Assessment of Morphological, Physical, Thermal, and Thermal Conductivity Properties of Polypropylene/Lignosulfonate Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blend Production
2.2. Blend Characterization
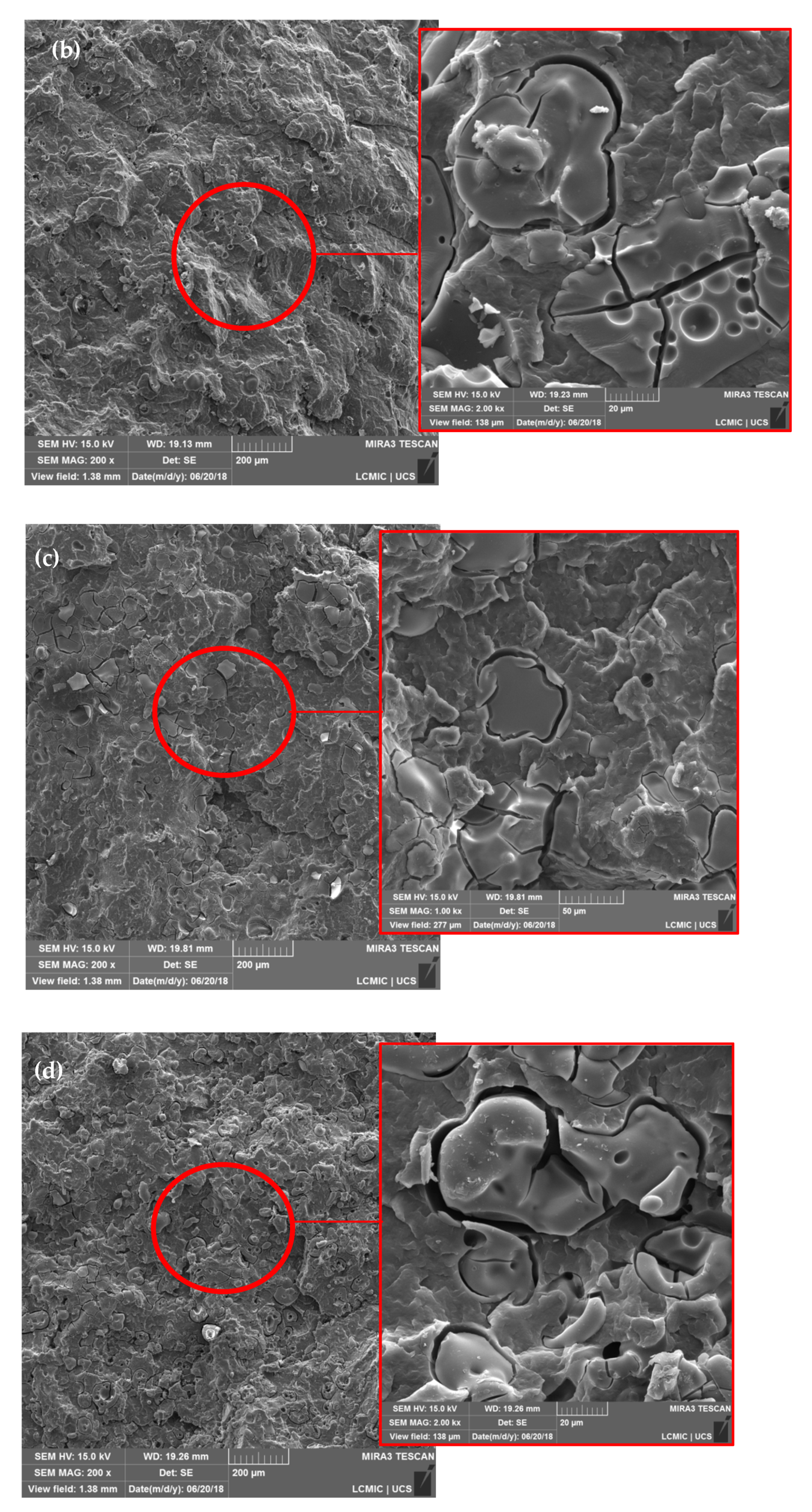
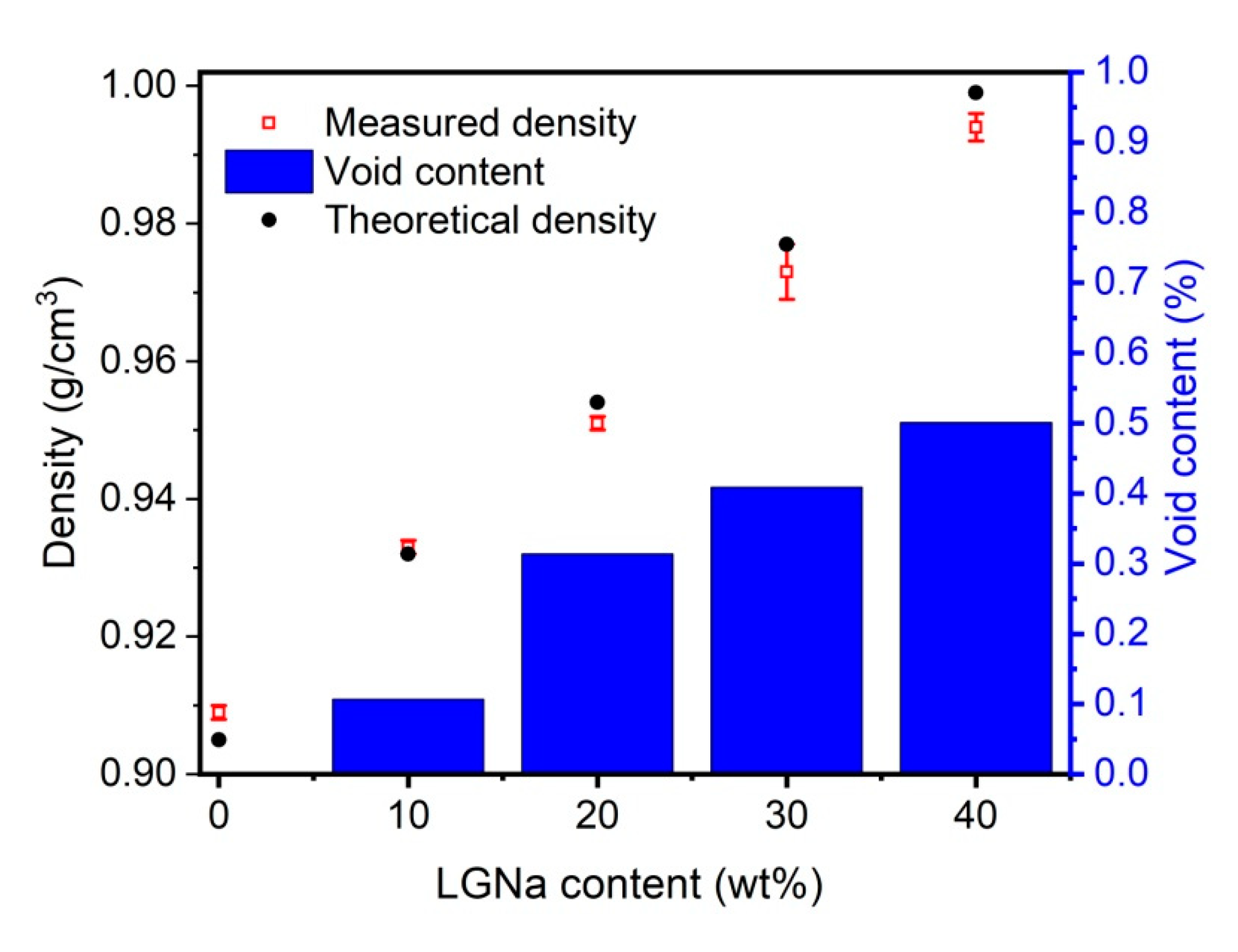
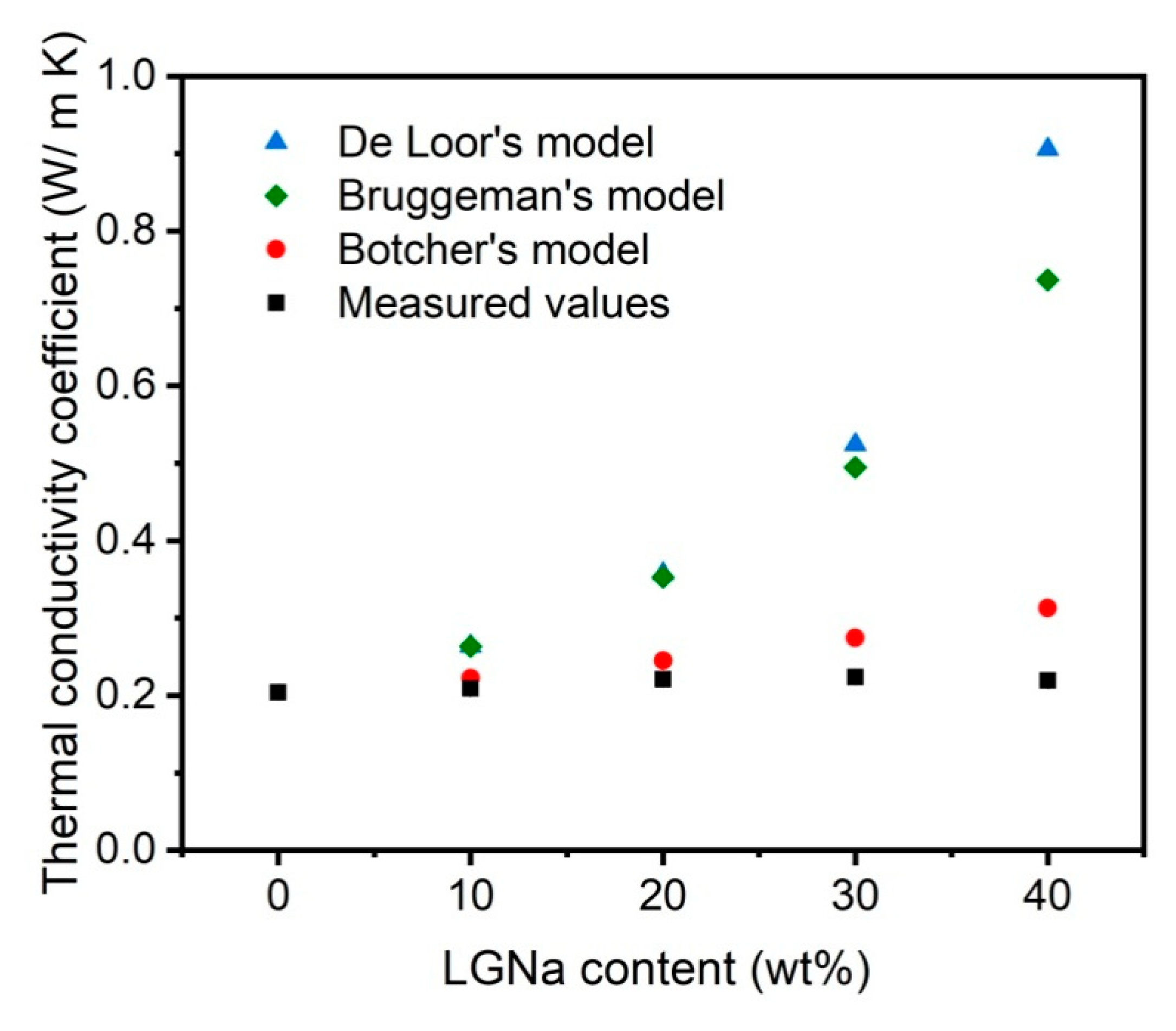
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michel Murillo, A.; Tutikian, B.F.; Christ, R.; Silva, L.F.O.; Maschen, M.; Leandro Gómez, P.; Oliveira, M.L.S. Analysis of the influence of thickness on fire reaction performance in polyisocyanurate core sandwich panels. J. Mater. Res. Technol. 2020, 9, 9487–9497. [Google Scholar] [CrossRef]
- Barbos, J.D.V.; Azevedo, J.B.; Cardoso, P.d.S.M.; da Costa Garcia Filho, F.; del Río, T.G. Development and characterization of WPCs produced with high amount of wood residue. J. Mater. Res. Technol. 2020, 9, 9684–9690. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Kim, Y.; Eberhardt, T.L.; Shmulsky, R. Lab-scale structural insulated panels with lignin-incorporated rigid polyurethane foams as core. Ind. Crop. Prod. 2019, 132, 292–300. [Google Scholar] [CrossRef]
- Parit, M.; Jiang, Z. Towards lignin derived thermoplastic polymers. Int. J. Biol. Macromol. 2020, 165, 3180–3197. [Google Scholar] [CrossRef] [PubMed]
- Naseem, A.; Tabasum, S.; Zia, K.M.; Zuber, M.; Ali, M.; Noreen, A. Lignin-derivatives based polymers, blends and composites: A review. Int. J. Biol. Macromol. 2016, 93, 296–313. [Google Scholar] [CrossRef]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef]
