Comparison of Foam Glass-Ceramics with Different Composition Derived from Ark Clamshell (ACS) and Soda Lime Silica (SLS) Glass Bottles Sintered at Various Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Materials Characterizations
3. Results and Discussion
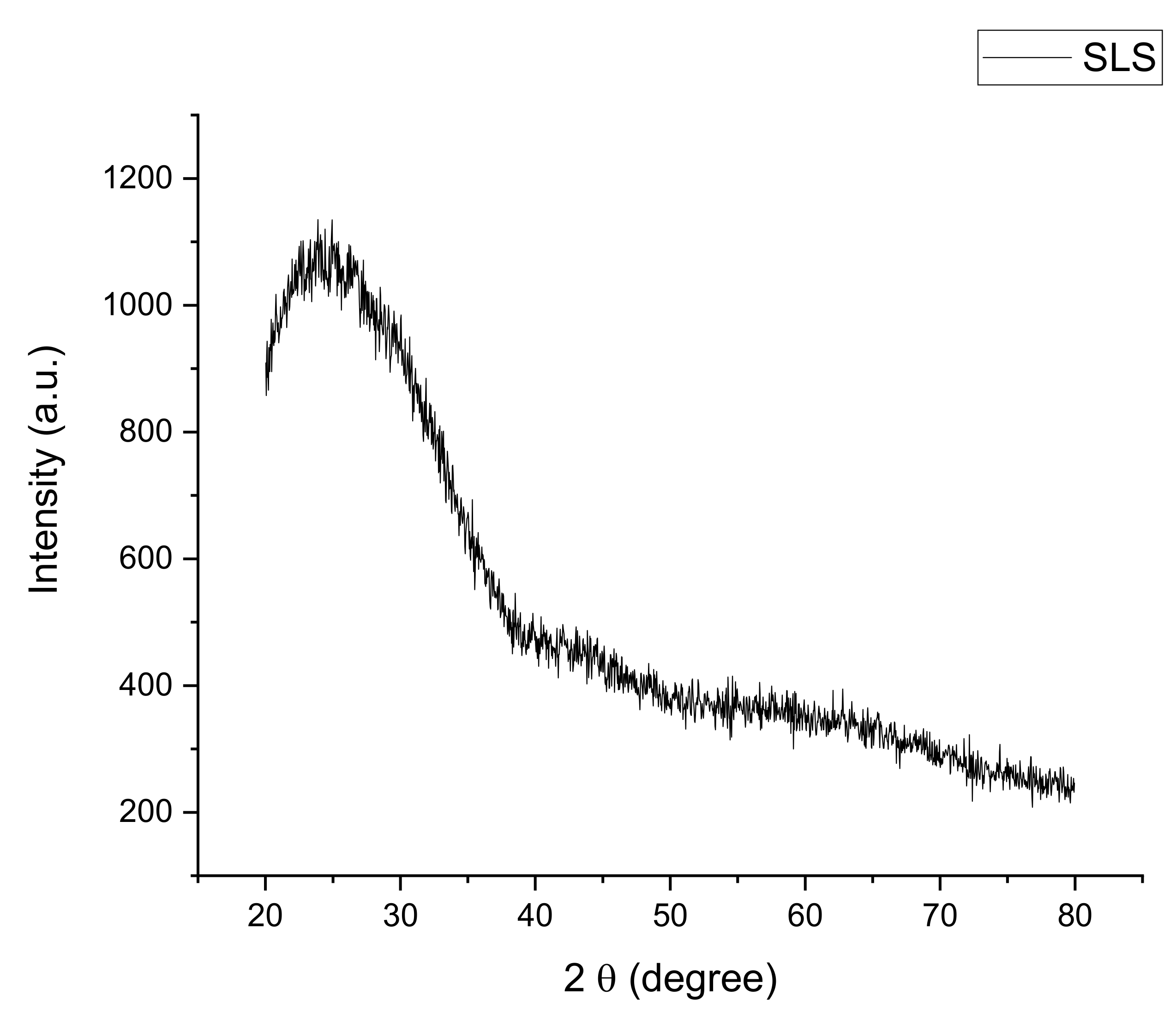
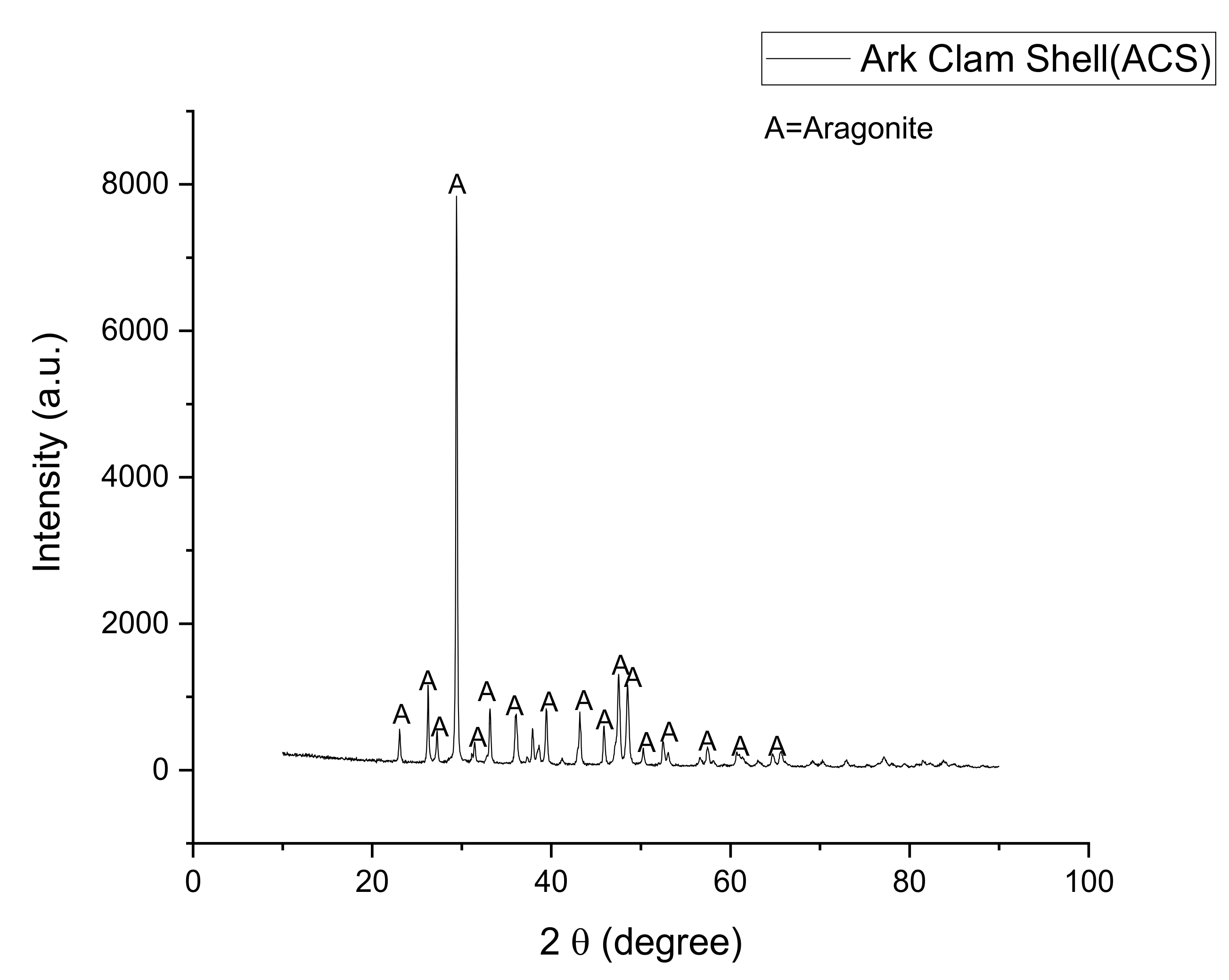
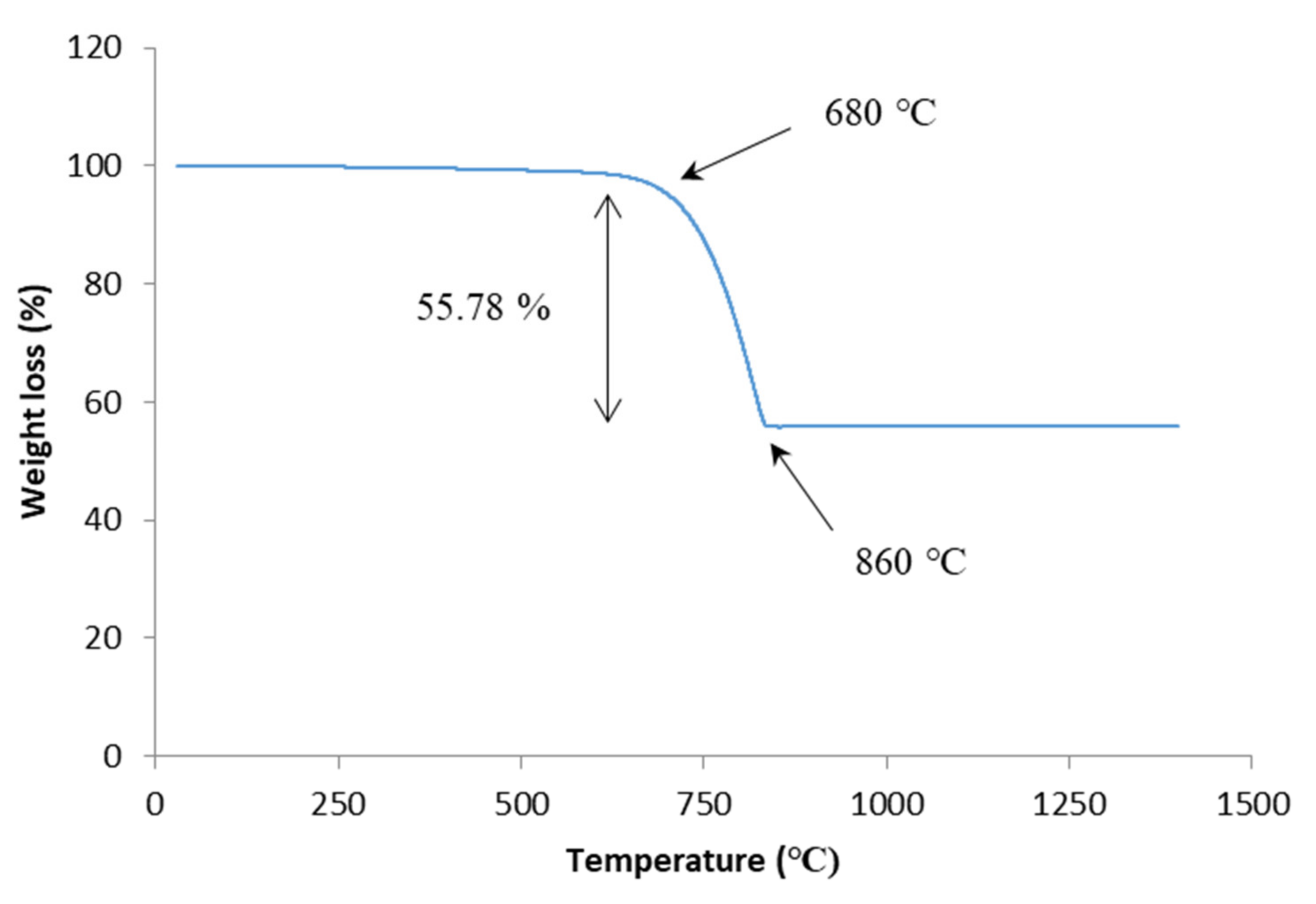
3.1. Raw Materials Analysis
3.2. Foam Glass-Ceramics Analysis
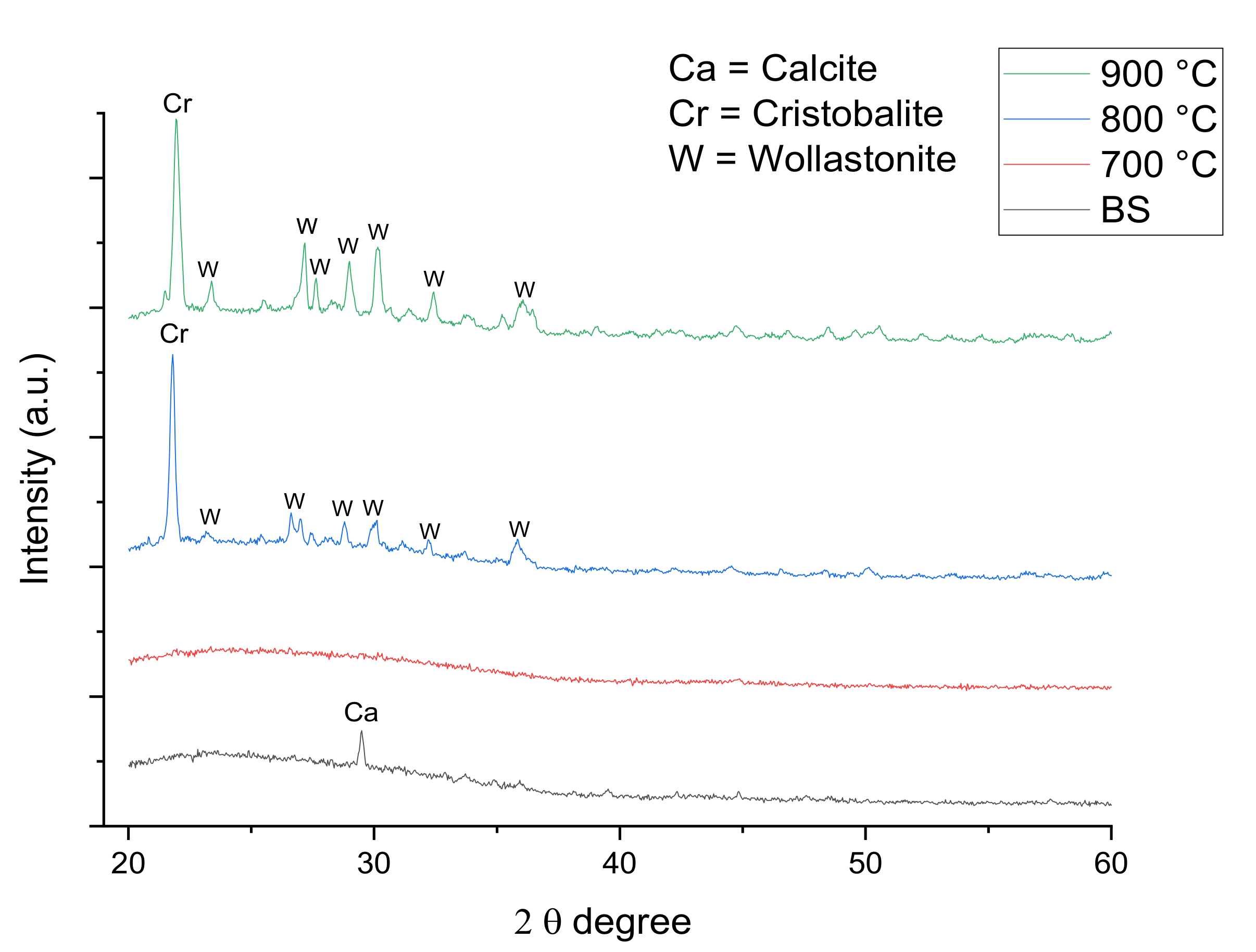
3.2.1. Structural Studies
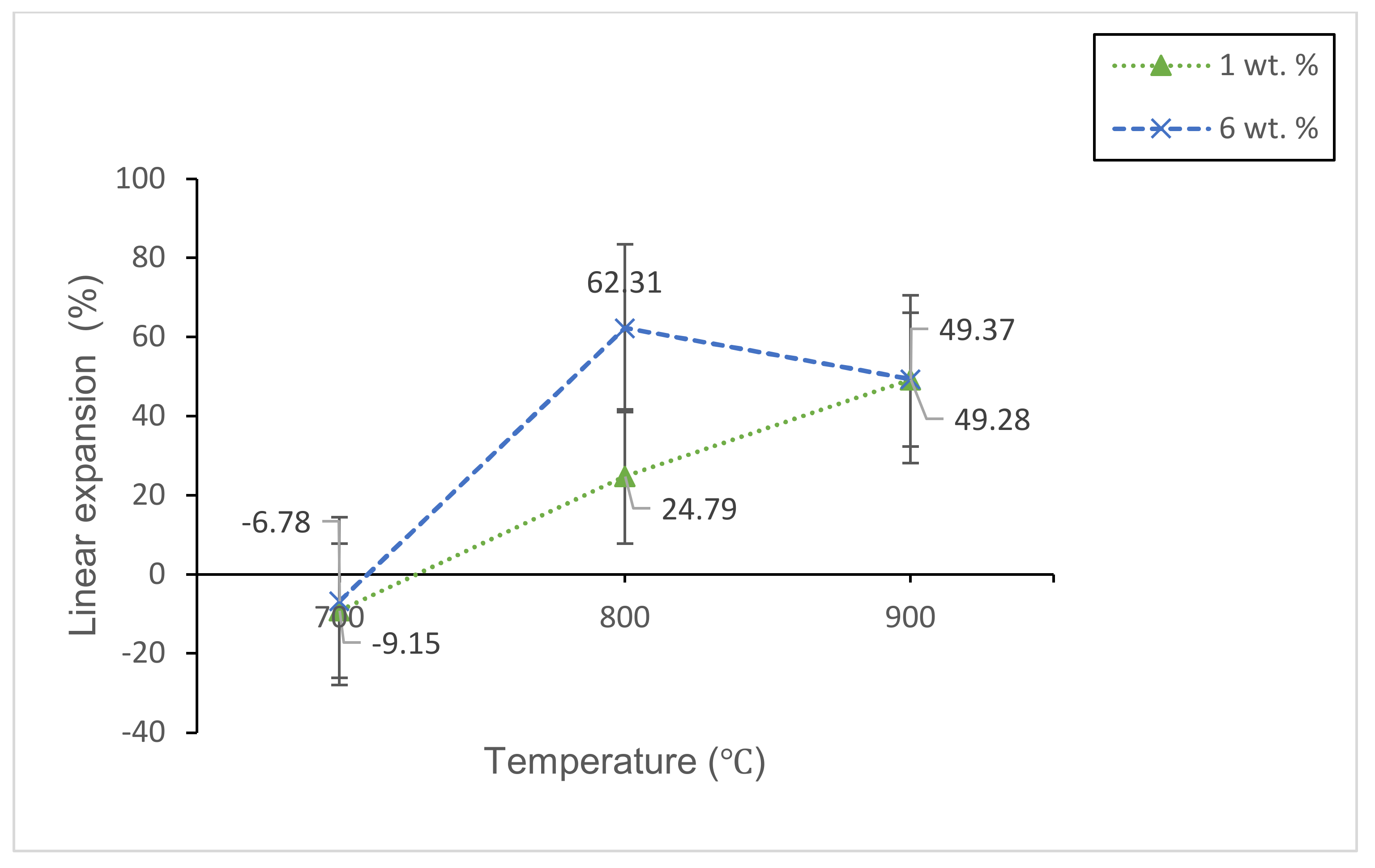
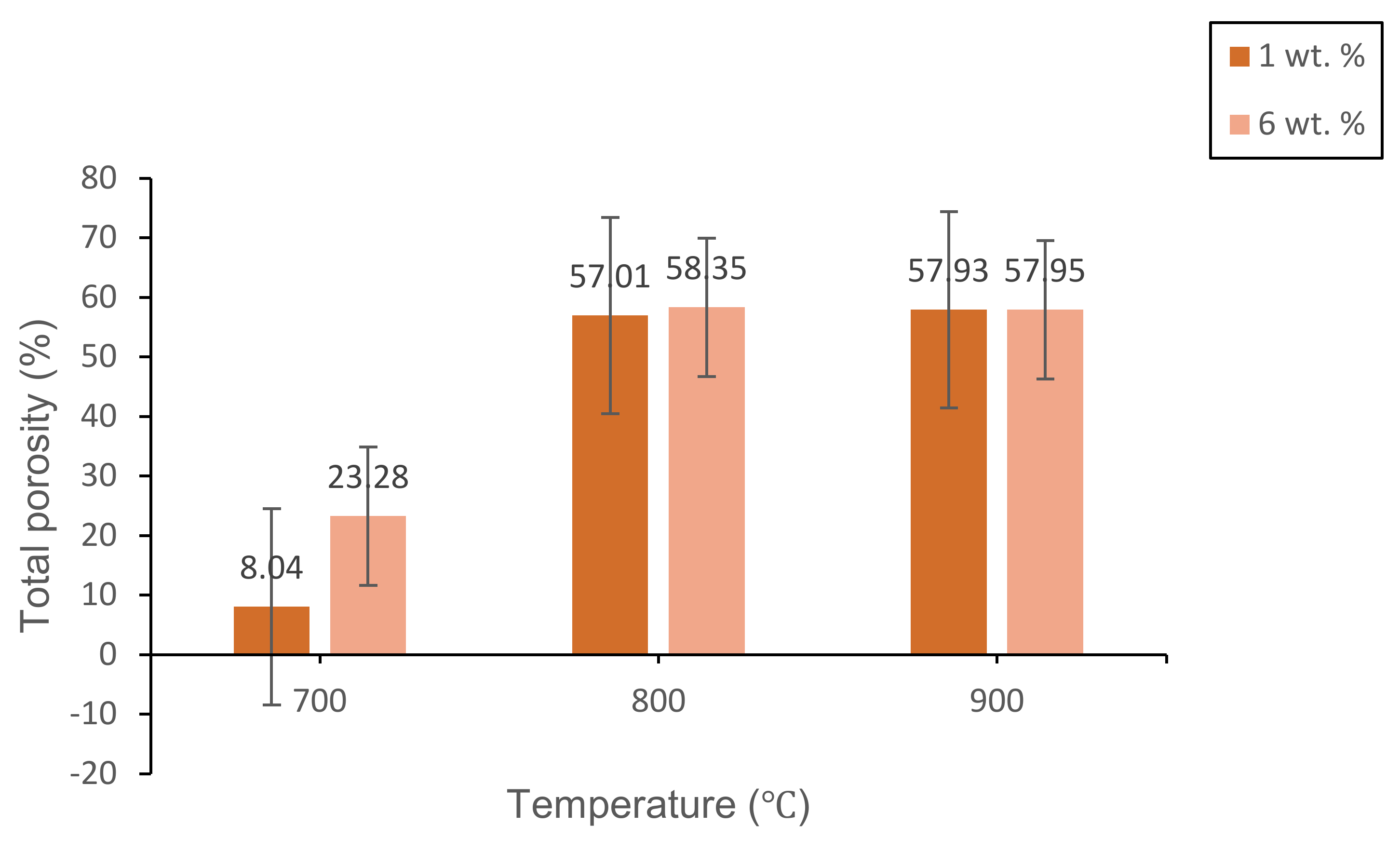
3.2.2. Physical Studies
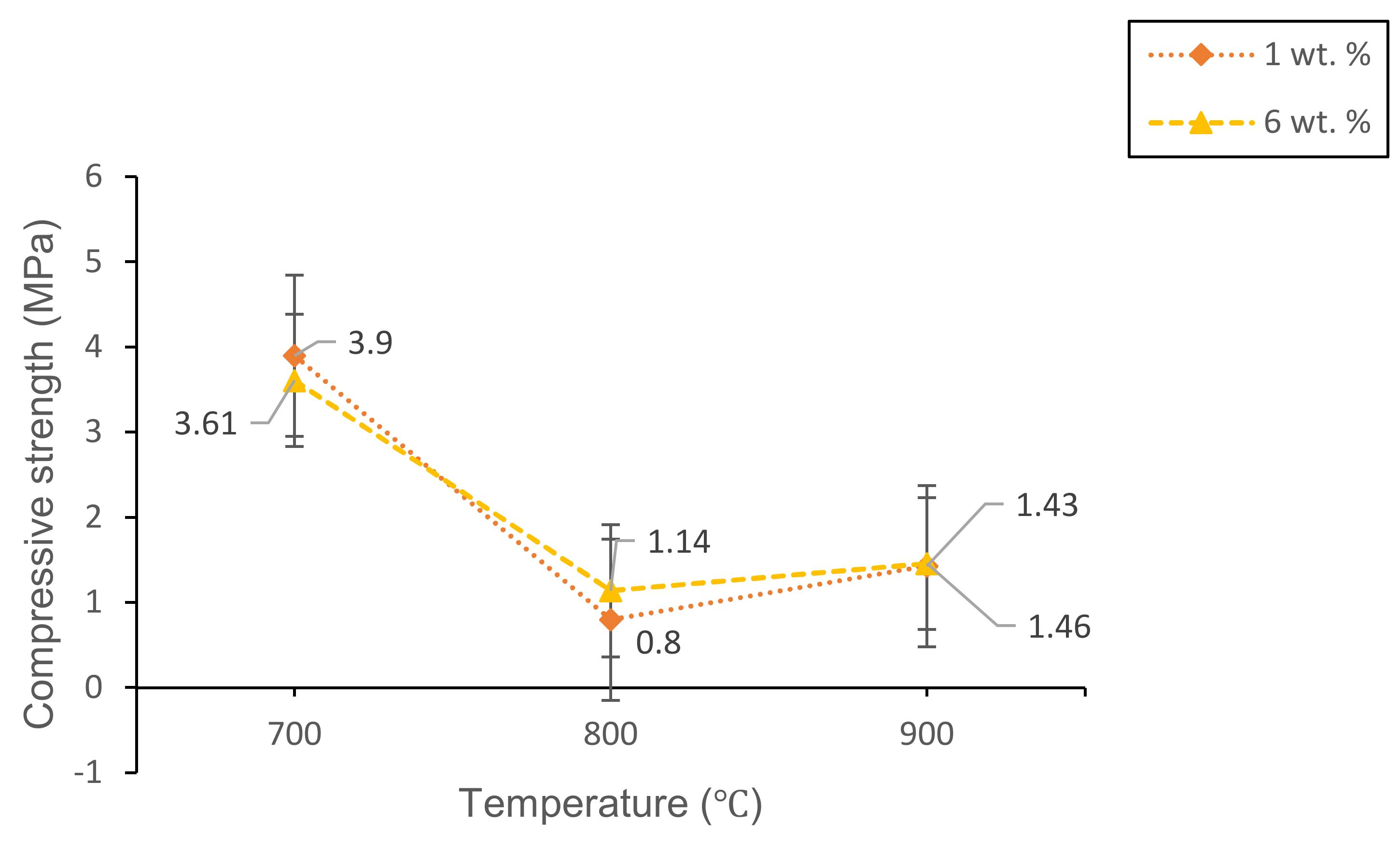
3.2.3. Mechanical Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souza, M.T.; Maia, B.G.O.; Teixeira, L.B.; Oliveira, K.G.; Teixeira, A.H.B.; Oliveira, A.P.N. Glass foams produced from glass bottles and eggshell wastes. Process Saf. Environ. 2017, 111, 60–64. [Google Scholar] [CrossRef]
- Da Silva Fernandes, F.A.; Arcaro, S.; Junior, E.F.T.; Serra, J.C.V.; Bergmann, C.P. Glass foams produced from soda-lime glass waste and rice husk ash applied as partial substitutes for concrete aggregates. Process Saf. Environ. 2019, 128, 77–84. [Google Scholar] [CrossRef]
- Lu, J.; Onitsuka, K. Construction utilisation of foamed waste glass. Int. J. Environ. Sci. 2004, 16, 302–307. [Google Scholar]
