Absorption of Nitrogen during Pulsed Wave L-PBF of 17-4 PH Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Nitrogen Covergas Absorption
3.2. Equilibrium Solubility
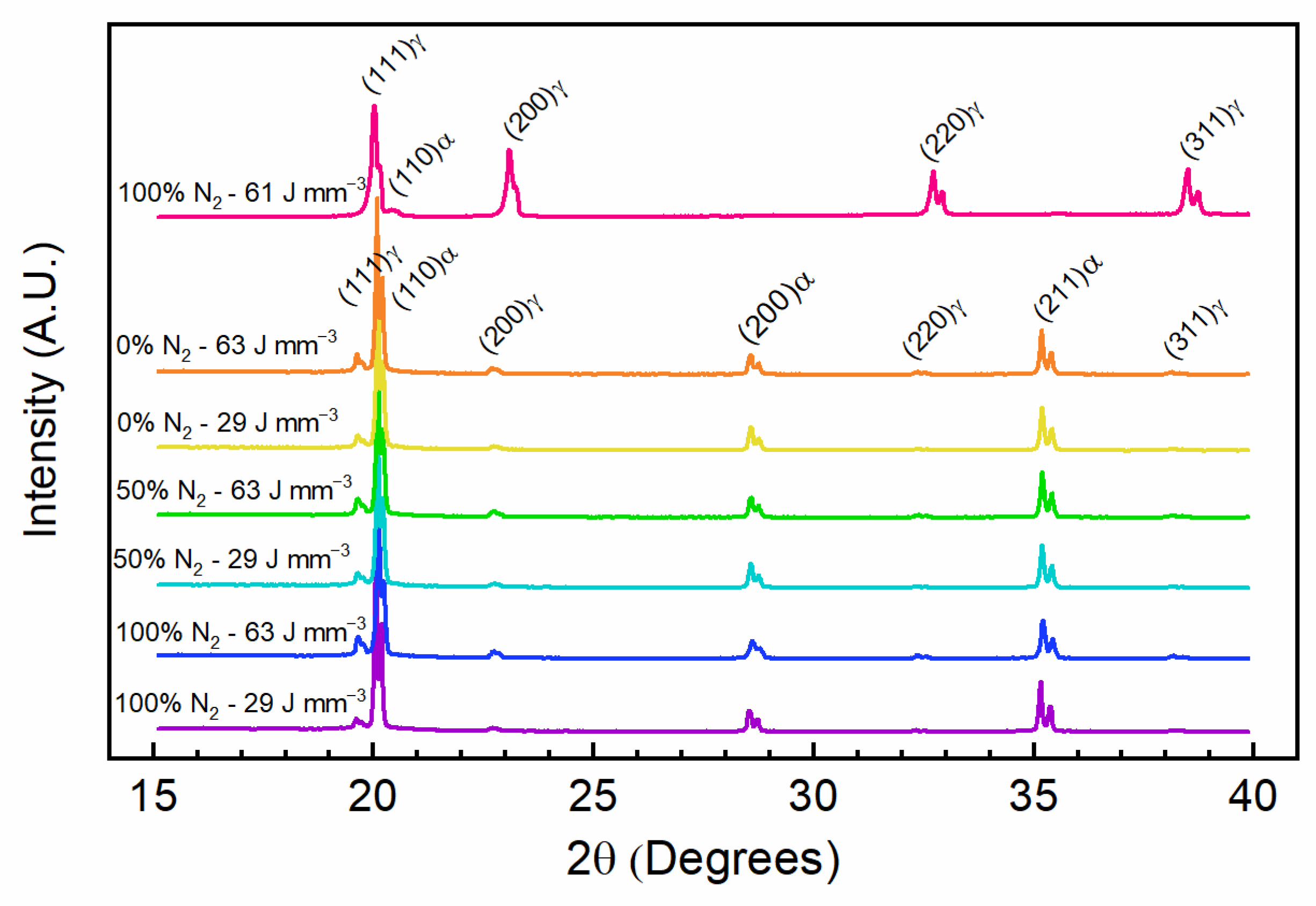
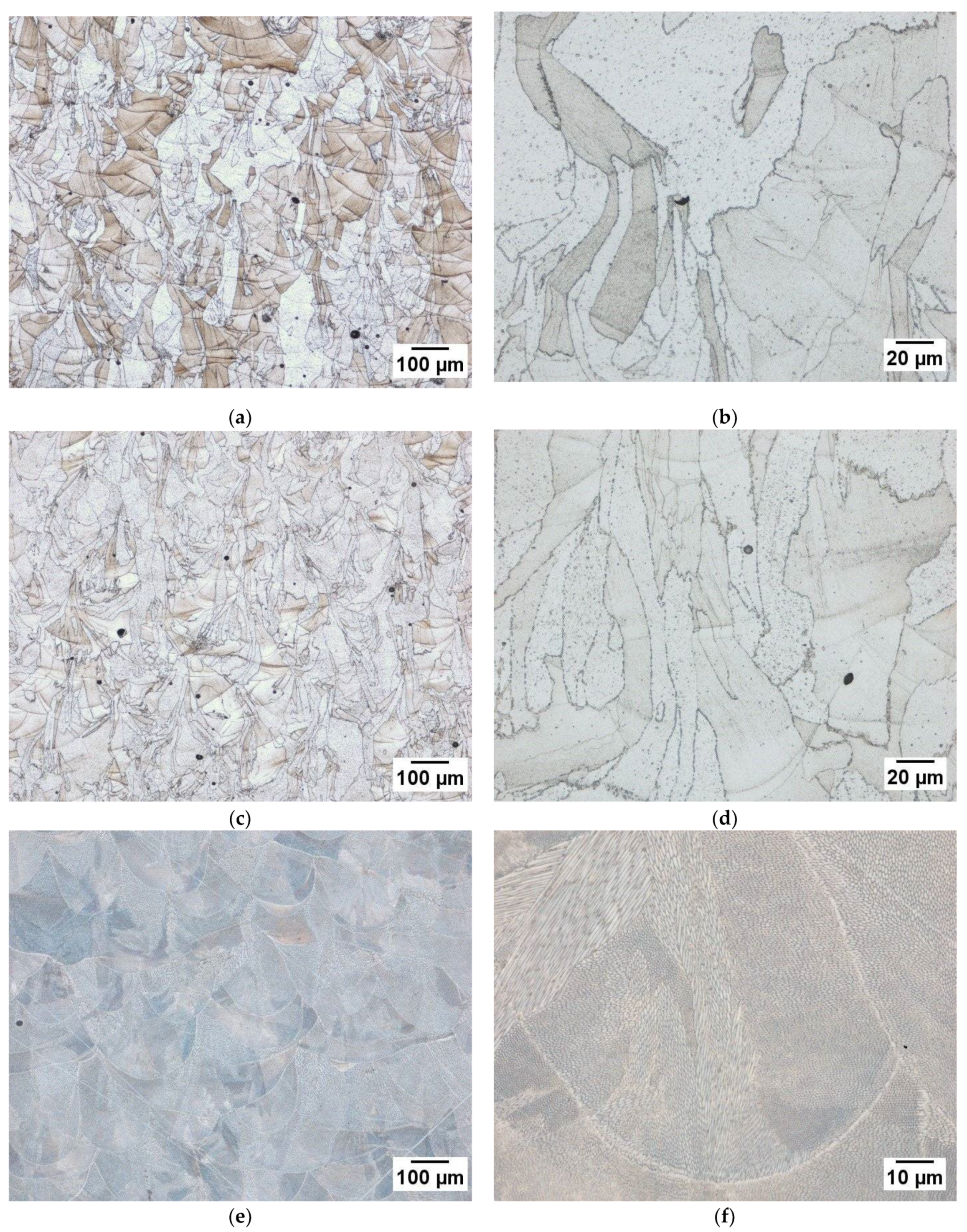
3.3. Microstructure Analysis
3.4. Mechanical Properties
4. Conclusions
- Nitrogen absorption, in addition to what was present in the base material, was shown to be dependent on the concentration of nitrogen in the cover gas as well as the laser exposure parameters used to fabricate samples.
- PW L-PBF exhibited less nitrogen absorption than CW L-PBF. Over the ranges tested the absorbed nitrogen was insufficient to result in any significant structure change
- It was found that the retained austenite in L-PBF 17-4 PH has no predominant preferred texture, yet detection was dependent on samples orientation.
- The small amount of absorbed nitrogen in the PW L-PBF samples resulted in a slight increase in ultimate tensile strength that is significantly less dramatic than the effects of large amounts of retained austenite.
