3D Scaffolds to Model the Hematopoietic Stem Cell Niche: Applications and Perspectives
Abstract
:1. Introduction
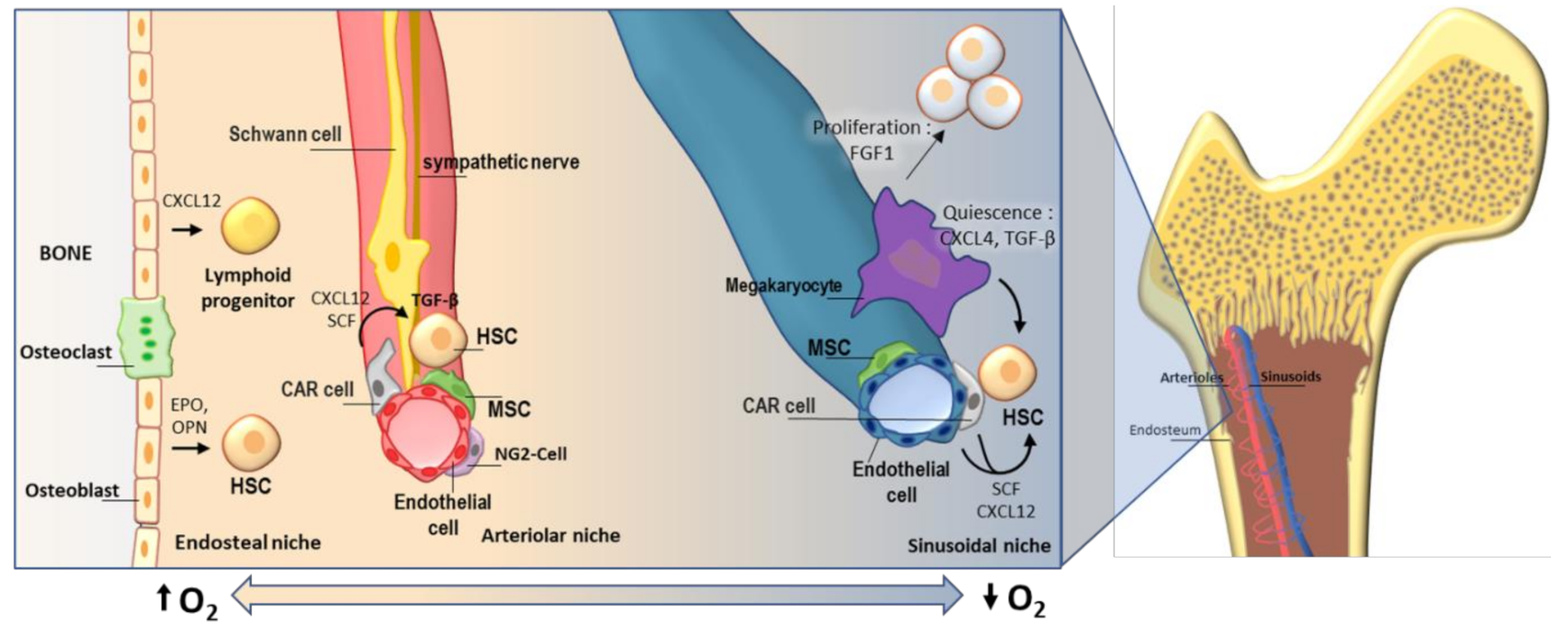
2. Hematopoietic Stem Cells and Their Niche
3. Applications of 3D Models of BM Niche for Cell Culture
3.1. Ex Vivo Expansion of HSC
3.2. Bone Marrow Study Model
3.3. Large Scale Drug Testing Platforms
4. Current 3D Models of the Bone Marrow Niche
4.1. Decellularized 3D Model of the Bone Marrow Niche
4.2. Other Models
4.2.1. Synthetic Scaffolds
4.2.2. Microspheroids/Organoids
4.2.3. 3D Printing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, L.; Morrison, S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013, 495, 231–235. [Google Scholar] [CrossRef]
- Chen, J.Y.; Miyanishi, M.; Wang, S.K.; Yamazaki, S.; Kao, K.S.; Nakauchi, H.; Weissman, I.L. Hoxb5 marks long-term haematopoietic stem cells revealing a homogenous perivascular niche. Nature 2016, 530, 223–227. [Google Scholar] [CrossRef]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Bruns, I.; Lucas, D.; Pinho, S.; Ahmed, J.; Lambert, M.P.; Kunisaki, Y.; Frenette, P.S. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 2014, 20, 1315–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, F.; Hirao, A.; Ohmura, M.; Sato, H.; Matsuoka, S.; Takubo, K.; Suda, T. Tie2/Angiopoietin-1 Signaling Regulates Hematopoietic Stem Cell Quiescence in the Bone Marrow Niche. Cell 2004, 118, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreger, P.; Viehmann, K.; Neuhoff, N.; Von Glaubitz, T.; Petzoldt, O.; Glass, B.; Schmitz, N. Autografting of highly purified peripheral blood progenitor cells following myeloablative therapy in patients with lymphoma: A prospective study of the long-term effects on tumor eradication, reconstitution of hematopoiesis and immune recovery. Bone Marrow Transplant. 1999, 24, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlush, L.I.; Zandi, S.; Mitchell, A.; Chen, W.C.; Joseph, M.; Gupta, V.; Brown, A.M.K. Identification of pre-leukemic hematopoietic stem cells in acute leukemia. Nature 2014, 506, 328–333. [Google Scholar] [CrossRef]
- Castagnoli, R.; Delmonte, O.M.; Calzoni, E.; Notarangelo, L.D. Hematopoietic Stem Cell Transplantation in Primary Immunodeficiency Diseases: Current Status and Future Perspectives. Front. Pediatr. 2019, 7, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mejia-Ramirez, E.; Florian, M.C. Understanding intrinsic hematopoietic stem cell aging. Haematologica 2020, 105, 22–37. [Google Scholar] [CrossRef]
- Lyman, G.H.; Edin, F.; Carolina, N. Impact of Chemotherapy Dose Intensity on Cancer Patient Outcomes. J. Natl. Compr. Cancer Netw. 2009, 7, 99–108. [Google Scholar] [CrossRef]
- Wang, S.; Qu, X.; Zhao, R.C. Clinical applications of mesenchymal stem cells. J. Hematol. Oncol. 2012, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone Marrow Mesenchymal Stem Cells: Aging and Tissue Engineering Applications to Enhance Bone Healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal Stem Cells: Cell Fate Decision to Osteoblast or Adipocyte and Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabi, S.; Pahlavan, S.; Ashtiani, M.K.; Ansari, H.; Abbasalizadeh, S.; Sayahpour, F.A.; Varzideha, F.; Kostin, S.; Aghdami, N.; Braun, T.; et al. Human embryonic stem cell-derived cardiovascular progenitor cells efficiently colonize in bFGF-tethered natural matrix to construct contracting humanized rat hearts. Biomaterials 2018, 154, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Uygun, B.E.; Soto-Gutierrez, A.; Yagi, H.; Izamis, M.; Guzzardi, M.A.; Shulman, C.; Uygun, K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010, 16. [Google Scholar] [CrossRef]
- Goh, S.; Bertera, S.; Olsen, P.; Candiello, J.E.; Halfter, W.; Uechi, G.; Banerjee, I. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013, 34, 6760–6772. [Google Scholar] [CrossRef] [Green Version]
