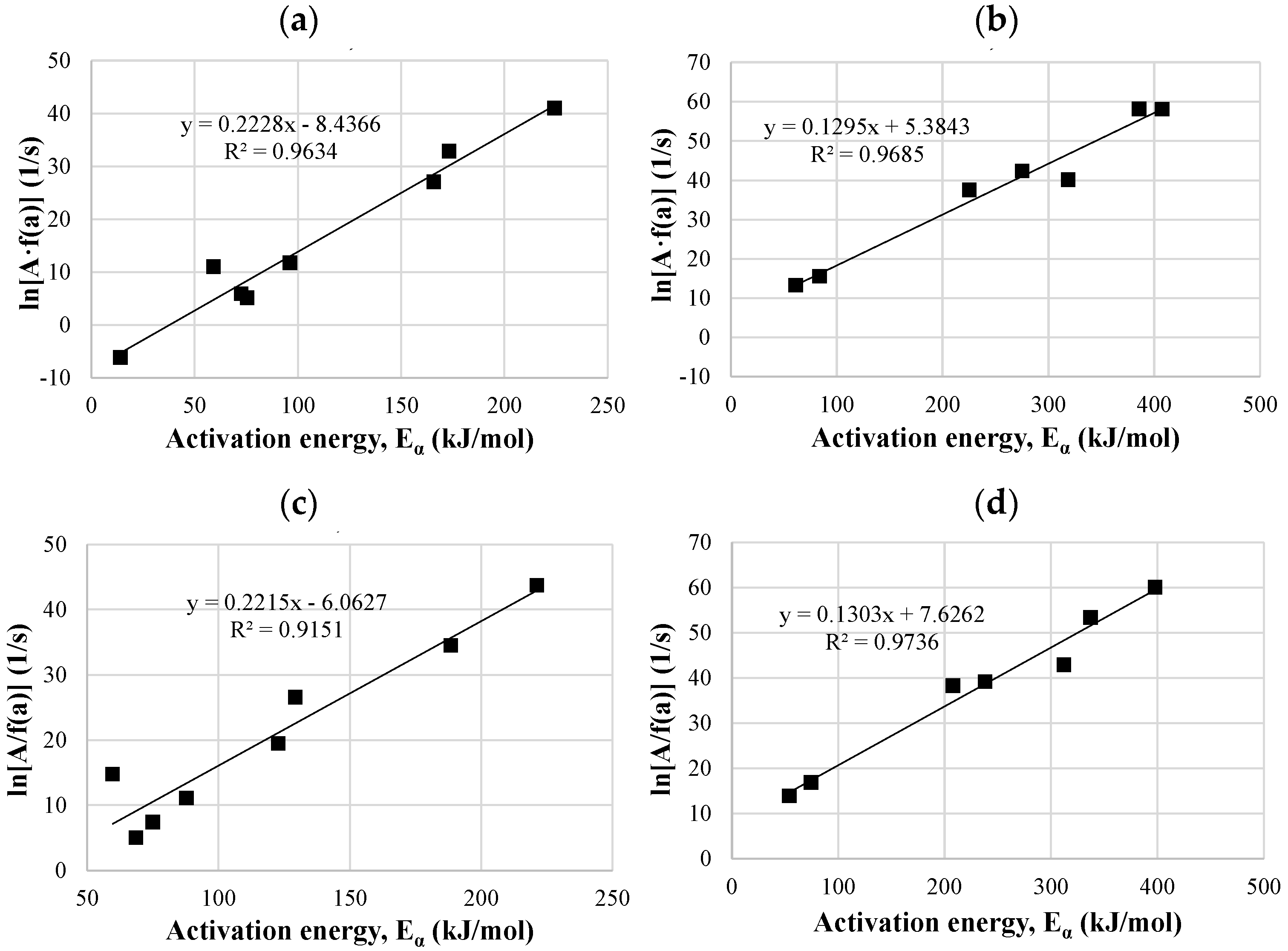
3.4.3. Kinetic Compensation Effect
3.5. Thermodynamic Parameters
3.5.1. Enthalpy (ΔH)
3.5.2. Entropy (ΔS)
3.5.3. Gibbs Free Energy (ΔG)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Roadmap 2050. Policy 2012, 1–9. [Google Scholar] [CrossRef]
- Jiang, Q.; Qi, Z.; Xue, L.; Bukovsky, M.; Madramootoo, A.C.; Smith, W. Assessing climate change impacts on greenhouse gas emissions, N losses in drainage and crop production in a subsurface drained field. Sci. Total Environ. 2020, 705, 135969. [Google Scholar] [CrossRef]
- European Commission. COM(2019) 640 final. Eur. Green Deal 2019, 53, 1689–1699. [Google Scholar] [CrossRef]
- Ramos-Carmona, S.; Martínez, J.D.; Pérez, J.F. Torrefaction of patula pine under air conditions: A chemical and structural characterization. Ind. Crops Prod. 2018, 118, 302–310. [Google Scholar] [CrossRef]
- Arriola, E.; Chen, W.H.; Chih, Y.K.; de Luna, M.D.; Show, P.L. Impact of post-torrefaction process on biochar formation from wood pellets and self-heating phenomena for production safety. Energy 2020, 207, 118324. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, M.; Jiang, E.; Wang, D.; Zhang, K.; Ren, Y.; Jiang, Y. Pyrolysis of Torrefied Biomass. Trends Biotechnol. 2018, 36, 1287–1298. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; He, C.; Yu, Z.; Jiang, L.; Zeng, Z.; Tian, X. Integrated process of lignocellulosic biomass torrefaction and pyrolysis for upgrading bio-oil production: A state-of-the-art review. Renew. Sustain. Energy Rev. 2019, 107, 20–36. [Google Scholar] [CrossRef]
- Tian, X.; Dai, L.; Wang, Y.; Zeng, Z.; Zhang, S.; Jiang, L.; Yang, X.; Yue, L.; Liu, Y.; Ruan, R. Influence of torrefaction pretreatment on corncobs: A study on fundamental characteristics, thermal behavior, and kinetic. Bioresour. Technol. 2020, 297, 122490. [Google Scholar] [CrossRef] [PubMed]
- de Mattos Carneiro-Junior, J.A.; de Oliveira, G.F.; Alves, C.T.; Andrade, H.M.C.; de Melo, S.A.B.V.; Torres, E.A. Valorization of prosopis juliflora woody biomass in northeast brazilian through dry torrefaction. Energies 2021, 14, 3465. [Google Scholar] [CrossRef]