- The presented results suggest the ability to use cover to manipulate the performance of L-PBF produced components. By controlling laser parameters and cover gas composition, absorption can be tuned for a particular application, either minimizing it allowing for either nitrogen or argon to be used interchangeably or maximizing it to improve mechanical performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfaify, M.; Saleh, A.F.; Al-Ahmari, A. Design for Additive Manufacturing. Sustainability 2020, 12, 7936. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive Manufactruing of Metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Murr, L.E.; Martinez, E.; Amato, K.N.; Gaytan, S.M.; Hernandez, J.; Ramirez, D.A.; Shindo, P.W.; Medina, F.; Wicker, R.B. Fabrication of Metal and Alloy Components by Additive Manufacturing: Examples of 3D Materials Science. J. Mater. Res. Technol. 2012, 1, 42–54. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal Fabrication by Additive Manufacturing Using Laser and Electron Beam Melting Technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Suri, P.; Smarslok, B.P.; German, R. Impact properties of sintered and wrought 17–4 PH stainless steel. Powder Met. 2006, 49, 40–47. [Google Scholar] [CrossRef]
- Hamlin, R.J.; Dupont, J.N. Microstructural Evolution and Mechanical Properties of Simulated Heat-Affected Zones in Cast Precipitation-Hardened Stainless Steels 17-4 and 13-8+Mo. Met. Mater. Trans. A 2016, 48, 246–264. [Google Scholar] [CrossRef]
- Hunt, J.; Derguti, F.; Todd, I. Selection of steels suitable for additive layer manufacturing. Ironmak. Steelmak. 2014, 41, 254–256. [Google Scholar] [CrossRef]
- Facchini, L.; Vicente, N., Jr.; Lonardelli, I.; Magalini, E.; Robotti, P.; Molinari, A. Metastable Austenite in 17-4 Precipita-tion-Hardening Stainless Steel Produced by Selective Laser Melting. Adv. Eng. Mater. 2010, 12, 184–188. [Google Scholar] [CrossRef]