- Mainka, H.; Täger, O.; Körner, E.; Hilfert, L.; Busse, S.; Edelmann, F.T.; Herrmann, A.S. Lignin—An alternative precursor for sustainable and cost-effective automotive carbon fiber. J. Mater. Res. Technol. 2015, 4, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Brudin, S.; Schoenmakers, P. Analytical methodology for sulfonated lignins. J. Sep. Sci. 2010, 33, 439–452. [Google Scholar] [CrossRef]
- Wang, C.; Kelley, S.S.; Venditti, R.A. Lignin-based thermoplastic materials. ChemSusChem 2016, 9, 770–783. [Google Scholar] [CrossRef]
- Canetti, M.; Bertini, F. Influence of the lignin on thermal degradation and melting behaviour of Poly(ethylene terephthalate) based composites. ePolymers 2009, 9. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef] [Green Version]
- Lemes, A.P.; Soto-Oviedo, M.A.; Waldman, W.R.; Innocentini-Mei, L.H.; Durán, N. Effect of lignosulfonate on the thermal and morphological behavior of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Polym. Environ. 2010, 18, 250–259. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Chen, F. Effects of lignin as a filler on properties of soy protein plastics. I. Lignosulfonate. J. Appl. Polym. Sci. 2003, 88, 3284–3290. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Wang, F.; Yang, X.; Zou, Y. Effect of the maleation of lignosulfonate on the mechanical and thermal properties of lignosulfonate/poly(ε-caprolactone) blends. J. Appl. Polym. Sci. 2016, 133, 1–7. [Google Scholar] [CrossRef]
- Ebadi-Dehaghani, H.; Reiszadeh, M.; Chavoshi, A.; Nazempour, M.; Vakili, M.H. The effect of zinc oxide and calcium carbonate nanoparticles on the thermal conductivity of polypropylene. J. Macromol. Sci. Part B 2014, 53, 93–107. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Lu, X.; Zhang, X.; He, H.; Pan, F.; Zhou, L.; Liu, X.; Ji, X.; Zhang, S. Cascade utilization of lignocellulosic biomass to high-value products. Green Chem. 2019, 21, 3499–3535. [Google Scholar] [CrossRef]