- Chen, Q.Z.; Thompson, I.D.; Boccaccin, A.R. 45S5 Bioglass®-derived glass-ceramic scaffold for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Belluci, D.; Cannilo, V.; Sola, A.; Chiellini, F.; Gazzarri, M.; Migone, C. Microporous Bioglass®-derived scaffolds for bone tissue regeneration. Ceram. Int. 2011, 37, 1575–1585. [Google Scholar] [CrossRef]
- Sheth, N.; Luo, J.; Banerjee, J.; Pantano, C.G.; Kim, S.H. Characterization of surface structures of dealkalized soda lime silica glass using x-ray photoelectron, specular reflection infrared, attenuated total reflection infrared and sum frequency generation spectroscopies. J. Non Cryst. Solids 2017, 474, 24–31. [Google Scholar] [CrossRef]
- Almutawa, F.; Vandal, R.; Wang, S.Q.; Lim, H.W. Current status of photoprotection by window glass, automobile glass, window films, and sunglasses. Photodermatol. Photoimmunol. Photomed. 2013, 29, 65–72. [Google Scholar] [CrossRef]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Juoi, J.M.; Arudra, D.; Rosli, Z.M.; Hussain, K.; Jaafar, A.J. Microstructural properties of glass composite material made from incinerated scheduled waste slag and soda lime silicate (SLS) waste glass. J. Non Cryst. Solids 2013, 367, 8–13. [Google Scholar] [CrossRef]