- Gan, Y.Y.; Ong, H.C.; Ling, T.C.; Chen, W.H.; Chong, C.T. Torrefaction of de-oiled Jatropha seed kernel biomass for solid fuel production. Energy 2019, 170, 367–374. [Google Scholar] [CrossRef]
- Kwoczynski, Z.; Čmelík, J. Characterization of biomass wastes and its possibility of agriculture utilization due to biochar production by torrefaction process. J. Clean. Prod. 2020, 280, 124302. [Google Scholar] [CrossRef]
- Álvarez, A.; Nogueiro, D.; Pizarro, C.; Matos, M.; Bueno, J.L. Non-oxidative torrefaction of biomass to enhance its fuel properties. Energy 2018, 158, 1–8. [Google Scholar] [CrossRef]
- Simonic, M.; Goricanec, D.; Urbancl, D. Impact of torrefaction on biomass properties depending on temperature and operation time. Sci. Total Environ. 2020, 740, 140086. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goricanec, D.; Krope, J.; Urbancl, D. Torrefaction pretreatment of lignocellulosic biomass for sustainable solid biofuel production. Energy 2021, 122483. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Colin, B.; Pétrissans, A.; Pétrissans, M. A study of hygroscopic property of biomass pretreated by torrefaction. Energy Procedia 2019, 158, 32–36. [Google Scholar] [CrossRef]
- Hu, J.; Song, Y.; Liu, J.; Evrendilek, F.; Buyukada, M.; Yan, Y.; Li, L. Combustions of torrefaction-pretreated bamboo forest residues: Physicochemical properties, evolved gases, and kinetic mechanisms. Bioresour. Technol. 2020, 304, 122960. [Google Scholar] [CrossRef]
- Bach, Q.V.; Chen, W.H.; Chu, Y.S.; Skreiberg, Ø. Predictions of biochar yield and elemental composition during torrefaction of forest residues. Bioresour. Technol. 2016, 215, 239–246. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, Z.; Rahman, M.M.; Guo, X.; Zhang, X.; Cai, J. Insight into torrefaction of woody biomass: Kinetic modeling using pattern search method. Energy 2020, 201, 117648. [Google Scholar] [CrossRef]
- Shoulaifar, T.K. Chemical Changes in Biomass during Torrefaction. Ph.D. Thesis, Åbo Akademi University, Turku, Finland, 2016. [Google Scholar]
- Bergman, P.C.; Boersma, A.R.; Zwart, R.W.R.; Kiel, J.H.A. Torrefaction for Biomass Co-Firing in Existing Coal-Fired Power Stations. 2005. Available online: http://www.ecn.nl/publications/PdfFetch.aspx?nr=ECN-C--05–013 (accessed on 8 November 2021).