- Murr, L.E.; Martinez, E.; Hernandez, J.; Collins, S.; Amato, K.N.; Gaytan, S.M.; Shindo, P.W. Microstructures and Properties of 17-4 PH Stainless Steel Fabricated by Selective Laser Melting. J. Mater. Res. Technol. 2012, 1, 167–177. [Google Scholar] [CrossRef]
- Rafi, H.K.; Pal, D.; Patil, N.; Starr, T.L.; Stucker, B.E. Microstructure and Mechanical Behavior of 17-4 Precipitation Har-denable Steel Processed by Selective Laser Melting. J. Mater. Eng. Perform. 2014, 23, 4421–4428. [Google Scholar] [CrossRef]
- Meredith, S.; Zuback, J.; Keist, J.; Palmer, T. Impact of composition on the heat treatment response of additively manufactured 17–4 PH grade stainless steel. Mater. Sci. Eng. A. 2018, 738, 44–56. [Google Scholar] [CrossRef]
- Starr, T.L.; Rafi, K.; Stucker, B.; Scherzer, C.M. Controlling Phase Composition in Selective Laser Melted Stainless Steels. In Proceedings of the Solid Freeform Fabrication Symposium, Austin, TX, USA, 6–8 August 2012; pp. 439–446. [Google Scholar]
- Averyanova, M.; Bertrand, P.; Verquin, B. Studying the Influence of Initial Power Characteristics on the Properties of Final Parts Manufactured by the Selective Laser Melting Technology. Virtual Phys. Prototyp. 2011, 6, 215–223. [Google Scholar] [CrossRef]
- Averyanova, M.; Cicala, E.; Bertrand, P.; Grevey, D. Experimental Design Approach to Optimize Selective Laser Melting of Martensitic 17-4 PH Powder: Part 1-Single LaserTracks and First Layer. Rapid Prototyp. J. 2012, 18, 28–37. [Google Scholar] [CrossRef]
- Lebrun, T.; Nakamoto, T.; Horikawa, K.; Kobayashi, H. Effect of retained austenite on subsequent thermal processing and resultant mechanical properties of selective laser melted 17–4 PH stainless steel. Mater. Des. 2015, 81, 44–53. [Google Scholar] [CrossRef]
- Lebrun, T.; Tanigaki, K.; Horikawa, K.; Kobayashi, H. Strain rate sensitivity and mechanical anisotropy of selective laser melted 17-4 PH stainless steel. Mech. Eng. J. 2014, 1. [Google Scholar] [CrossRef]
- Dong, W.; Kokawa, H.; Sato, Y.S.; Tsukamoto, S.; Ogawa, M. Mechanism governing nitrogen absorption by steel weld metal during laser welding. Met. Mater. Trans. A 2004, 35, 331–338. [Google Scholar] [CrossRef]