- Juoi, J.M.; Ojovan, M.I.; Lee, W.E. Microstructure and leaching durability of glass composite wasteforms for spent clinoptilolite immobilisation. J. Nucl. Mat. 2008, 372, 358–366. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Matori, K.A.; Zaid, M.H.M.; Zainuddin, N.; Ab Aziz, S.; Khiri, M.Z.A.; Jalil, R.A.; Jusoh, W.N.W. Fabrication of alumino-silicate-fluoride based bioglass derived from waste clam shell and soda lime silica glasses. Results Phys. 2019, 12, 743–747. [Google Scholar] [CrossRef]
- Butler, J.H.; Hooper, P.D. Glass waste. In Waste: A Handbook for Management, 2nd ed.; Letcher, T.M., Vallero, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 307–322. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, J.; Petersen, R.R.; Yue, Y. Influence of the glass–calcium carbonate mixture’s characteristics on the foaming process and the properties of the foam glass. J. Eur. Ceram. Soc. 2014, 34, 1591–1598. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Tulyaganov, D.U.; Ferreira, J.M.F. Preparation and characterisation of foams from sheet glass and fly ash using carbonates as foaming agents. Ceram. Int. 2009, 35, 229–235. [Google Scholar] [CrossRef]
- Ayadi, A.; Stiti, N.; Boumchedda, K.; Rennai, H.; Lerari, Y. Elaboration and characterisation of porous granules based on waste glass. Powder Technol. 2011, 208, 423–426. [Google Scholar] [CrossRef]