- Han, T.T.Y.; Toutounji, S.; Amsden, B.G.; Flynn, L.E. Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials 2015, 72, 125–137. [Google Scholar] [CrossRef]
- Bianco, J.E.R.; Rosa, R.G.; Congrains-Castillo, A.; Joazeiro, P.P.; Waldman, S.D.; Weber, J.F.; Saad, S.T.O. Characterization of a novel decellularized bone marrow scaffold as an inductive environment for hematopoietic stem cells. Biomater. Sci. 2019. [Google Scholar] [CrossRef]
- Yu, C.; Bianco, J.; Brown, C.; Fuetterer, L.; Watkins, J.; Samani, A.; Flynn, L. Porous decellularized adipose tissue foams for soft tissue regeneration. Biomaterials 2013, 34, 3290–3302. [Google Scholar] [CrossRef]
- Nombela-Arrieta, C.; Pivarnik, G.; Winkel, B.; Canty, K.J.; Harley, B.; Mahoney, J.E.; Silberstein, L.E. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat. Cell Biol. 2013, 15. [Google Scholar] [CrossRef]
- Taichman, R.; Reilly, M.J.; Emerson, S.G. Human osteoblasts support human hematopoietic progenitor cells in vitro. Blood 1996, 87. [Google Scholar] [CrossRef]
- Kiel, M.J.; Radice, G.L.; Morrison, S.J. Lack of Evidence that Hematopoietic Stem Cells Depend on N-Cadherin-Mediated Adhesion to Osteoblasts for Their Maintenance. Cell Stem Cell 2007, 1, 204–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stier, S.; Ko, Y.; Forkert, R.; Lutz, C.; Neuhaus, T.; Grünewald, E.; Scadden, D.T. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. JEM 2005, 201, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Rankin, E.B.; Wu, C.; Khatri, R.; Wilson, T.L.S.; Andersen, R.; Araldi, E.; Giaccia, A.J. The HIF Signaling Pathway in Osteoblasts Directly Modulates Erythropoiesis through the Production of EPO. Cell 2012, 149, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, R.B.; Link, D.C. Megakaryocytes in the hematopoietic stem cell niche. Nat. Med. 2014, 20, 1233–1234. [Google Scholar] [CrossRef]
- Sugiyama, T.; Kohara, H.; Noda, M. Maintenance of the Hematopoietic Stem Cell Pool by CXCL12-CXCR4 Chemokine Signaling in Bone Marrow Stromal Cell Niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Miyoshi, H.; Shioda, S.; Yamazaki, S.; Ema, H.; Taketo, M.M.; Nakauchi, H. Nonmyelinating Schwann Cells Maintain Hematopoietic Stem Cell Hibernation in the Bone Marrow Niche. Cell 2011, 147, 1146–1158. [Google Scholar] [CrossRef] [Green Version]
- Acar, M.; Kocherlakota, K.S.; Murphy, M.M.; Peyer, J.G.; Oguro, H.; Inra, C.N.; Morrison, S.J. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 2015, 526, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Pinho, S.; Marchand, T.; Yang, E.; Wei, Q.; Nerlov, C.; Frenette, S.; Unit, M.H. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev. Cell. 2018, 44, 634–641. [Google Scholar] [CrossRef] [Green Version]
- Pang, W.W.; Price, E.A.; Sahoo, D.; Beerman, I.; Maloney, W.J.; Rossi, D.J.; Weissman, I.L. Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc. Natl. Acad. Sci. USA 2011, 108, 20012–20017. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Sun, H.; Chen, H.; Liang, J.; Yu, X.; Wu, C.; Wang, Z. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc. Natl. Acad. Sci. USA 2014, 111, 4221–4226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Perry, J.M.; Marshall, H.; Venkatraman, A.; Qian, P.; He, X.C.; Li, L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 2014, 20, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Runnels, J.M.; Zaher, W.; Lin, C.P. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiva, O.; Leon, C.; Kah, S.; Pierre, N.; Gachet, C.; Ravid, K. The role of extracellular matrix stiffness in megakaryocyte and platelet development and function. Am. J. Hematol. 2018, 93, 430–441. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.; Buxboim, A.; Spinler, K.R.; Swift, J.; Christian, D.A.; Hunter, C.A.; Discher, D.E. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell 2015, 14, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Zoldan, J.; Karagiannis, E.D.; Lee, C.Y.; Anderson, D.G.; Langer, R.; Levenberg, S. The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials 2011, 32, 9612–9621. [Google Scholar] [CrossRef] [Green Version]