- Cazacu, G.; Mihaies, M.; Pascu, M.C.; Profire, L.; Kowarskik, A.L.; Vasile, C. Polyolefin/lignosulfonate blends, 9 functionalized polyolefin/lignin blends. Macromol. Mater. Eng. 2004, 289, 880–889. [Google Scholar] [CrossRef]
- Wypych, A. Databook of Adhesion Promoters, 1st ed.; Chem Tech Publishing: Scarborough, ON, Canada, 2018; Chapter 3.14 Lignin; p. B978–B1. [Google Scholar] [CrossRef]
- Poletto, M. Effect of styrene maleic anhydride on physical and mechanical properties of recycled polystyrene wood flour composites. Maderas Cienc. Tecnol. 2016, 18, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Köhnke, J.; Gierlinger, N.; Mateu, B.P.; Unterweger, C.; Solt, P.; Mahler, A.K.; Schwaiger, E.; Liebner, F.; Gindl-Altmutter, W. Comparison of four technical lignins as a resource for electrically conductive carbon particles. BioResources 2019, 14, 1091–1109. [Google Scholar] [CrossRef]
- Pang, J.; Zhang, W.; Zhang, H.; Zhang, J.; Zhang, H.; Cao, G.; Han, M.; Yang, Y. Sustainable nitrogen-containing hierarchical porous carbon spheres derived from sodium lignosulfonate for high-performance supercapacitors. Carbon 2018, 132, 280–293. [Google Scholar] [CrossRef]
- Araújo, J.R.; Waldman, W.R.; De Paoli, M.A. Thermal properties of high density polyethylene composites with natural fibres: Coupling agent effect. Polym. Degrad. Stab. 2008, 93, 1770–1775. [Google Scholar] [CrossRef]