- Francis, A.A.; Abdel Rahman, M.K.; Daoud, A. Processing, structures, and compressive properties of porous glass-ceramic composites prepared from secondary by-product materials. Ceram. Int. 2013, 39, 7089–7095. [Google Scholar] [CrossRef]
- Benzerga, R.; Laur, V.; Lebullenger, R.; Le Gendre, L.; Genty, S.; Sharaiha, A.; Queffelec, P. Waste-glass recycling: A step toward microwave applications. Mater. Res. Bull. 2015, 67, 261–265. [Google Scholar] [CrossRef]
- König, J.; Peterson, R.R.; Yue, Y. Fabrication of highly insulating foam glass made from CRT panel glass. Ceram. Int. 2015, 41, 9793–9800. [Google Scholar] [CrossRef]
- Peterson, R.R.; Konig, J.; Yue, Y. The mechanism of foaming and thermal conductivity of glasses foamed with MnO2. J. Non Cryst. Solids 2015, 425, 74–82. [Google Scholar] [CrossRef]
- Scarinci, G.; Brusatin, G.; Bernado, E. Glass Foam. In Cellular Ceramics: Structure, Manufacturing, Properties and Applications; Scheffler, M., Colombo, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 158–176. [Google Scholar]
- Marin, F. Mollusc shellomes: Past, present and future. J. Struct. Biol. 2020, 212, 107583. [Google Scholar] [CrossRef]