- Levy-Mishali, M.; Zoldan, J.; Levenberg, S. Effect of Scaffold Stiffness on Myoblast Differentiation. Tissue Eng. Part A 2009, 15, 23–27. [Google Scholar] [CrossRef]
- Peyton, S.R.; Kalcioglu, Z.I.; Cohen, J.C.; Runkle, A.P.; Vliet, K.J.; Van Lauffenburger, D.A.; Griffith, L.G. Marrow-Derived Stem Cell Motility in 3D Synthetic Scaffold Is Governed by Geometry along with Adhesivity and Stiffness. Biotechnol. Bioeng. 2011, 108, 1181–1193. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Zhang, C.; Li, J.; Han, J.; Liu, X.; Yang, H. The physical microenvironment of hematopoietic stem cells and its emerging roles in engineering applications. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mattei, G.; Ferretti, C.; Tirella, A.; Ahluwalia, A.; Mattioli, M. Decoupling the role of stiffness from other hydroxyapatite signalling cues in periosteal derived stem cell differentiation. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring. Harb. Perspect. Biol. 2011, 3, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Naba, A.; Clauser, K.R.; Hoersch, S.; Liu, H.; Carr, S.A.; Hynes, R.O. The Matrisome: In Silico Definition and In Vivo Characterization by Proteomics of Normal and Tumor Extracellular Matrices. Mol. Cell. Proteom. 2012, 11, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaday, G.G.; Lider, O. Extracellular matrix moieties, cytokines, and enzymes: Dynamic effects on immune cell behavior and inflammation. J. Leukoc. Biol. 2000, 67, 149–159. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, A.E.; Madsen, C.D.; Cox, T.R.; Horton, E.R.; Venning, F.A.; Erler, J.T. ISDoT: In situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat. Med. 2017, 23, 890–898. [Google Scholar] [CrossRef]
- Mauri, E.; Sacchetti, A.; Vicario, N.; Peruzzotti-Jametti, L.; Rossi, F.; Pluchino, S. Evaluation of RGD functionalization in hybrid hydrogels as 3D neural stem cell culture systems. Biomater. Sci. 2018, 501–510. [Google Scholar] [CrossRef]
- Tahlawi, A.; Klontzas, M.E.; Allenby, M.C.; Morais, J.C.; Panoskaltsis, N.; Mantalaris, A. RGD-functionalized polyurethane scaffolds promote umbilical cord blood mesenchymal stem cell expansion and osteogenic differentiation. J. Tissue Eng. Regen. Med. 2019, 13, 232–243. [Google Scholar] [CrossRef]
- Raic, A.; Rödling, L.; Kalbacher, H.; Lee-thedieck, C. Biomaterials Biomimetic macroporous PEG hydrogels as 3D scaffolds for the multiplication of human hematopoietic stem and progenitor cells. Biomaterials 2014, 35, 929–940. [Google Scholar] [CrossRef]
- Gragert, L.; Eapen, M.; Williams, E.; Freeman, J.; Spellman, S.; Baitty, R.; Maiers, M. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. N. Eng. J. Med. 2014, 371, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Iscove, N.N.; Nawa, K. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Curr. Biol. 1997, 7, 805–808. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Zaldivar, H.; Santos, L.; De Leon Rodriguez, A. Expansion of human hematopoietic stem cells for transplantation: Trends and perspectives. Cytotechnology 2008, 56, 151–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Laer, D.; Corovic, A.; Vogt, B.; Fehse, B.; Roscher, S.; Rimek, A.; Ostertag, W. Loss of CD38 antigen on CD34+ CD38+ cells during short-term culture. Leukemia 2000, 14, 947–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caiazzo, M.; Okawa, Y.; Ranga, A.; Piersigilli, A.; Tabata, Y.; Lutolf, M.P. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater. 2016, 15, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, T.; Huang, X.; Zhao, Y.; Wang, B.; Yin, Y.; Dai, J. Substrate-independent immunomodulatory characteristics of mesenchymal stem cells in three-dimensional culture. PLoS ONE 2018, 13, e0206811. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Park, J.; Lee, S.E.; Yoon, C.-H.; Jang, J.H.; Yang, J.M.; Kim, H.-S. Human peripheral blood-born hematosphere as a niche for hematopoietic stem cell expansion. Cell Res. 2011, 45, 987–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dao, M.A.; Hashino, K.; Kato, I.; Nolta, J.A. Adhesion to fibronectin maintains regenerative capacity during ex vivo culture and transduction of human hematopoietic stem and progenitor cells. Blood 1998, 92, 4612–4621. [Google Scholar] [CrossRef]