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. More efficient biomass gasification via torrefaction. Energy 2006, 31, 3458–3470. [Google Scholar] [CrossRef]
- Dacres, O.D.; Tong, S.; Li, X.; Sun, Y.; Wang, F.; Luo, G.; Liu, H.; Hu, H.; Yao, H. Gas-pressurized torrefaction of biomass wastes: The effect of varied pressure on pyrolysis kinetics and mechanism of torrefied biomass. Fuel 2020, 276, 118132. [Google Scholar] [CrossRef]
- Tong, S.; Sun, Y.; Li, X.; Hu, Z.; Dacres, O.D.; Worasuwannarak, N.; Luo, G.; Liu, H.; Hu, H.; Yao, H. Gas-pressurized torrefaction of biomass wastes: Roles of pressure and secondary reactions. Bioresour. Technol. 2020, 313, 123640. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Che, Q.; Yang, Q.; Draper, C.; Yang, H.; Zhang, S.; Chen, H. Torrefaction of different parts from a corn stalk and its effect on the characterization of products. Ind. Crops Prod. 2016, 92, 26–33. [Google Scholar] [CrossRef]
- Doddapaneni, T.R.K.C.; Konttinen, J.; Hukka, T.I.; Moilanen, A. Influence of torrefaction pretreatment on the pyrolysis of Eucalyptus clone: A study on kinetics, reaction mechanism and heat flow. Ind. Crops Prod. 2016, 92, 244–254. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, K.; Li, L.; Zhao, Z.; Jiang, L.; Huang, Z.; Wei, G.; He, F.; Li, H. Quantitative comparison of different chemical pretreatment methods on chemical structure and pyrolysis characteristics of corncobs. J. Energy Inst. 2018, 91, 676–682. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, S.; Liu, L.; Xu, D.; Xiong, Y. Investigation of representative components of flue gas used as torrefaction pretreatment atmosphere and its effects on fast pyrolysis behaviors. Bioresour. Technol. 2018, 267, 584–590. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Gazińska, M.; Fedyna, M.; Karcz, R.; Lorenc-Grabowska, E.; Rutkowski, P. Pyrolysis and torrefaction of waste wood chips and cone-like flowers derived from black alder (Alnus glutinosa L. Gaertn.) for sustainable solid fuel production. Biomass Bioenergy 2020, 143, 105842. [Google Scholar] [CrossRef]
- Shang, L.; Ahrenfeldt, J.; Holm, J.K.; Bach, L.S.; Stelte, W.; Henriksen, U.B. Kinetic model for torrefaction of wood chips in a pilot-scale continuous reactor. J. Anal. Appl. Pyrolysis 2014, 108, 109–116. [Google Scholar] [CrossRef]
- Liu, Y.; Rokni, E.; Yang, R.; Ren, X.; Sun, R.; Levendis, Y.A. Torrefaction of corn straw in oxygen and carbon dioxide containing gases: Mass/energy yields and evolution of gaseous species. Fuel 2021, 285, 119044. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Cao, X.; Li, Y.; Zhang, Y.; Ma, H. Restudy on torrefaction of corn stalk from the point of view of deoxygenation and decarbonization. J. Anal. Appl. Pyrolysis 2018, 135, 85–93. [Google Scholar] [CrossRef]
- Nguyen, Q.; Nguyen, D.D.; Vothi, H.; He, C.; Goodarzi, M.; Bach, Q.V. Isothermal torrefaction kinetics for sewage sludge pretreatment. Fuel 2020, 277, 118103. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Rashid, S.R.M.; Samad, N.A.F.A.; Saleh, S. Upgrading Physicochemical Properties using Torrefaction Process and Anhydrous Weight Loss Modelling for Palm Mesocarp Fiber. Mater. Today Proc. 2019, 19, 1703–1711. [Google Scholar] [CrossRef]
- Bach, Q.V.; Chen, W.H.; Eng, C.F.; Wang, C.W.; Liang, K.C.; Kuo, J.Y. Pyrolysis characteristics and non-isothermal torrefaction kinetics of industrial solid wastes. Fuel 2019, 251, 118–125. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, S.; Zhang, X.; Liu, H.; Sun, B.; Guo, S. Influence of air oxidative and non-oxidative torrefaction on the chemical properties of corn stalk. Bioresour. Technol. 2021, 332, 125120. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T.; Smȩdzik, M. The influence of torrefaction temperature on hydrophobic properties ofwaste biomass from food processing. Energies 2019, 12, 4609. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Onsree, T.; Tippayawong, N. Analysis of reaction kinetics for torrefaction of pelletized agricultural biomass with dry flue gas. Energy Rep. 2020, 6, 61–65. [Google Scholar] [CrossRef]