- Dong, W.; Kokawa, H.; Sato, Y.S.; Tsukamoto, S. Nitrogen absorption by iron and stainless steels during CO2 laser welding. Met. Mater. Trans. A 2003, 34, 75–82. [Google Scholar] [CrossRef]
- Keskitalo, M.; Mantyjarvi, K.; Sundqvist, J.; Powell, J.M.; Kaplan, A. Laser welding of duplex stainless steel with nitrogen as shielding gas. J. Mater. Process. Technol. 2015, 216, 381–384. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, K.; Zou, J.; Jiang, F.; Han, X. A study of the behavior and effects of nitrogen take-up from protective gas shielding in laser welding of stainless steel. J. Manuf. Process. 2018, 34, 477–485. [Google Scholar] [CrossRef]
- Kokawa, H. Nitrogen absorption and desorption by steels during arc and laser welding. Weld. Int. 2004, 18, 277–287. [Google Scholar] [CrossRef]
- Caballero, A.; Suder, W.; Chen, X.; Pardal, G.; Williams, S. Effect of shielding conditions on bead profile and melting behaviour in laser powder bed fusion additive manufacturing. Addit. Manuf. 2020, 34, 101342. [Google Scholar] [CrossRef]
- Simmons, J. Overview: High-nitrogen alloying of stainless steels. Mater. Sci. Eng. A 1996, 207, 159–169. [Google Scholar] [CrossRef]
- Elmer, J.; Vaja, J.; Carlton, H.; Pong, R. The Effect of Ar and N2 Shielding Gas on Laser Weld Porosity in Steel, Stainless Steels, and Nickel. Weld. J. 2015, 94, 313S–325S. [Google Scholar]
- Colombo, D.P.; Previtali, B. From Pulsed to Continuous Wave Emission in SLM with Contemporary Fiber Laser Sources: Effect of Temporal and Spatial Pulse Overlap in Part Quality. Int. J. Adv. Manuf. Technol. 2017, 91, 2701–2714. [Google Scholar]
- Glardon, R.; Karapatis, N.; Romano, V.; Levy, G. Influence of Nd:YAG Parameters on the Selective Laser Sintering of Metallic Powders. CIRP Ann. 2001, 50, 133–136. [Google Scholar] [CrossRef]
- Caprio, L.; Demir, A.G.; Previtali, B. Comparative study between CW and PW emissions in selective laser melting. J. Laser Appl. 2018, 30, 032305. [Google Scholar] [CrossRef]