- Kräter, M.; Jacobi, A.; Otto, O.; Tietze, S.; Müller, K.; David, M.; Wobus, M. Bone marrow niche-mimetics modulate HSPC function via integrin signaling. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Ventura, M.S.; Jahnen-dechent, W.; Labude, N.; Bovi, M.; Hieronymus, T.; Zenke, M.; Neurs, S. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials 2012, 33, 6987–6997. [Google Scholar] [CrossRef]
- Severn, C.E.; Hugo, M.; Eagle, M.J.; Rooney, P.; Mantalaris, A.; Toye, A.M. Polyurethane scaffolds seeded with CD34+ cells maintain early stem cells whilst also facilitating prolonged egress of haematopoietic progenitors. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Tomimori, Y.; Takagi, M.; Yoshida, T. The construction of an in vitro three-dimensional hematopoietic microenvironment for mouse bone marrow cells employing porous carriers. Cytotechnology 2000, 34, 121–130. [Google Scholar] [CrossRef]
- Bourgine, P.E.; Klein, T.; Paczulla, A.M.; Shimizu, T.; Kunz, L.; Kokkaliaris, K.D. In vitro biomimetic engineering of a human hematopoietic niche with functional properties. Proc. Natl. Acad. Sci. USA 2018, 115, 5689–5693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Zhu, B.; Wang, X.; Xiao, R.; Wang, C. Three-dimensional co-culture of mesenchymal stromal cells and differentiated osteoblasts on human bio-derived bone scaffolds supports active multi-lineage hematopoiesis in vitro: Functional implication of the biomimetic HSC niche. Int. J. Mol. Med. 2016, 38, 1141–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isern, J.; Martin-Antonio, B.; Ghazanfari, R.; Martin, A.M.; Lopez, J.A.; Toro, R.; Mendez-Ferre, S. Self-Renewing Human Bone Marrow Mesenspheres Promote Hematopoietic Stem Cell Expansion. Cell Rep. 2013, 3, 1714–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, Y.; Funamoto, S.; Kimura, T.; Nam, K.; Fujisato, T.; Kishida, A. The effect of decellularized bone/bone marrow produced by high-hydrostatic pressurization on the osteogenic differentiation of mesenchymal stem cells. Biomaterials 2011, 32, 7060–7067. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Spagnoli, G.C.; Martin, I.; Ploegert, S.; Demougin, P.; Heberer, M.; Reschner, A. Three-Dimensional Culture of Melanoma Cells Profoundly Affects Gene Expression Profile: A High Density Oligonucleotide Array Study. J. Cell. Physiol. 2005, 224, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Luca, A.; Mersch, S.; Deenen, R.; Schmidt, S.; Messner, I.; Schäfer, K.-L.; Stoecklein, N.H. Impact of the 3D Microenvironment on Phenotype, Gene Expression, and EGFR Inhibition of Colorectal Cancer Cell Lines. PLoS ONE 2013, 8, e59689. [Google Scholar] [CrossRef]
- Kobayashi, H.; Morikawa, T.; Okinaga, A.; Hamano, F.; Hashidate-Yoshida, T. Environmental Optimization Enables Maintenance of Quiescent Hematopoietic Stem Cells Ex Vivo. Cell Rep. 2019, 28, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Abarrategi, A.; Mufti, G.; Bonnet, D.; Abarrategi, A.; Foster, K.; Hamilton, A.; Bonnet, D. Versatile humanized niche model enables study of normal and malignant human hematopoiesis Versatile humanized niche model enables study of normal and malignant human hematopoiesis. J. Clin. Investig. 2017, 127, 543–548. [Google Scholar] [CrossRef] [Green Version]
- Leisten, I.; Kramann, R.; Ventura, M.S.; Bovi, M.; Neuss, S.; Ziegler, P.; Schneider, R.K. 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials 2012, 33, 1736–1747. [Google Scholar] [CrossRef]