- Delgado, B.; González, D.L.; Godbout, S.; Lagacé, R.; Giroir-Fendler, A.; Avalos Ramirez, A. A study of torrefied cardboard characterization and applications: Composition, oxidation kinetics and methane adsorption. Sci. Total Environ. 2017, 593–594, 406–417. [Google Scholar] [CrossRef]
- Castells, B.; Amez, I.; Medic, L.; Fernandez-Anez, N.; Garcia-Torrent, J. Study of lignocellulosic biomass ignition properties estimation from thermogravimetric analysis. J. Loss Prev. Process Ind. 2021, 71, 104425. [Google Scholar] [CrossRef]
- Xu, F.; Linnebur, K.; Wang, D. Torrefaction of Conservation Reserve Program biomass: A techno-economic evaluation. Ind. Crops Prod. 2014, 61, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Barzegar, R.; Yozgatligil, A.; Olgun, H.; Atimtay, A.T. TGA and kinetic study of different torrefaction conditions of wood biomass under air and oxy-fuel combustion atmospheres. J. Energy Inst. 2020, 93, 889–898. [Google Scholar] [CrossRef]
- Bui, H.H.; Tran, K.Q.; Chen, W.H. Pyrolysis of microalgae residues—A Kinetic study. Bioresour. Technol. 2015, 199, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Qu, B.; Wang, W.; Wang, W.; Ji, G.; Li, A. Rice husk and rice straw torrefaction: Properties and pyrolysis kinetics of raw and torrefied biomass. Environ. Technol. Innov. 2021, 24, 101872. [Google Scholar] [CrossRef]
- Osman, A.I.; Abdelkader, A.; Farrell, C.; Rooney, D.; Morgan, K. Reusing, recycling and up-cycling of biomass: A review of practical and kinetic modelling approaches. Fuel Process. Technol. 2019, 192, 179–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Lu, J.; Yu, H.; Zhu, J.; Zhou, J.; Zhang, X.; Liu, F.; Wang, Y.; Hao, B.; et al. An effective strategy for dual enhancements on bioethanol production and trace metal removal using Miscanthus straws. Ind. Crops Prod. 2020, 152, 112393. [Google Scholar] [CrossRef]
- Fradj, N.B.; Rozakis, S.; Borzęcka, M.; Matyka, M. Miscanthus in the European bio-economy: A network analysis. Ind. Crops Prod. 2020, 148, 112281. [Google Scholar] [CrossRef]
- Hrnčič, M.K.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop compounds: Extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontek, B.; Jedrejek, D.; Oleszek, W.; Olas, B. Antiradical and antioxidant activity in vitro of hops-derived extracts rich in bitter acids and xanthohumol. Ind. Crops Prod. 2021, 161, 113208. [Google Scholar] [CrossRef]
- U.E. 14774-1:2009. Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 1: Total Moisture—Reference Method; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Jóźwiak, P.; Hercog, J.; Kiedrzyńska, A.; Badyda, K. CFD analysis of natural gas substitution with syngas in the industrial furnaces. Energy 2019, 179, 593–602. [Google Scholar] [CrossRef]
- U.E. 15289:2011. Solid Biofuels—Determination of Total Content of Sulfur and Chlorine; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- U.E. 15104:2011. Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen—Instrumental Methods; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- A.D. 7582:2015. Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- U.E. 14918:2010. Solid Biofuels—Determination of Calorific Value; International Organization for Standardization: Geneva, Switzerland, 2018. [Google Scholar]
- I. > 75 > 75. 16. > 75.160.10 and I. 1928:2009. Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Nwabunwanne, N.; Vuyokazi, T.; Olagoke, A.; Mike, O.; Patrick, M.; Anthony, O. Improving the solid fuel properties of non-lignocellulose and lignocellulose materials through torrefaction. Materials 2021, 14, 2072. [Google Scholar] [CrossRef]
- Noszczyk, T.; Dyjakon, A.; Koziel, J.A. Kinetic parameters of nut shells pyrolysis. Energies 2021, 14, 682. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T. Alternative fuels from forestry biomass residue: Torrefaction process of horse chestnuts, oak acorns, and spruce cones. Energies 2020, 13, 2468. [Google Scholar] [CrossRef]