- Martínez-García, C.; González-Fonteboa, B.; Carro-López, D.; Martínez-Abella, F. Recycled mollusc shells. In New Trends in Eco-Efficient and Recycled Concrete; de Brito, J., Agrela, F., Eds.; Woodhead Publishing: Cambridge, UK, 2005; pp. 191–205. [Google Scholar]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z.; Lee, S.C.; Goh, W.I.; Yuen, C.W. Recycling of seashell waste in concrete: A review. Constr. Build. Mater. 2018, 162, 751–764. [Google Scholar] [CrossRef]
- Siriprom, W.; Chumnanvej, N.; Choeysuppaket, A.; Limsuwan, P. A biomonitoring study: Trace metal elements in perna viridis shell. Procedia Eng. 2012, 32, 1123–1126. [Google Scholar] [CrossRef]
- Küther, J.; Seshadri, R.; Knoll, W.; Tremel, W. Templated growth of calcite, vaterite and aragonite crystals onself-assembled monolayers of substituted alkylthiols on gold. J. Mater. Chem. 1998, 8, 641–650. [Google Scholar] [CrossRef]
- Hanshaw, B.B.; Back, W. Major geochemical processes in the evolution of carbonate aquifer systems. J. Hydrol. 1979, 43, 287–312. [Google Scholar] [CrossRef]
- Tang, B.; Lin, J.; Qian, S.; Wang, J.; Zhang, S. Preparation of glass–ceramic foams from the municipal solid waste slag produced by plasma gasification process. Mater. Lett. 2014, 28, 68–70. [Google Scholar] [CrossRef]
- Núñez, D.; Elgueta, E.; Varaprasad, K.; Oyarzún, P. Hydroxyapatite nanocrystals synthesized from calcium rich bio-wastes. Mater. Lett. 2018, 230, 64–68. [Google Scholar] [CrossRef]