- Seet, C.S.; He, C.; Bethune, M.T.; Li, S.; Chick, B.; Gschweng, E.H.; Montel-Hagen, A. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat. Method 2017, 14. [Google Scholar] [CrossRef]
- Parekh, C.; Crooks, G.M. Critical Differences in Hematopoiesis and Lymphoid Development Between Humans and Mice. J. Clin. Immunol. 2013, 33, 711–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prewitz, M.C.; Seib, F.P.; Bonin, M.; Von Friedrichs, J.; Stißel, A.; Niehage, C.; Werner, C. Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat. Method 2013, 10, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, J.; Miller, P. Phase II and Phase III attrition rates. Nat. Rev. Drug Discov. 2013, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Nefedova, Y.; Landowski, T.H.; Dalton, W.S. Bone marrow stromal-derived soluble factors and direct cell contact contribute to de novo drug resistance of myeloma cells by distinct mechanisms. Leukemia 2003, 17, 1175–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, A.; Loutherback, K.; Lambert, G.; Estévez-salmerón, L.; Tlsty, T.D.; Austin, R.H.; Sturm, J.C. Cell motility and drug gradients in the emergence of resistance to chemotherapy. Proc. Natl. Acad. Sci. USA 2013, 110, 16103–16108. [Google Scholar] [CrossRef] [Green Version]
- Stegmaier, K.; Ross, K.N.; Colavito, S.A.; Malley, S.O.; Stockwell, B.R.; Golub, T.R. Gene expression—Based high-throughput screening (GE-HTS) and application to leukemia differentiation. Nat. Genet. 2004, 36, 257–264. [Google Scholar] [CrossRef]
- Nierode, G.J.; Perea, B.C.; Mcfarland, S.K.; Pascoal, J.F.; Clark, D.S.; Schaffer, D.V.; Dordick, J.S. High-Throughput Toxicity and Phenotypic Screening of 3D Human Neural Progenitor Cell Cultures on a Microarray Chip Platform. Stem Cell Rep. 2016, 7, 970–982. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Y.; Cheng, P.; Chan, D.W. A simple affinity spin tube filter method for removing high-abundant common proteins or enriching low-abundant biomarkers for serum proteomic analysis. Proteomics 2003, 3, 243. [Google Scholar] [CrossRef]
- Wagner, D.E.; Bonenfant, N.R.; Sokocevic, D.; Desarno, M.J.; Borg, Z.D.; Parsons, C.S.; Weiss, D.J. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials 2014, 35, 2664–2679. [Google Scholar] [CrossRef] [Green Version]
- Brien, F.J.O. Biomaterials scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Weber, B.; Dijkmand, P.E.; Scherman, J.; Sanders, B.; Emmert, M.Y.; Grünenfelder, J.; Verbeek, R.; Bracher, M.; Black, M.; Franz, T.; et al. Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials 2013, 34, 7269–7280. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.M.; Lowdell, M.W.; Urbani, L.; Ansari, T.; Burns, A.J.; Turmaine, M.; De Coppi, P. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc. Nat. Acad. Sci. USA 2013, 110, 14360–14365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, M.; Emerson, S.; Palsson, B. Large-scale Expansion of Human Stem and Progenitor Cells from Bone Marrow Mononuclear Cells in Continuous Perfusion Cultures Large-scale expansion of human stem and progenitor cells from bone marrow mononuclear cells in continuous perfusion cultures. Blood 1993, 82, 378–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meissner, P.; Schröder, B.; Herfurth, C.; Biselli, M.; Jülich, F.; Gmbh, J. Development of a fixed bed bioreactor for the expansion of human hematopoietic progenitor cells. Cytotechnology 1999, 30, 227–234. [Google Scholar] [CrossRef]
- Schmal, O.; Seifert, J.; Schäffer, T.E.; Walter, C.B.; Aicher, W.K.; Klein, G. Hematopoietic Stem and Progenitor Cell Expansion in Contact with Mesenchymal Stromal Cells in a Hanging Drop Model Uncovers Disadvantages of 3D Culture. Stem Cell Int. 2016, 2016. [Google Scholar] [CrossRef]