- Chaudhry, A.U.; Mabrouk, A.; Abdala, A. Thermally enhanced pristine polyolefins: Fundamentals, progress and prospective. J. Mater. Res. Technol. 2020, 9, 10796–10806. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Review of thermal conductivity in composites: Mechanisms, parameters and theory. Prog. Polym. Sci. 2016, 61, 1–28. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Zhai, S.; Zhang, P.; Xian, Y.; Zeng, J.; Shi, B. Effective thermal conductivity of polymer composites: Theoretical models and simulation models. Int. J. Heat Mass Transf. 2018, 117, 358–374. [Google Scholar] [CrossRef]
- Rizzo, M.; Zeni, M.; Fernanda, M.; Nunes, D.O.; Maria, A. Reacao ao fogo, isolamento t ́ermico e desempenhoac ́ustico de aglomerados de poliuretano rıgido comadicao de fibras naturais. Sci. Cum Ind. 2015, 3, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Atsonios, I.; Mandilaras, I.; Founti, M. Thermal assessment of a novel drywall system insulated with VIPs. Energies 2019, 12, 2373. [Google Scholar] [CrossRef] [Green Version]
- Kucukdogan, N.; Aydin, L.; Sutcu, M. Theoretical and empirical thermal conductivity models of red mud filled polymer composites. Thermochim. Acta 2018, 665, 76–84. [Google Scholar] [CrossRef]
- Odebiyi, O.S.; Onitiri, M.A.; Akinlabi, E.T. Theoretical investigation into the thermal conductivity of particle filled polypropylene composites. Mater. Today Proc. 2018, 5, 74–78. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Aguedo, M.; Richel, A.; Brohez, S.; Delvosalle, C.; Dubois, P. Phosphorus and nitrogen derivatization as efficient route for improvement of lignin flame retardant action in PLA. Eur. Polym. J. 2016, 84, 652–667. [Google Scholar] [CrossRef]


| wt% PP/wt% LGNa | Ti (3 wt% Weight Loss) (°C) | Calculated Ti (3 wt% Weight Loss) (°C) | Tmax LGNa (°C) | Tmax PP (°C) |
|---|---|---|---|---|