- Saparuddin, D.I.; Hisham, N.A.N.; Ab Aziz, S.; Matori, K.A.; Honda, S.; Iwamoto, Y.; Zaid, M.H.M. Effect of sintering temperature on the crystal growth, microstructure and mechanical strength of foam glass-ceramic from waste materials. J. Mater. Res. 2020, 9, 5640–5647. [Google Scholar]
- Zhao, X.; Gao, C.; Li, B. Effect of CeO2 on sintering behavior, crystallization, and properties of CaO-Al2O3-SiO2 glass–ceramics for packages. J. Mater. Sci. 2020, 31, 17718–17725. [Google Scholar] [CrossRef]
- Casas-Luna, M.; Torres-Rodríguez, J.A.; Valdés-Martínez, O.U.; Obradović, N.; Slámečka, K.; Maca, K.; Kaiser, J.; Montúfar, E.B.; Čelko, L. Robocasting of controlled porous CaSiO3–SiO2 structures: Architecture–strength relationship and material catalytic behavior. Ceram. Int. 2020, 46, 8853–8861. [Google Scholar] [CrossRef]
- Bateni, H.; Karimi, K. Biodiesel production from castor plant integrating ethanol production via a biorefinery approach. Chem. Eng. Res. Des. 2016, 107, 4–12. [Google Scholar] [CrossRef]
- Jusoh, W.N.W.; Matori, K.A.; Zaid, M.H.M.; Zainuddin, N.; Khiri, M.Z.; Rahman, N.A.; Jalil, R.A.; Kul, E. Effect of sintering temperature on physical and structural properties of alumino-silicate-fluoride glass-ceramics fabricated from clam shell and soda lime silicate glass. Results Phys. 2019, 12, 1909–1914. [Google Scholar] [CrossRef]