- Eliasson, P.; Rehn, M.; Hammar, P.; Larsson, P.; Sirenko, O.; Flippin, L.A.; Jo, J. Hypoxia mediates low cell-cycle activity and increases the proportion of long-term reconstituting hematopoietic stem cells during in vitro culture. Exp. Hematol. 2010, 38, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Eliasson, P.; Jonsson, J.-I. The Hematopoietic Stem Cell Niche: Low in Oxygen but a Nice Place to be. J. Cell. Physiol. 2010, 222, 17–22. [Google Scholar] [CrossRef]
- Sharma, M.B.; Limaye, L.S.; Kale, V.P. Mimicking the functional hematopoietic stem cell niche in vitro: Recapitulation of marrow physiology by hydrogel-based three-dimensional cultures of mesenchymal stromal cells. Hematopoiesis 2012, 97, 651–660. [Google Scholar] [CrossRef] [Green Version]
- Rödling, L.; Schwedhelm, I.; Kraus, S.; Bieback, K.; Hansmann, J.; Lee-Thedieck, C. 3D models of the hematopoietic stem cell niche under steady-state and active conditions. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Bai, T.; Li, J.; Sinclair, A.; Imren, S.; Merriam, F.; Sun, F.; Delaney, C. Expansion of primitive human hematopoietic stem cells by culture in a zwitterionic hydrogel. Nat. Med. 2019, 25, 1566–1575. [Google Scholar] [CrossRef]
- Lai, W.Y.; Li, Y.Y.; Mak, S.K.; Ho, F.C.; Chow, S.T.; Chooi, W.H.; Chan, B.P. Reconstitution of bone-like matrix in osteogenically differentiated mesenchymal stem cell—Collagen constructs: A three-dimensional in vitro model to study hematopoietic stem cell niche. J. Tissue Eng. 2013, 4, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pievani, A.; Sacchetti, B.; Corsi, A.; Rambaldi, B.; Donsante, S.; Scagliotti, V.; Riminucci, M. Human umbilical cord blood-borne fibroblasts contain marrow niche precursors that form a bone/marrow organoid in vivo. Development 2017, 144, 1035–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, D.; Chen, L.; Ding, J.; Zhang, X.; Nie, Z.; Li, X. A 3D engineered scaffold for hematopoietic progenitor/stem cell co-culture in vitro. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Braham, M.V.J.; Ahlfeld, T.; Akkineni, A.R.; Minnema, M.C.; Dhert, W.J.A.; Robin, C.; Alblas, J. Endosteal and Perivascular Subniches in a 3D Bone Marrow Model for Multiple Myeloma. Tissue Eng. Part C Methods 2018, 24, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Grauss, R.W.; Hazekamp, M.G.; Vliet, S.; Van Groot, A.C.G.; Deruiter, M.C. Decellularization of rat aortic valve allografts reduces leaflet destruction and extracellular matrix remodeling. J. Thorac. Cardiovasc. Surg. 2003, 126, 2003–2010. [Google Scholar] [CrossRef] [Green Version]
- Kitahara, H.; Yagi, H.; Tajima, K.; Okamoto, K.; Yoshitake, A.; Aeba, R.; Shimizu, H. Heterotopic transplantation of a decellularized and recellularized whole porcine heart †. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Badylak, S.F.; Gilbert, T.W. Immune Response to Biologic Scaffold Materials. Semin. Immunol. 2008, 20, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Duisit, J.; Orlando, G.; Debluts, D.; Maistriaux, L.; Xhema, D.; Bisthoven, Y.J.; De Gianello, P. Decellularization of the Porcine Ear Generates a Biocompatible, Nonimmunogenic Extracellular Matrix Platform for Face. Ann. Surg. 2018, 267, 1191–1201. [Google Scholar] [CrossRef]
- Harris, G.M.; Raitman, I.; Schwarzbauer, J.E.; States, U.; States, U. Cell-derived decellularized extracellular matrices. Methods Cell Biol. 2018, 143, 97–114. [Google Scholar] [CrossRef]
- Brown, B.N.; Freund, J.M.; Han, L.; Rubin, J.P.; Reing, J.E.; Badylak, S.F. Comparison of Three Methods for the Derivation of a Biologic Scaffold Composed of Adipose. Tissue Eng. Part C 2011, 17, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Rieder, E.; Kasimir, M.-T.; Silberhumer, G.; Seebacher, G.; Wolner, E.; Simon, P.; Weigel, G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J. Thorac. Cardiovasc. Surg. 2004, 127, 399–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.H.; Chen, J.; Kirilak, Y.; Willers, C.; Xu, J.; Wood, D. Porcine Small Intestine Submucosa (SIS) Is Not an Acellular Collagenous Matrix and Contains Porcine DNA: Possible Implications in Human Implantation. J. Biomed. Mater. Res. 2005, 73, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Toner, M.; Cravalho, E.G.; Karel, M.; Karel, M. Thermodynamics and kinetics of intracellular ice formation during freezing of biological cells. J. Appl. Phys. 1990, 67, 1582–1593. [Google Scholar] [CrossRef]