- Brachi, P.; Miccio, F.; Miccio, M.; Ruoppolo, G. Isoconversional kinetic analysis of olive pomace decomposition under torrefaction operating conditions. Fuel Process. Technol. 2015, 130, 147–154. [Google Scholar] [CrossRef]
- Arenas, C.N.; Navarro, M.V.; Martínez, J.D. Pyrolysis kinetics of biomass wastes using isoconversional methods and the distributed activation energy model. Bioresour. Technol. 2019, 288, 121485. [Google Scholar] [CrossRef]
- Bada, S.O.; Falcon, R.M.S.; Falcon, L.M. Investigation of combustion and co-combustion characteristics of raw and thermal treated bamboo with thermal gravimetric analysis. Thermochim. Acta 2014, 589, 207–214. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Lisboa, S.N.; Mate, R.; Manjate, A.; Sitoe, A. Applying the icat sustainable development methodology to assess the impacts of promoting a greater sustainability of the charcoal value chain in mozambique. Sustainability 2020, 12, 10390. [Google Scholar] [CrossRef]
- Mallick, D.; Poddar, M.K.; Mahanta, P.; Moholkar, V.S. Discernment of synergism in pyrolysis of biomass blends using thermogravimetric analysis. Bioresour. Technol. 2018, 261, 294–305. [Google Scholar] [CrossRef]
- Ma, P.; Yang, J.; Xing, X.; Weihrich, S.; Fan, F.; Zhang, X. Isoconversional kinetics and characteristics of combustion on hydrothermally treated biomass. Renew. Energy 2017, 114, 1069–1076. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghari, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Isoconversional determination of the apparent reaction models governing pyrolysis of wood, straw and sewage sludge, with an approach to rate modelling. Renew. Energy 2020, 161, 972–987. [Google Scholar] [CrossRef]
- Kojić, M.M.; Petrović, J.T.; Petrović, M.S.; Stanković, S.M.; Porobić, S.J.; Marinović-Cincović, M.T.; Mihajlović, M.L. Hydrothermal carbonization of spent mushroom substrate: Physicochemical characterization, combustion behavior, kinetic and thermodynamic study. J. Anal. Appl. Pyrolysis 2021, 155, 105028. [Google Scholar] [CrossRef]
- Du, J.; Gao, L.; Yang, Y.; Chen, G.; Guo, S.; Omran, M.; Chen, J.; Ruan, R. Study on thermochemical characteristics properties and pyrolysis kinetics of the mixtures of waste corn stalk and pyrolusite. Bioresour. Technol. 2021, 324, 124660. [Google Scholar] [CrossRef]
- Singh, R.K.; Patil, T.; Sawarkar, A.N. Pyrolysis of garlic husk biomass: Physico-chemical characterization, thermodynamic and kinetic analyses. Bioresour. Technol. Rep. 2020, 12, 100558. [Google Scholar] [CrossRef]
- Lee, B.-H.; Jeong, T.-Y.; Trinh, V.T.; Jeon, C.-H. Thermal degradation of kenaf (Hibiscus cannabinus L.): Impact of torrefaction on pyrolysis kinetics and thermal behavior. Energy Rep. 2021, 7, 951–959. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Rizzo, A.M.; Prussi, M.; Tedeschi, S.; Zimbardi, F.; Braccio, G.; Viola, E.; Pardelli, P.T. 2nd generation lignocellulosic bioethanol: Is torrefaction a possible approach to biomass pretreatment? Biomass Convers. Biorefinery 2011, 1, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Bridgeman, T.G.; Jones, J.M.; Williams, A.; Waldron, D.J. An investigation of the grindability of two torrefied energy crops. Fuel 2010, 89, 3911–3918. [Google Scholar] [CrossRef] [Green Version]
- Gismatulina, Y.A.; Budaeva, V.V. Chemical composition of five Miscanthus sinensis harvests and nitric-acid cellulose therefrom. Ind. Crops Prod. 2017, 109, 227–232. [Google Scholar] [CrossRef]
- Eyres, G.; Dufour, J.P. Hop essential oil: Analysis, chemical composition and odor characteristics. Beer Heal. Dis. Prev. 2009, 239–254. [Google Scholar] [CrossRef]