- Caprio, L.; Demir, A.G.; Previtali, B. Influence of pulsed and continuous wave emission on melting efficiency in selective laser melting. J. Mater. Process. Technol. 2019, 266, 429–441. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, S.-W. Lasers in additive manufacturing: A review. Int. J. Precis. Eng. Manuf. Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Biffi, C.; Fiocchi, J.; Bassani, P.; Tuissi, A. Continuous wave vs pulsed wave laser emission in selective laser melting of AlSi10Mg parts with industrial optimized process parameters: Microstructure and mechanical behaviour. Addit. Manuf. 2018, 24, 639–646. [Google Scholar] [CrossRef]
- Simchi, A.; Pohl, H. Effects of laser sintering processing parameters on the microstructure and densification of iron powder. Mater. Sci. Eng. A. 2003, 359, 119–128. [Google Scholar] [CrossRef]
- Brown, B. Characterization of 304L Stainless Steel by Means of Minimum Input Energy on the Selective Laser Melting Platform. Masters’s Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2014. [Google Scholar]
- Katayama, S.; Kawahito, Y.; Mizutani, M. Elucidation of laser welding phenomena and factors affecting weld penetration and welding defects. Phys. Procedia 2010, 5, 9–17. [Google Scholar] [CrossRef]
- Dong, W.; Kokawa, H.; Sato, Y.S.; Tsukamoto, S. Nitrogen desorption by high-nitrogen steel weld metal during CO2 laser welding. Met. Mater. Trans. A 2005, 36, 677–681. [Google Scholar] [CrossRef]
- Gu, D.; Hagedorn, Y.-C.; Meiners, W.; Meng, G.; Batista, R.J.S.; Wissenbach, K.; Poprawe, R. Densification behavior, microstructure evolution, and wear performance of selective laser melting processed commercially pure titanium. Acta Mater. 2012, 60, 3849–3860. [Google Scholar] [CrossRef]
- Mullis, A.M.; Farrell, L.; Cochrane, R.F.; Adkins, N.J. Estimation of Cooling Rates During Close-Coupled Gas Atomization Using Secondary Dendrite Arm Spacing Measurement. Met. Mater. Trans. A 2013, 44, 992–999. [Google Scholar] [CrossRef]