- Flynn, L.; Semple, J.L.; Woodhouse, K.A. Decellularized placental matrices for adipose tissue engineering. J. Biomed. Mater. Res. 2006, 79, 359–369. [Google Scholar] [CrossRef]
- Farrokhi, A.; Pakyari, M.; Nabai, L.; Pourghadiri, A. Evaluation of Detergent-Free and Detergent-Based Methods for Decellularization of Murine Skin. Tissue Eng. Part A 2018, 24, 955–967. [Google Scholar] [CrossRef]
- Erten, E.; Arslan, S.; Emre, Y. Cartilage for chondrogenic differentiation of human adipose mesenchymal stem cells in vitro. 2016, 6, 94236–94246. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Ashton, R.S.; Banerjee, A.; Punyani, S.; Schaffer, D.V.; Kane, R.S. Scaffolds based on degradable alginate hydrogels and poly (lactide-co-glycolide) microspheres for stem cell culture. Biomaterials 2007, 28, 5518–5525. [Google Scholar] [CrossRef]
- Yu, J.; Du, K.T.; Fang, Q.; Gu, Y.; Mihardja, S.S.; Sievers, R.E.; Lee, R.J. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials 2010, 31, 7012–7020. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Miller, J.S. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019, 464, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Lee, S.; Choi, Y.; Hee, D.; Gao, G.; Wang, H.; Cho, D. 3D cell-printing of biocompatible and functional meniscus constructs using meniscus-derived bioink. Biomaterials 2021, 267, 120466. [Google Scholar] [CrossRef]
- Dasgupta, Q.; Black, L.D. A Fresh Slate for 3D bioprinting. Science 2019, 365, 446–447. [Google Scholar] [CrossRef] [PubMed]


| System | Description | Main Results |
|---|---|---|
| Synthetic scaffolds | ||
| Poly (lactic-co-glycolic acid) (PLGA) | PLGA is a biocompatible and biodegradable material. | It does not support CD34+ cells growth [58]. |
| Polycaprolactone (PCL) | elastic mechanical properties and slow degradation rate | Supports CD34+ adhesion and proliferation [58]. |
| Polyurethane (PU) | PU is a polymer with attractive mechanical properties and biocompatible. | Supports Cd34+ proliferation, differentiation and egress [59]. |
| Non-woven polyester fiber/polypropylene mesh | Fibrous material, multiple fibrous layers of polymers. | Supports CD34+ proliferation [60]. |
| Biodegradable zwitterionic hydrogel | Poly-carboxybetaine acrylamide (pCBAA) hydrogel, with zwitterionic segments of 20 alternating K and E residues and a metalloproteinase-cleavable motif for degradation. | Prevents differentiation, maintains self-renewal and reduces metabolic activity of HSCs. Shows superior expansion of primitive HSCs [90]. |
| Bio-functionalized scaffolds | ||
| Ceramic scaffold bio-functionalized with mesenchymal cells and osteoblasts | Ceramic scaffold is cultured with hMSC and osteoblast to produce ECM and cytokines previous to HSC culture. | MSCs and osteoblasts produced a bone marrow-like environment. Functionalization increased expansion of HSCs capable of hematopoietic reconstitution [61]. |
| Polyethylene glycol (PEG) bio-functionalized hydrogels | PEG-acrylate hydrogel was bio-functionalized by including a modified RGD peptide (involved in ECM-cell adhesion) | Supports CD34+ expansion and stemness better than 2D culture [48]. |
| Bio-derived bone scaffolds (BDBS) | Scaffold from human bone is biofunctionalized with MSCs and osteoblasts. | Supports adhesion, expansion and maintenance of stemness in HSCs better than 2D co-culture [62]. |