- Zhu, M.; Ji, R.; Li, Z.; Wang, H.; Liu, L.; Zhang, Z. Preparation of glass ceramic foams for thermal insulation applications from coal fly ash and waste glass. Constr. Build. Mater. 2016, 112, 398–405. [Google Scholar] [CrossRef]
- Zhang, Q.; He, F.; Shu, H.; Qiao, Y.; Mei, S.; Jin, M.; Xie, J. Preparation of high strength glass ceramic foams from waste cathode ray tube and germanium tailings. Constr. Build. Mater. 2016, 111, 105–110. [Google Scholar] [CrossRef]
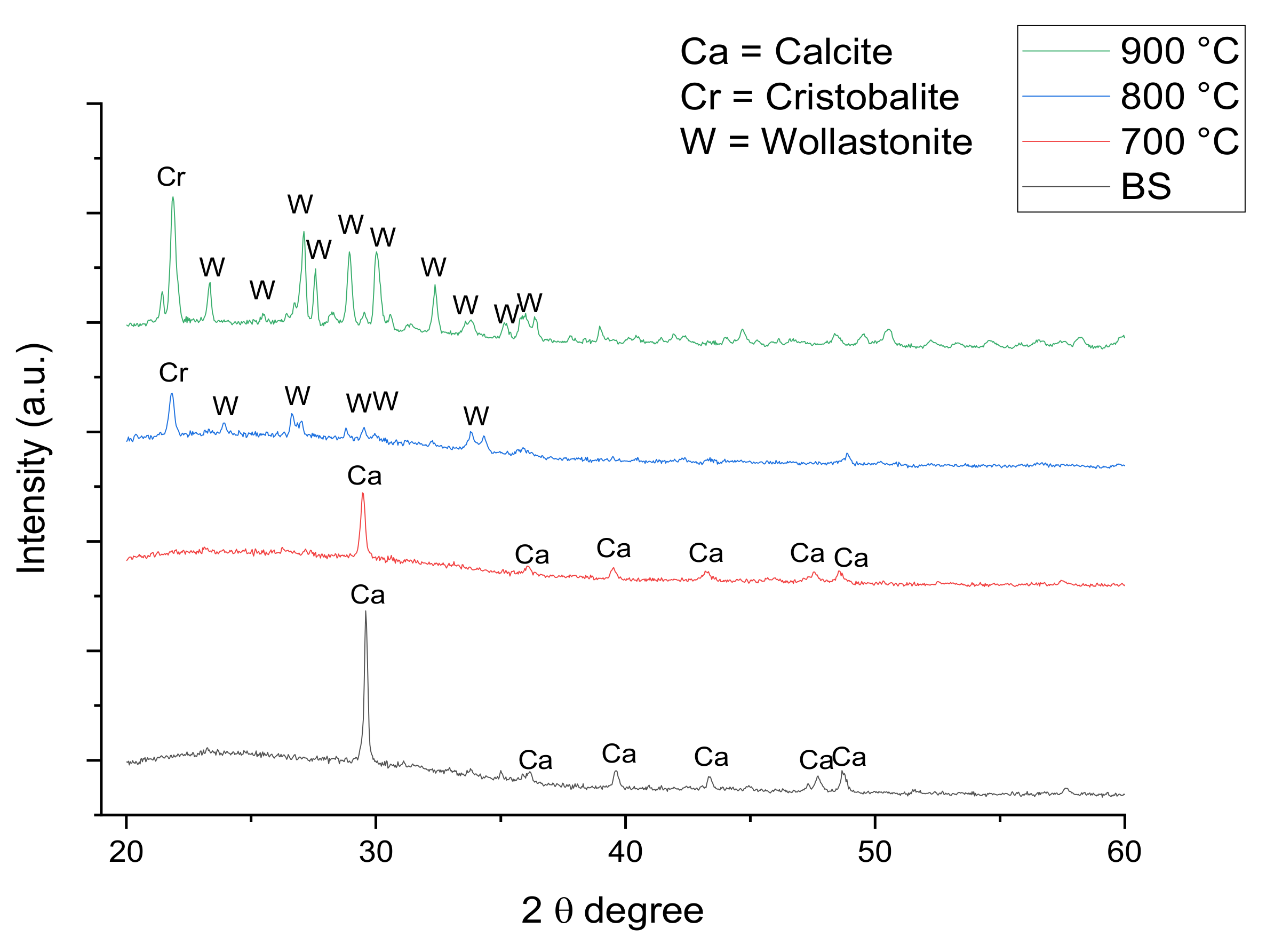
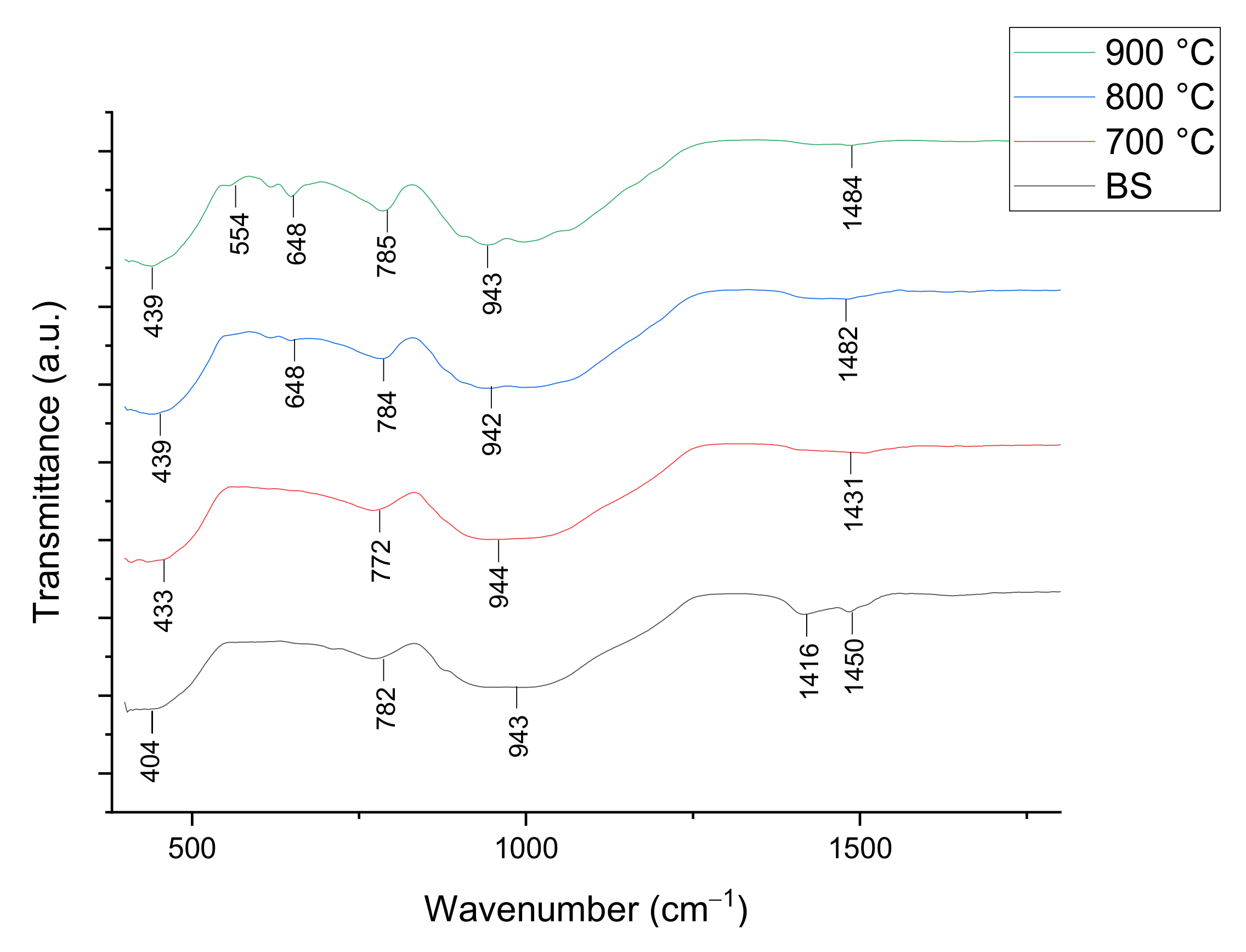
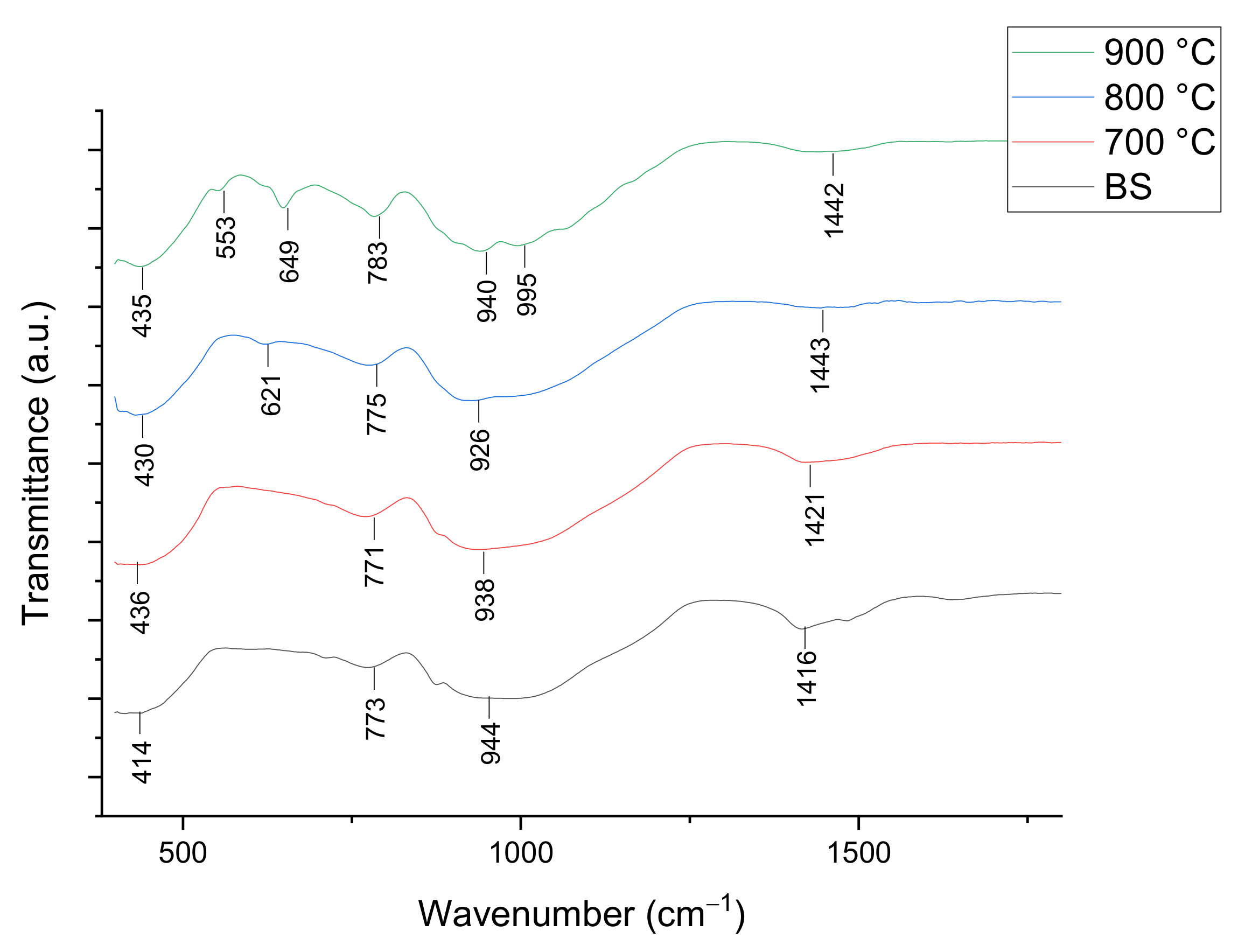

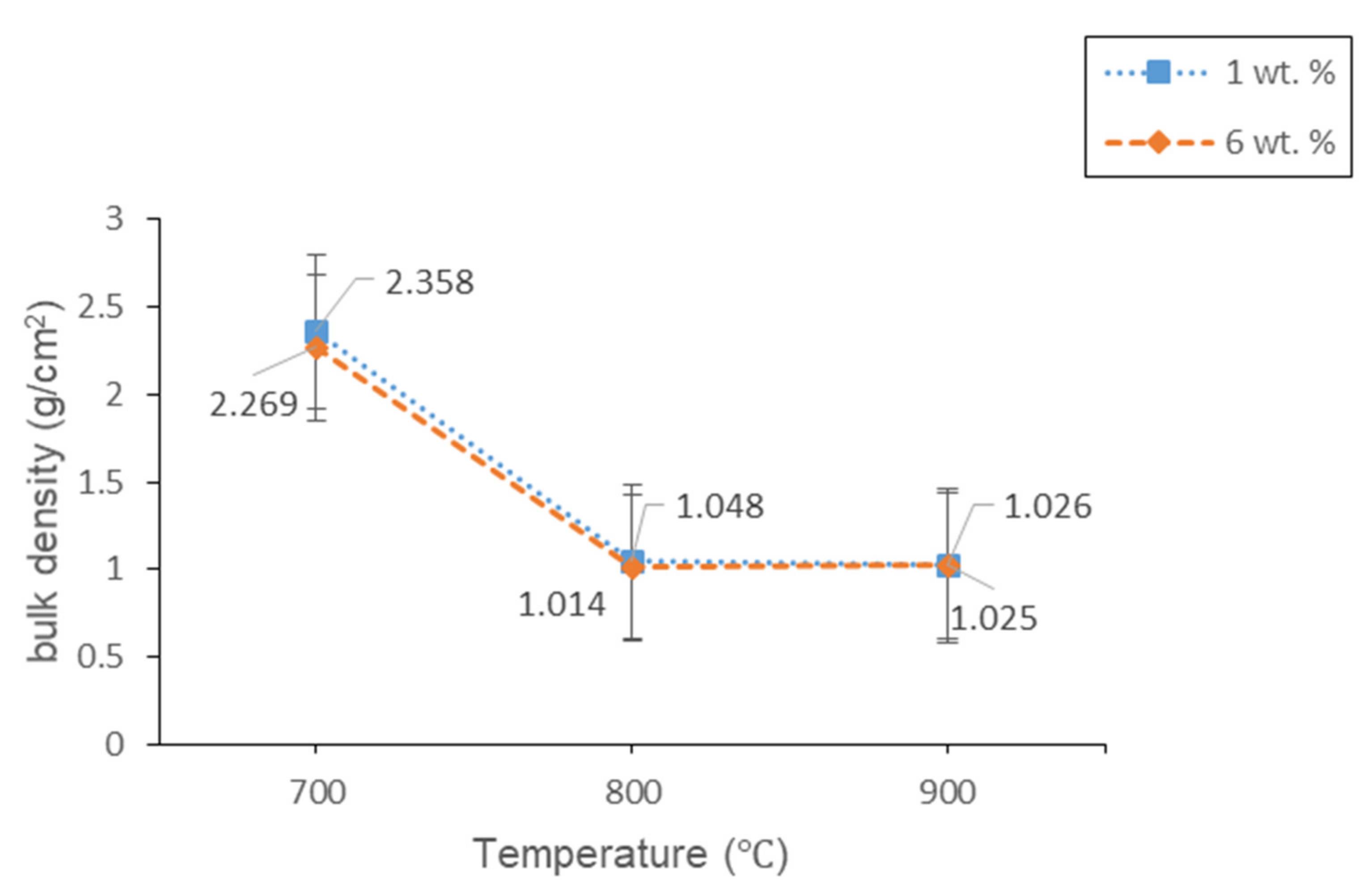
| Empirical Formula [ACS]x[SLS]100−x | Weight (g) | ||
|---|---|---|---|
| ACS | SLS | ACS-SLS | |
| 1(ACS)99(SLS) | 0.01 | 0.99 | 1.00 |
| 6(ACS)94(SLS) | 0.06 | 0.94 | 1.00 |
| Oxides (wt%) | Raw Materials | |
|---|---|---|
| SLS ± 0.1 | ACS ± 0.1 | |
| SiO2 | 71.90 | 0.13 |
| CaO | 11.69 | 53.41 |
| Na2O | 13.00 | 0.68 |
| Al2O3 | 1.39 | - |
| MgO | 1.43 | 0.18 |
| K2O | 0.15 | - |
| P2O5 | 0.05 | 0.06 |
| SO3 | 0.15 | 0.08 |
| Fe2O3 | 0.15 | 0.04 |
| ZnO | 0.03 | 0.05 |
| SrO | 0.01 | 0.14 |
| Others | 0.05 | 45.23 |
| Total | 100 | 100 |
| Wavenumber (cm−1) | Vibrational Mode Assignment |
|---|---|
| 400–600 | Ca-O stretching mode, O-Si-O bending mode |
| 600–650 | Ca-O-Si vibration band |
| 800–1250 | Si-O symmetric stretching mode, O-Si-O bending mode |