- Lupis, C.H.P. Chemical Thermodynamics of Materials; Prentice Hall: Upper Saddle River, NJ, USA, 1983. [Google Scholar]
- Boyce, B.L.; Salzbrenner, B.C.; Rodelas, J.M.; Swiler, L.P.; Madison, J.D.; Jared, B.H.; Shen, Y.L. Extreme-Value Statistics Reveal Rare Failure-Critical Defects in Additive Manufaturing. Adv. Eng. Mater. 2017, 19, 1–10. [Google Scholar] [CrossRef]
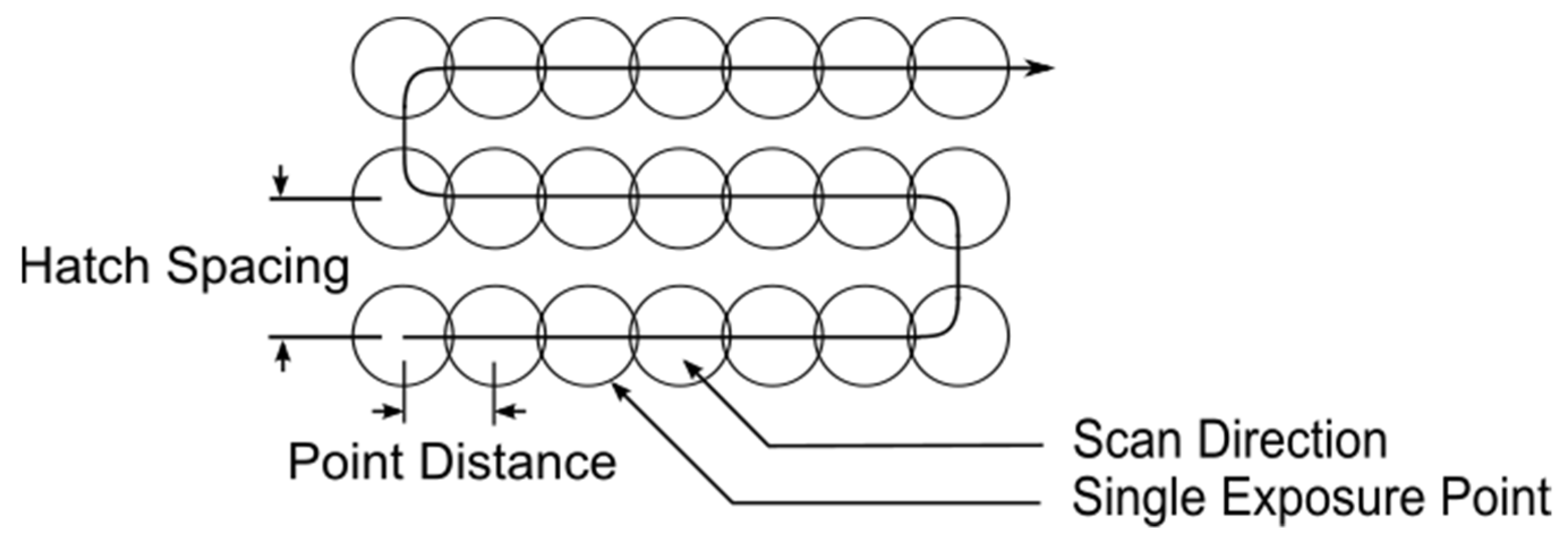
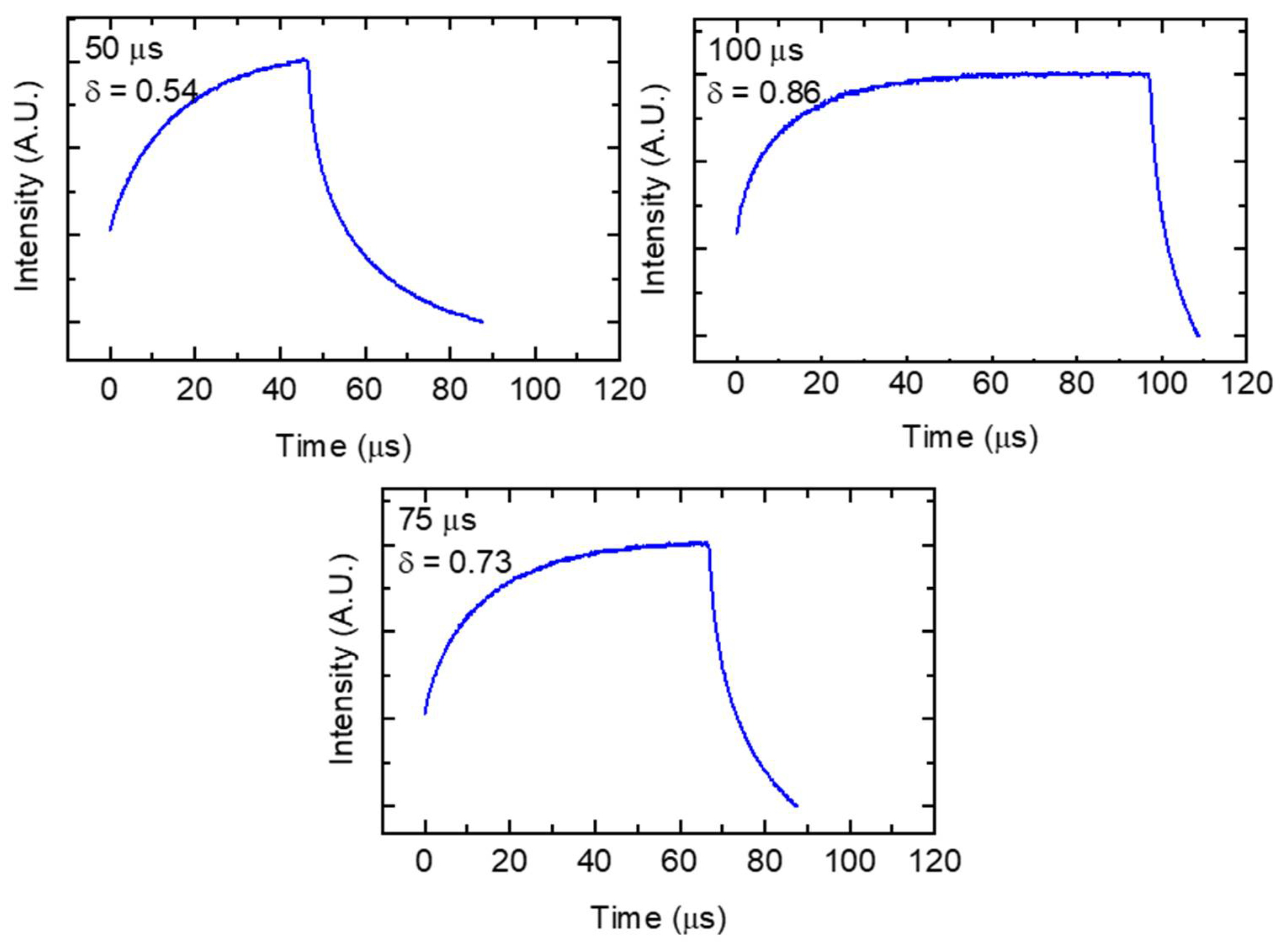
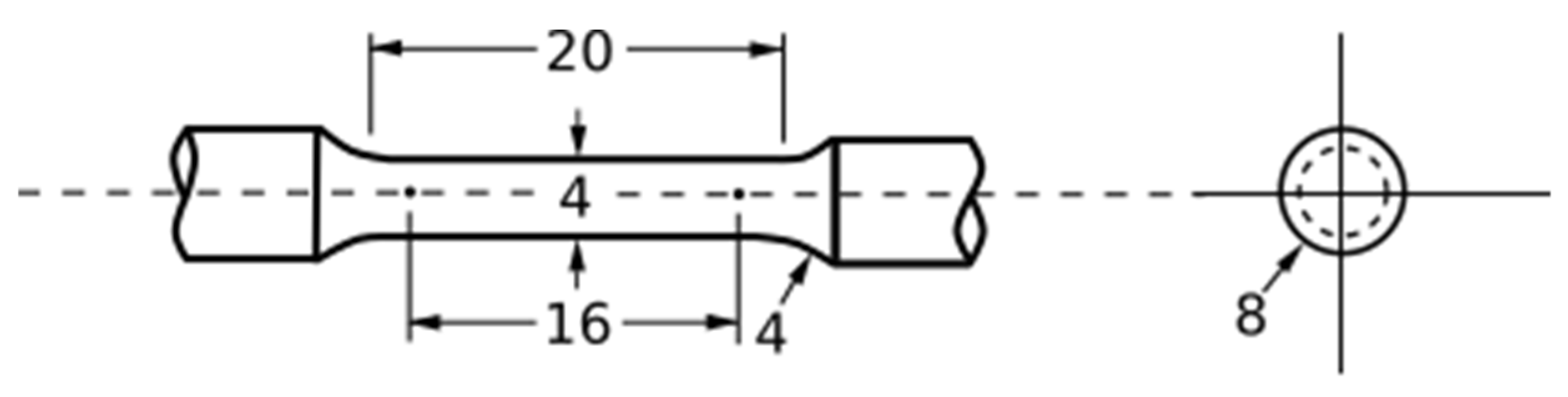
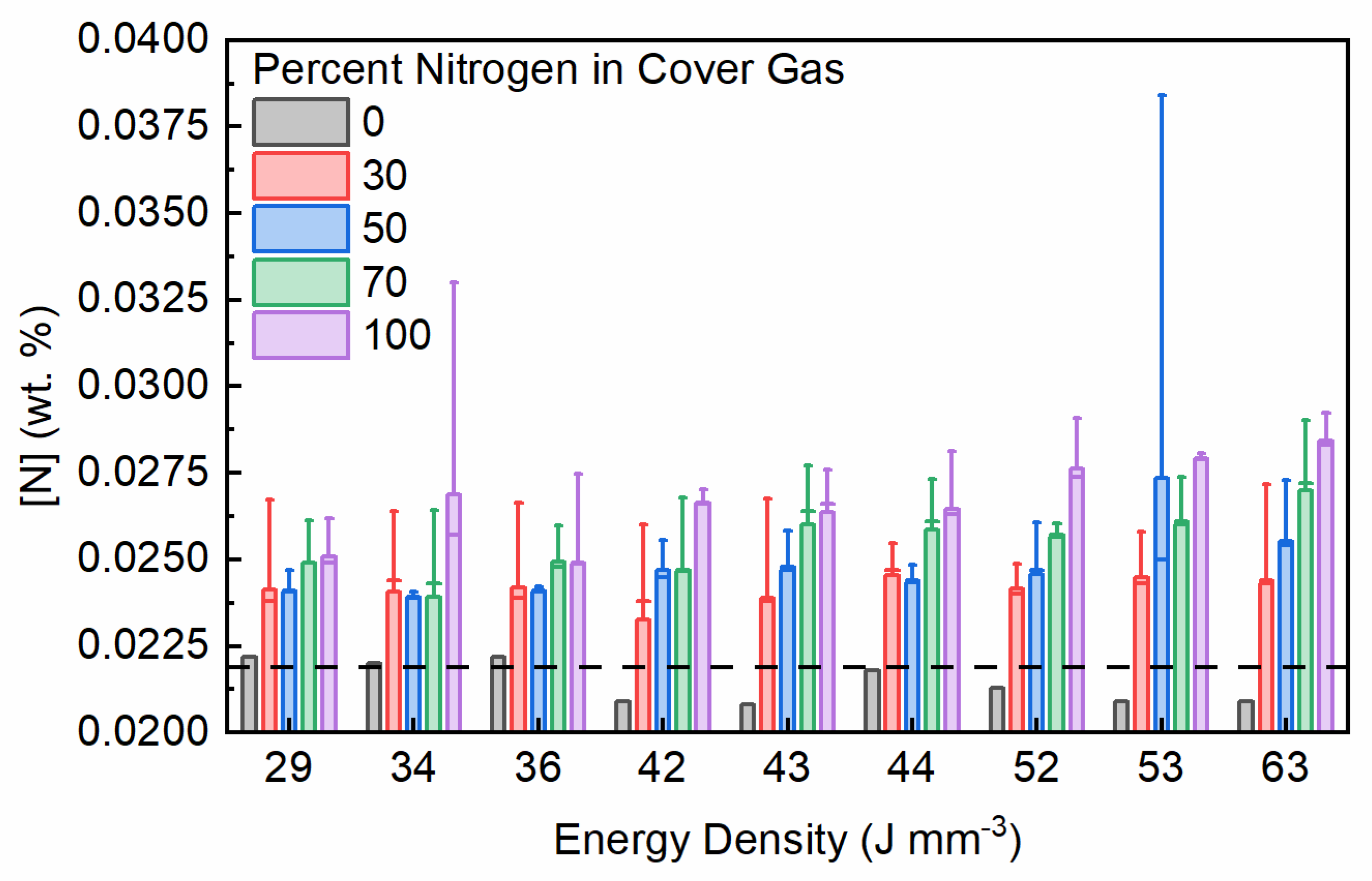

| Parameter Combination | Laser Power (W) | Hatch Spacing (μm) | Layer Thickness (μm) | Point Distance (μm) | Exposure Time (μs) | Scan Velocity (mm s−1) | Duty Cycle | Energy Density (J mm−3) |
|---|---|---|---|---|---|---|---|---|
| 1 | 200 | 90 | 50 | 45 | 85 | 529 | 0.75 | 63 |
| 2 | 200 | 90 | 50 | 45 | 75 | 600 | 0.71 | 53 |
| 3 | 200 | 90 | 50 | 45 | 65 | 692 | 0.65 | 42 |
| 4 | 200 | 90 | 50 | 55 | 85 | 647 | 0.75 | 52 |
| 5 | 200 | 90 | 50 | 55 | 75 | 733 | 0.71 | 43 |
| 6 | 200 | 90 | 50 | 55 | 65 | 846 | 0.65 | 34 |
| 7 | 200 | 90 | 50 | 65 | 85 | 765 | 0.75 | 44 |
| 8 | 200 | 90 | 50 | 65 | 75 | 867 | 0.71 | 36 |
| 9 | 200 | 90 | 50 | 65 | 65 | 1000 | 0.65 | 29 |
| 10 | 195 | 100 | 40 | ~ | ~ | 800 | 1.0 | 61 |
| Element | ASTM A564 (wt.%) | Argon Atomized Powder (wt.%) | Nitrogen Atomized Powder (wt.%) |
|---|---|---|---|
| Cr | 15.00–17.50 | 16.25 | 15.32 |
| Ni | 3.00–5.00 | 4.336 | 4.53 |
| Cu | 3.00–5.00 | 4.21 | 4.41 |
| Mn | 1.0 Max. | 0.1968 | 0.81 |
| Si | 1.0 Max. | 0.39 | 0.37 |
| Nb | 0.15–0.45 | 0.3 | 0.24 |
| C | 0.07 Max | 0.0171 | 0.06 |
| P | 0.04 Max. | 0.0117 | 0.012 |
| S | 0.03 Max. | 0.00149 | 0.004 |
| O | - | 0.0422 | 0.038 |
| N | - | 0.0219 | 0.184 |
| Co | - | 0.0024 | - |
| Mo | - | 0.0068 | 0.094 |
| V | - | 0.05 | 0.038 |
| W | - | 0.001 | - |
| Al | - | 0.002 | - |
| Fe | Bal. | Bal. | Bal. |
| Element | Interaction Coefficient |
| Cr | −0.046 |
| Ni | 0.0063 |
| Cu | 0.009 |
| Mn | −0.036 |
| Si | 0.047 |
| Nb | −0.067 |
| C | 0.103 |
| P | 0.045 |
| O | 0.05 |
| N | 0 |