| Gelatin-based porous scaffold (Gelfoam) functionalized with several stromal cells | Scaffold was cultured with MSC, endothelial, osteoblasts previous to HSC on the Gel foam. | This functionalized scaffold allowed adhesion and growth of different niche cells. Supported expansion and maintenance of HSC [68]. |
| Natural Materials | ||
| Collagen | Elastic, biodegradable, natural component of the ECM | Co-culture in collagen supports CD34+ differentiation and expansion [69]. |
| Fibrin | Natural protein, highly biocompatible. | Supports CD34+ adherence and proliferation [58]. |
| Cellulose | Abundant, low-cost, non-biodegradable. Could be natural or synthetic. | Cellulose beads did not support CD34+ cell adhesion and proliferation [60]. |
| Microspheres/organoids | ||
| Collagen microspheres | MSCs were encapsulated in collagen microspheres, osteogenic differentiation was induced and subsequent decellularization to use it as scaffold for HSCs culture. | Supported mice HSC and MSC proliferation and adhesion [91]. |
| Mesenspheres | Spheres of a low-adherence population of MSCs formed spontaneously in ultra-low adherent dishes | BM Mesenspheres support expansion of HSC [63] in co-culture. |
| Hematosphere | Peripheral blood mononuclear cells formed spheres in ultra-low attach surfaces. | Spheres formed from PBMNCs support extensive expansion of primitive Lin(−)CD34(+)CD38(−) HSCs [55]. |
| Bone marrow organoid | Cord blood fibroblasts form a cellular pellet, this pellet was differentiated in vitro to a chondroid rudiment. After implantation in mice these rudiments remodeled into a functional BM niche. | The implanted organoid resembled the natural HSC niche. Host cells formed vascular structures and HSC engrafted in the organoid [92]. |
| Decellularized ECM/tissue/organ scaffolds | ||
| Decellularized ECM | Obtained by decellularization of ECM produced by stromal cells in vitro | Enhanced HSC adhesion and expansion of CD34+ cells [57]. |
| Decellularized bovine bone marrow (DeBM) | Detergent-free decellularized bovine bone marrow with highly preserved bone marrow architecture | DeBM supported adhesion, focal localization and proliferation of mesenchymal and HSCs [18]. |
| Decellularized porcine bone marrow | High-hydrostatic Pressurization method for decellularization of BM. Cultured with MSCs. | Supported MSC growth and differentiation. Implantation in mice induced HSC recruitment [64]. |
| 3D printing | ||
| 3D printing of MSCs-laden alginate-gelatin bioink | HSCs were cultured in a printed 3D scaffold fabricated using a mix of alginate, gelatin and MSCs. | Enhanced expansion and stemness of HSCs. Induced expression of integrins and adhesion [93]. |
| 3D printing model of endosteal and perivascular niches | 3D printing of pasty calcium phosphate cement in cylinder-like format and seeded with osteogenic MSC to emulate the endosteal niche and endothelial cell laden Matrigel to mimic the perivascular niche. | Supported proliferation of CD138+ myeloma cells [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Congrains, A.; Bianco, J.; Rosa, R.G.; Mancuso, R.I.; Saad, S.T.O. 3D Scaffolds to Model the Hematopoietic Stem Cell Niche: Applications and Perspectives. Materials 2021, 14, 569. https://doi.org/10.3390/ma14030569
Congrains A, Bianco J, Rosa RG, Mancuso RI, Saad STO. 3D Scaffolds to Model the Hematopoietic Stem Cell Niche: Applications and Perspectives. Materials. 2021; 14(3):569. https://doi.org/10.3390/ma14030569
Chicago/Turabian StyleCongrains, Ada, Juares Bianco, Renata G. Rosa, Rubia I. Mancuso, and Sara T. O. Saad. 2021. "3D Scaffolds to Model the Hematopoietic Stem Cell Niche: Applications and Perspectives" Materials 14, no. 3: 569. https://doi.org/10.3390/ma14030569
APA StyleCongrains, A., Bianco, J., Rosa, R. G., Mancuso, R. I., & Saad, S. T. O. (2021). 3D Scaffolds to Model the Hematopoietic Stem Cell Niche: Applications and Perspectives. Materials, 14(3), 569. https://doi.org/10.3390/ma14030569