- Yang, W.; Shimanouchi, T.; Iwamura, M.; Takahashi, Y.; Mano, R.; Takashima, K.; Tanifuji, T.; Kimura, Y. Elevating the fuel properties of Humulus lupulus, Plumeria alba and Calophyllum inophyllum L. through wet torrefaction. Fuel 2015, 146, 88–94. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Pyrolysis kinetics and thermodynamic parameters of castor (Ricinus communis) residue using thermogravimetric analysis. Bioresour. Technol. 2018, 250, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.R.; Dupont, C.; Heux, L.; Da Silva Perez, D.; Commandre, J.M.; Gourdon, C. Study of solid chemical evolution in torrefaction of different biomasses through solid-state 13C cross-polarization/magic angle spinning NMR (nuclear magnetic resonance) and TGA (thermogravimetric analysis). Energy 2016, 97, 381–390. [Google Scholar] [CrossRef]
- Luo, J.; Xue, W.; Shao, H. Thermo-economic comparison of coal-fired boiler-based and groundwater-heat-pump based heating and cooling solution—A case study on a greenhouse in Hubei, China. Energy Build. 2020, 223, 110214. [Google Scholar] [CrossRef]
- Mihajlović, M.; Petrović, J.; Maletić, S.; Isakovski, M.K.; Stojanović, M.; Lopičić, Z.; Trifunović, S. Hydrothermal carbonization of Miscanthus × giganteus: Structural and fuel properties of hydrochars and organic profile with the ecotoxicological assessment of the liquid phase. Energy Convers. Manag. 2018, 159, 254–263. [Google Scholar] [CrossRef]
- Quosai, P.; Anstey, A.; Mohanty, A.K.; Misra, M. Characterization of biocarbon generated by high- and low-temperature pyrolysis of soy hulls and coffee chaff: For polymer composite applications. R. Soc. Open Sci. 2018, 5, 171970. [Google Scholar] [CrossRef] [Green Version]
- Osman, A.I.; Abdelkader, A.; Johnston, C.R.; Morgan, K.; Rooney, D.W. Thermal Investigation and Kinetic Modeling of Lignocellulosic Biomass Combustion for Energy Production and Other Applications. Ind. Eng. Chem. Res. 2017, 56, 12119–12130. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Fast co-pyrolysis of sewage sludge and lignocellulosic biomass in a conical spouted bed reactor. Fuel 2015, 159, 810–818. [Google Scholar] [CrossRef]
- Haque, M.A.; Barman, D.N.; Kang, T.H.; Kim, M.K.; Kim, J.; Kim, H.; Yun, H.D. Effect of dilute alkali on structural features and enzymatic hydrolysis of barley straw (Hordeum vulgare) at boiling temperature with low residence time. J. Microbiol. Biotechnol. 2012, 22, 1681–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anstey, A.; Vivekanandhan, S.; Rodriguez-Uribe, A.; Misra, M.; Mohanty, A.K. Oxidative acid treatment and characterization of new biocarbon from sustainable Miscanthus biomass. Sci. Total Environ. 2016, 550, 241–247. [Google Scholar] [CrossRef]
- Wilk, M.; Magdziarz, A. Hydrothermal carbonization, torrefaction and slow pyrolysis of Miscanthus giganteus. Energy 2017, 140, 1292–1304. [Google Scholar] [CrossRef]
- Xue, Y.; Li, Q.; Gu, Y.; Yu, H.; Zhang, Y.; Zhou, X. Improving biodegradability and biogas production of miscanthus using a combination of hydrothermal and alkaline pretreatment. Ind. Crops Prod. 2020, 144, 111985. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Zhong, L.; Orndroff, W.; Zhao, H.; Cao, Y.; Zhang, K.; Pan, W.P. Synergistic effects of mineral matter on the combustion of coal blended with biomass. J. Therm. Anal. Calorim. 2013, 113, 489–496. [Google Scholar] [CrossRef]
- He, Q.; Ding, L.; Gong, Y.; Li, W.; Wei, J.; Yu, G. Effect of torrefaction on pinewood pyrolysis kinetics and thermal behavior using thermogravimetric analysis. Bioresour. Technol. 2019, 280, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Bedoić, R.; Bulatović, V.O.; Čuček, L.; Ćosić, B.; Špehar, A.; Pukšec, T.; Duić, N. A kinetic study of roadside grass pyrolysis and digestate from anaerobic mono-digestion. Bioresour. Technol. 2019, 292, 121935. [Google Scholar] [CrossRef] [PubMed]
- Wilk, M.; Magdziarz, A.; Gajek, M.; Zajemska, M.; Jayaraman, K.; Gokalp, I. Combustion and kinetic parameters estimation of torrefied pine, acacia and Miscanthus giganteus using experimental and modelling techniques. Bioresour. Technol. 2017, 243, 304–314. [Google Scholar] [CrossRef]