| Mo | −0.011 |
| Powder Composition | Measured Nitrogen Concentration in Powder (wt.%) | Calculated Equilibrium Nitrogen Concentration (wt.%) |
|---|---|---|
| Nitrogen Atomized Powder | 0.184 | 0.2032 |
| Argon Atomized Powder | 0.0219 | 0.2178 |
| Energy Density (J mm−3) | Nitrogen in Cover Gas (%) | Powder Atomization Gas | Austenite Phase Fraction (%) | Martensite Phase Fraction (%) | Austenite Lattice Parameter (Å) | Martensite Lattice Parameter (Å) |
|---|---|---|---|---|---|---|
| 61 | 100 | N2 | 97.3 | 2.7 | 3.557 | 2.832 |
| 63 | 0 | Ar | 3.1 | 96.9 | 3.605 | 2.876 |
| 29 | 0 | Ar | 2.4 | 97.6 | 3.601 | 2.874 |
| 63 | 50 | Ar | 2.4 | 97.6 | 3.599 | 2.874 |
| 29 | 50 | Ar | 1.6 | 98.4 | 3.599 | 2.874 |
| 63 | 100 | Ar | 3.7 | 96.3 | 3.600 | 2.872 |
| 29 | 100 | Ar | 1.9 | 98.1 | 3.608 | 2.878 |
| Laser Exposure Type | Powder Atomization Gas | L-PBF Cover Gas | Elastic Modulus (GPa) | 0.2% YS (MPa) | UTS (MPa) | Strain at Failure (%) |
|---|---|---|---|---|---|---|
| PW | Ar | 100% Ar | 174.3 (165.1–183.49 | 777.3 (756.3–798.3) | 821.3 (800.7–841.8) | 17.3 (15.8–18.9) |
| PW | Ar | 100% N2 | 181.6 (173.2–189.9) | 815.7 (791.1–840.3) | 864.2 (834.9–893.4) | 14.1 (9.9–18.2) |
| CW | N2 | 100% N2 | 200.6 (188.8–212.4) | 547 (533.4–561.2) | 913 (911.0–916.1) | 43.3 (42.6–44.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, B.; Newkirk, J.; Liou, F. Absorption of Nitrogen during Pulsed Wave L-PBF of 17-4 PH Steel. Materials 2021, 14, 560. https://doi.org/10.3390/ma14030560
Brown B, Newkirk J, Liou F. Absorption of Nitrogen during Pulsed Wave L-PBF of 17-4 PH Steel. Materials. 2021; 14(3):560. https://doi.org/10.3390/ma14030560
Chicago/Turabian StyleBrown, Ben, Joseph Newkirk, and Frank Liou. 2021. "Absorption of Nitrogen during Pulsed Wave L-PBF of 17-4 PH Steel" Materials 14, no. 3: 560. https://doi.org/10.3390/ma14030560
APA StyleBrown, B., Newkirk, J., & Liou, F. (2021). Absorption of Nitrogen during Pulsed Wave L-PBF of 17-4 PH Steel. Materials, 14(3), 560. https://doi.org/10.3390/ma14030560