| 1440–1450 | C-O bond |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hisham, N.A.N.; Zaid, M.H.M.; Aziz, S.H.A.; Muhammad, F.D. Comparison of Foam Glass-Ceramics with Different Composition Derived from Ark Clamshell (ACS) and Soda Lime Silica (SLS) Glass Bottles Sintered at Various Temperatures. Materials 2021, 14, 570. https://doi.org/10.3390/ma14030570
Hisham NAN, Zaid MHM, Aziz SHA, Muhammad FD. Comparison of Foam Glass-Ceramics with Different Composition Derived from Ark Clamshell (ACS) and Soda Lime Silica (SLS) Glass Bottles Sintered at Various Temperatures. Materials. 2021; 14(3):570. https://doi.org/10.3390/ma14030570
Chicago/Turabian StyleHisham, Noor Aizat Noor, Mohd Hafiz Mohd Zaid, Sidek Hj Ab Aziz, and Farah Diana Muhammad. 2021. "Comparison of Foam Glass-Ceramics with Different Composition Derived from Ark Clamshell (ACS) and Soda Lime Silica (SLS) Glass Bottles Sintered at Various Temperatures" Materials 14, no. 3: 570. https://doi.org/10.3390/ma14030570
APA StyleHisham, N. A. N., Zaid, M. H. M., Aziz, S. H. A., & Muhammad, F. D. (2021). Comparison of Foam Glass-Ceramics with Different Composition Derived from Ark Clamshell (ACS) and Soda Lime Silica (SLS) Glass Bottles Sintered at Various Temperatures. Materials, 14(3), 570. https://doi.org/10.3390/ma14030570