| 100/0 | 332 | − | − | 432 |
| 0/100 | 93 | − | 267 | − |
| 90/10 | 327 | 278 | 267 | 435 |
| 80/20 | 305 | 250 | 266 | 438 |
| 70/30 | 273 | 241 | 268 | 418 |
| 60/40 | 222 | 230 | 266 | 421 |
| Criteria Conditions | PP | 90/10 | 80/20 | 70/30 | 60/40 |
|---|---|---|---|---|---|
| Afterflame time for each individual specimen, t1 or t2 1 | ≥30 s | ≤30 s | ≤30 s | ≥30 s | ≥30 s |
| Total afterflame time for any condition set (t1 plus t2 for the five specimens) | ≥250 s | ≤250 s | ≤250 s | ≥250 s | ≥250 s |
| Afterflame plus afterglow time for each individual specimen after the second flame application (t2 + t3) | ≥60 s | ≤60 s | ≤60 s | ≥60 s | ≥60 s |
| Afterflame or afterglow of any specimen up to the holding clamp | Yes | No | No | Yes | Yes |
| Cotton indicator ignited by flaming particles or drops | Yes | Yes | Yes | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, M.; Finimundi, N.; Podzorova, M.; Pantyukhov, P.; Poletto, M. Assessment of Morphological, Physical, Thermal, and Thermal Conductivity Properties of Polypropylene/Lignosulfonate Blends. Materials 2021, 14, 543. https://doi.org/10.3390/ma14030543
Schneider M, Finimundi N, Podzorova M, Pantyukhov P, Poletto M. Assessment of Morphological, Physical, Thermal, and Thermal Conductivity Properties of Polypropylene/Lignosulfonate Blends. Materials. 2021; 14(3):543. https://doi.org/10.3390/ma14030543
Chicago/Turabian StyleSchneider, Mariane, Noriê Finimundi, Maria Podzorova, Petr Pantyukhov, and Matheus Poletto. 2021. "Assessment of Morphological, Physical, Thermal, and Thermal Conductivity Properties of Polypropylene/Lignosulfonate Blends" Materials 14, no. 3: 543. https://doi.org/10.3390/ma14030543