- Tian, H.; Hu, Q.; Wang, J.; Liu, L.; Yang, Y.; Bridgwater, A.V. Steam gasification of Miscanthus derived char: The reaction kinetics and reactivity with correlation to the material composition and microstructure. Energy Convers. Manag. 2020, 219, 113026. [Google Scholar] [CrossRef]
- Tian, H.; Hu, Q.; Wang, J.; Chen, D.; Yang, Y.; Bridgwater, A.V. Kinetics Study on the CO2 Gasification of Biochar Derived from Miscanthus at Different Processing Conditions. Energy 2020, 217, 119341. [Google Scholar] [CrossRef]
- Grams, J.; Kwapińska, M.; Jędrzejczyk, M.; Rzeźnicka, I.; Leahy, J.J.; Ruppert, A.M. Surface characterization of Miscanthus × giganteus and Willow subjected to torrefaction. J. Anal. Appl. Pyrolysis 2019, 138, 231–241. [Google Scholar] [CrossRef]
- Cortés, A.M.; Bridgwater, A.V. Kinetic study of the pyrolysis of miscanthus and its acid hydrolysis residue by thermogravimetric analysis. Fuel Process. Technol. 2015, 138, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.M.; de Oliveira, L.S.; Rocha, E.P.A.; Franca, A.S. Thermal conversion of defective coffee beans for energy purposes: Characterization and kinetic modeling. Renew. Energy 2020, 147, 1275–1291. [Google Scholar] [CrossRef]
- Ali, I. Misuse of pre-exponential factor in the kinetic and thermodynamic studies using thermogravimetric analysis and its implications. Bioresour. Technol. Rep. 2018, 2, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Sbirrazzuoli, N. Model-free isothermal and nonisothermal predictions using advanced isoconversional methods. Thermochim. Acta 2020, 697, 178855. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.; Liu, Q.; Qi, X.; Qu, J.; Zhang, S.; Wang, X.; Jia, K.; Zhu, M. Study on chemical components and sources of PM2.5 during heavy air pollution periods at a suburban site in Beijing of China. Atmos. Pollut. Res. 2021, 12, 188–199. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.S.; Kim, S.H. Investigation of thermodynamic parameters in the thermal decomposition of plastic waste-waste lube oil compounds. Environ. Sci. Technol. 2010, 44, 5313–5317. [Google Scholar] [CrossRef]



| Method | Kinetic Expression |
|---|---|
| Friedman method (FR) | |
| Kissinger–Akahira–Sunose (KAS) |
| Method | Kinetic Expression |
|---|---|
| Pre-exponential factor (A) | |
| Change of enthalpy (ΔH) | |
| Entropy (ΔS) | |
| Gibbs free energy (ΔG) |
| Material | Torrefaction Temperature (°C) | Elemental Analysis (wt.%, Dry Basis) | MC (wt.%, Dry Basis) | Proximate Analysis (wt.%, Dry Basis) | HHV (MJ/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | H/C | O/C | FC | VM | Ash | ||||
| Miscanthus raw | / | 46.2 | 4.1 | 0.8 | 48.9 | 0.03 | 1.0 | 0.8 | 9.3 | 3.9 | 83.9 | 2.9 | 16.4 |
| Miscanthus torrefied | 250 | 55.5 | 3.9 | 0.6 | 39.9 | 0.01 | 0.9 | 0.5 | 0 | 14.6 | 72.4 | 5.8 | 21.1 |
| Hops waste raw | / | 42.4 | 4.9 | 2.5 | 36.9 | 0.02 | 1.4 | 0.7 | 11.8 | 1.9 | 82.9 | 3.4 | 16.5 |
| Hops waste torrefied | 250 | 48.6 | 3.4 | 3.1 | 24.2 | 0.01 | 0.8 | 0.4 | 0 | 7.5 | 74.8 | 6.7 | 18.9 |
| Sample | 10 °C min−1 | 15 °C min−1 | 20 °C min−1 | ||||
|---|---|---|---|---|---|---|---|
| Tp | Weight Loss (wt. %) | Tp | Weight Loss (wt. %) | Tp | Weight Loss (wt. %) | ||
| Miscanthus | Raw | 336.2 | 80.5 | 337.8 | 82.3 | 351.5 | 77.9 |
| Torrefied at 250 °C | 436.8 | 47.1 | 434.2 | 47.3 | 440.6 | 48.0 | |
| Hops waste | Raw | 308.8 | 63.5 | 317.0 | 63.5 | 321.7 | 61.1 |
| Torrefied at 250 °C | 480.6 | 48.2 | 443.3 | 45.3 | 448.5 | 46.2 | |
| Conversion Degree, α | Friedman Method | KAS Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | A (1/s) | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (J/mol·K) | R2 | A (1/s) | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (J/mol·K) | |
| Miscanthus raw | ||||||||||
| 0.1 | 0.97 | 2.22 × 108 | 117.67 | 178.22 | −99.39 | 0.63 | 3.50 × 10 | 43.08 | 182.96 | −229.62 |
| 0.2 | 0.76 | 2.59 × 107 | 107.24 | 178.67 | −117.26 | 0.67 | 1.75 × 105 | 83.17 | 179.89 | −158.78 |
| 0.3 | 0.89 | 6.83 × 109 | 134.39 | 177.57 | −70.90 | 0.68 | 2.36 × 106 | 95.67 | 179.22 | −137.15 |
| 0.4 | 0.93 | 1.38 × 1013 | 171.74 | 176.37 | −7.60 | 0.91 | 1.07 × 1010 | 136.59 | 177.50 | −67.14 |
| 0.5 | 0.85 | 8.46 × 108 | 124.19 | 177.96 | −88.26 | 1.00 | 6.10 × 1010 | 145.10 | 177.20 | −52.69 |
| 0.6 | 0.98 | 3.36 × 108 | 119.69 | 178.14 | −95.95 | 0.99 | 4.50 × 109 | 132.35 | 177.65 | −74.36 |
| 0.7 | 0.72 | 2.03 × 109 | 128.46 | 177.79 | −80.98 | 1.00 | 4.63 × 109 | 132.48 | 177.64 | −74.13 |
| 0.8 | 0.34 | 2.32 × 102 | 140.37 | 177.36 | −60.72 | 0.84 | 1.06 × 109 | 125.29 | 177.92 | −86.39 |
| Miscanthus torrefied (250 °C) | ||||||||||
| 0.1 | 0.56 | 7.25 × 10−2 | 19.35 | 219.70 | −282.26 | 0.53 | 5.07 | 40.79 | 216.08 | −246.94 |
| 0.2 | 0.94 | 3.02 × 109 | 152.81 | 208.86 | −78.96 | 0.93 | 1.93 × 107 | 124.15 | 210.03 | −120.99 |
| 0.3 | 0.72 | 6.58 × 1010 | 170.38 | 208.24 | −53.33 | 0.82 | 3.29 × 108 | 140.21 | 209.34 | −97.39 |
| 0.4 | 0.70 | 2.19 × 1011 | 177.25 | 208.01 | −43.33 | 0.62 | 8.30 × 108 | 145.47 | 209.14 | −89.70 |
| 0.5 | 0.69 | 1.15 × 1014 | 213.15 | 206.95 | 8.73 | 0.64 | 4.19 × 1012 | 194.14 | 207.49 | −18.80 |
| 0.6 | 0.83 | 1.68 × 1014 | 215.32 | 206.90 | 11.87 | 0.82 | 2.21 × 1014 | 216.91 | 206.85 | 14.17 |
| 0.7 | 0.58 | 5.25 × 1016 | 248.42 | 206.07 | 59.65 | 0.56 | 3.46 × 1015 | 232.74 | 206.45 | 37.05 |
| Conversion Degree, α | Friedman Method | KAS Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | A (1/s) | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (J/mol·K) | R2 | A (1/s) | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (J/mol·K) | |
| Hops waste raw | ||||||||||
| 0.1 | 0.83 | 7.22 × 102 | 54.34 | 173.06 | −204.07 | 0.85 | 8.22 × 102 | 54.92 | 173.01 | −202.98 |
| 0.2 | 0.98 | 3.56 × 1013 | 168.24 | 167.87 | 0.64 | 0.96 | 3.12 × 109 | 124.46 | 169.28 | −77.03 |
| 0.3 | 0.93 | 1.79 × 1018 | 219.33 | 166.61 | 90.62 | 0.97 | 9.41 × 1017 | 216.30 | 166.68 | 85.30 |
| 0.4 | 0.78 | 7.32 × 1012 | 160.80 | 168.08 | −12.51 | 0.89 | 9.27 × 1014 | 183.60 | 167.45 | 27.74 |
| 0.5 | 0.38 | 2.44 × 106 | 91.29 | 170.71 | −136.52 | 0.66 | 7.62 × 108 | 117.90 | 169.53 | −88.75 |
| 0.6 | 0.82 | 1.38 × 104 | 67.64 | 172.08 | −179.52 | 0.93 | 3.99 × 105 | 82.97 | 171.15 | −151.56 |
| 0.7 | 0.90 | 2.66 × 104 | 70.62 | 171.88 | −174.07 | 0.89 | 2.42 × 104 | 70.18 | 171.91 | −174.87 |
| 0.8 | 0.35 | 1.54 × 10−2 | 9.25 | 180.00 | −293.50 | 0.96 | 5.85 × 103 | 63.75 | 172.34 | −186.67 |
| Hops waste torrefied (250 °C) | ||||||||||
| 0.1 | 0.71 | 3.80 × 10 | 54.97 | 228.82 | −230.69 | 0.71 | 1.05 × 10 | 47.72 | 229.61 | −241.36 |
| 0.2 | 0.94 | 1.86 × 103 | 77.38 | 226.86 | −198.37 | 0.99 | 3.85 × 102 | 68.24 | 227.59 | −211.45 |
| 0.3 | 0.85 | 3.22 × 1013 | 218.90 | 220.66 | −2.34 | 0.91 | 1.78 × 1012 | 201.28 | 221.17 | −26.40 |
| 0.4 | 0.88 | 1.13 × 1017 | 268.78 | 219.40 | 65.52 | 0.87 | 2.64 × 1014 | 231.73 | 220.31 | 15.16 |
| 0.5 | 0.56 | 7.34 × 1024 | 379.37 | 217.29 | 215.09 | 0.56 | 2.73 × 1021 | 330.74 | 218.13 | 149.43 |
| 0.6 | 0.69 | 2.42 × 1026 | 400.93 | 216.95 | 244.15 | 0.70 | 5.10 × 1025 | 391.32 | 217.10 | 231.20 |
| 0.7 | 0.43 | 1.33 × 1020 | 312.17 | 218.49 | 124.32 | 0.52 | 4.63 × 1019 | 305.76 | 218.62 | 115.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanovski, M.; Petrovic, A.; Ban, I.; Goricanec, D.; Urbancl, D. Determination of the Kinetics and Thermodynamic Parameters of Lignocellulosic Biomass Subjected to the Torrefaction Process. Materials 2021, 14, 7877. https://doi.org/10.3390/ma14247877
Ivanovski M, Petrovic A, Ban I, Goricanec D, Urbancl D. Determination of the Kinetics and Thermodynamic Parameters of Lignocellulosic Biomass Subjected to the Torrefaction Process. Materials. 2021; 14(24):7877. https://doi.org/10.3390/ma14247877
Chicago/Turabian StyleIvanovski, Maja, Aleksandra Petrovic, Irena Ban, Darko Goricanec, and Danijela Urbancl. 2021. "Determination of the Kinetics and Thermodynamic Parameters of Lignocellulosic Biomass Subjected to the Torrefaction Process" Materials 14, no. 24: 7877. https://doi.org/10.3390/ma14247877






