Copper Nitride Nanowire Arrays—Comparison of Synthetic Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
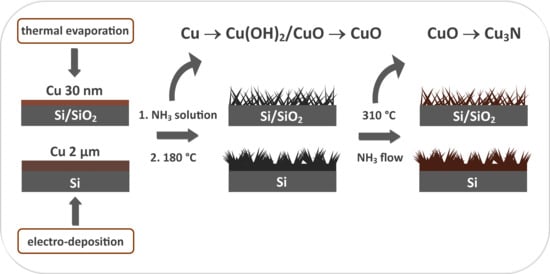
2.2. Cu3N Thin Films on Electrodeposited Copper Surfaces
2.3. Cu3N Thin Films on Copper Surfaces Deposited by Thermal Evaporation
2.4. Characterization
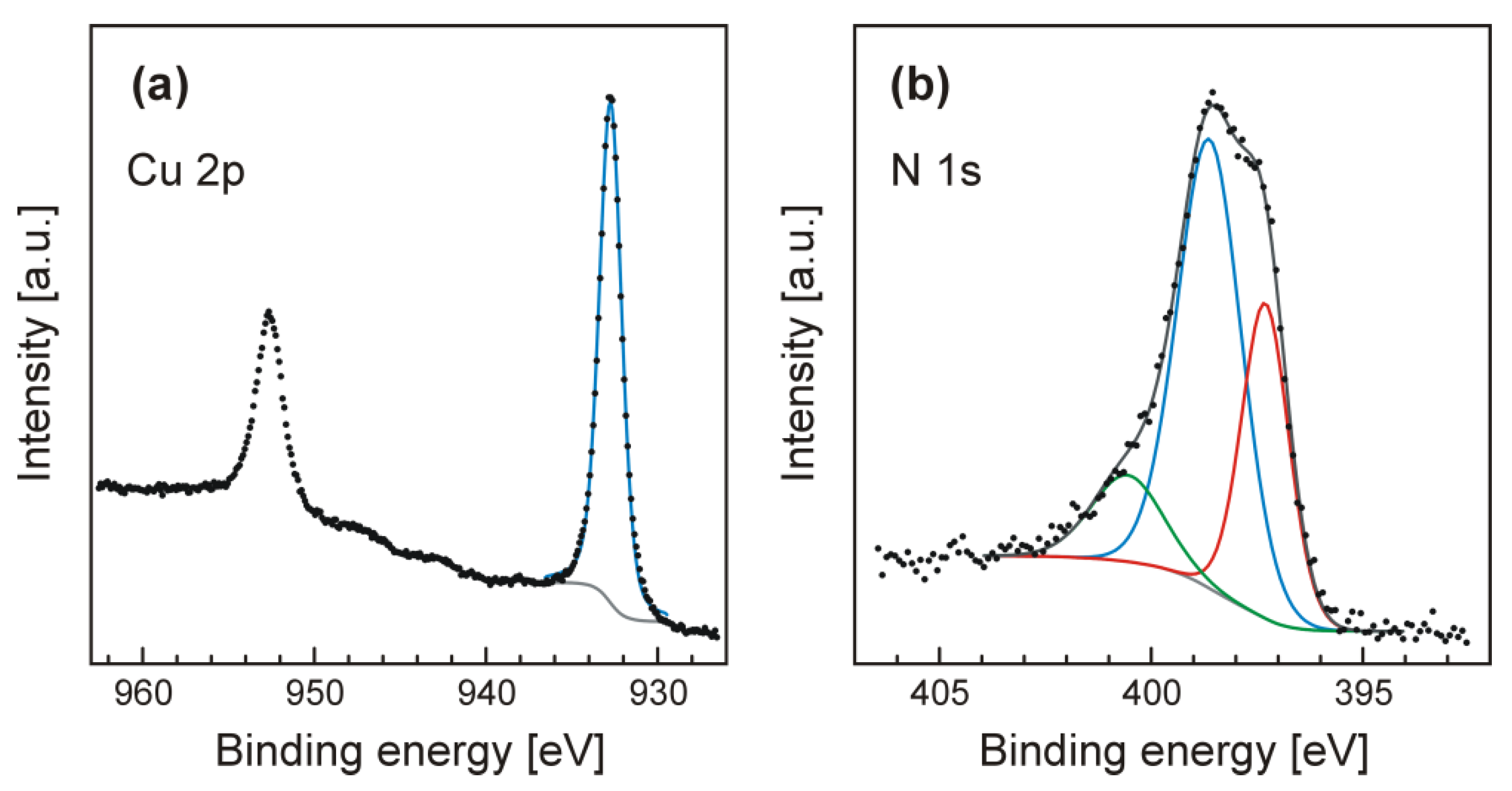
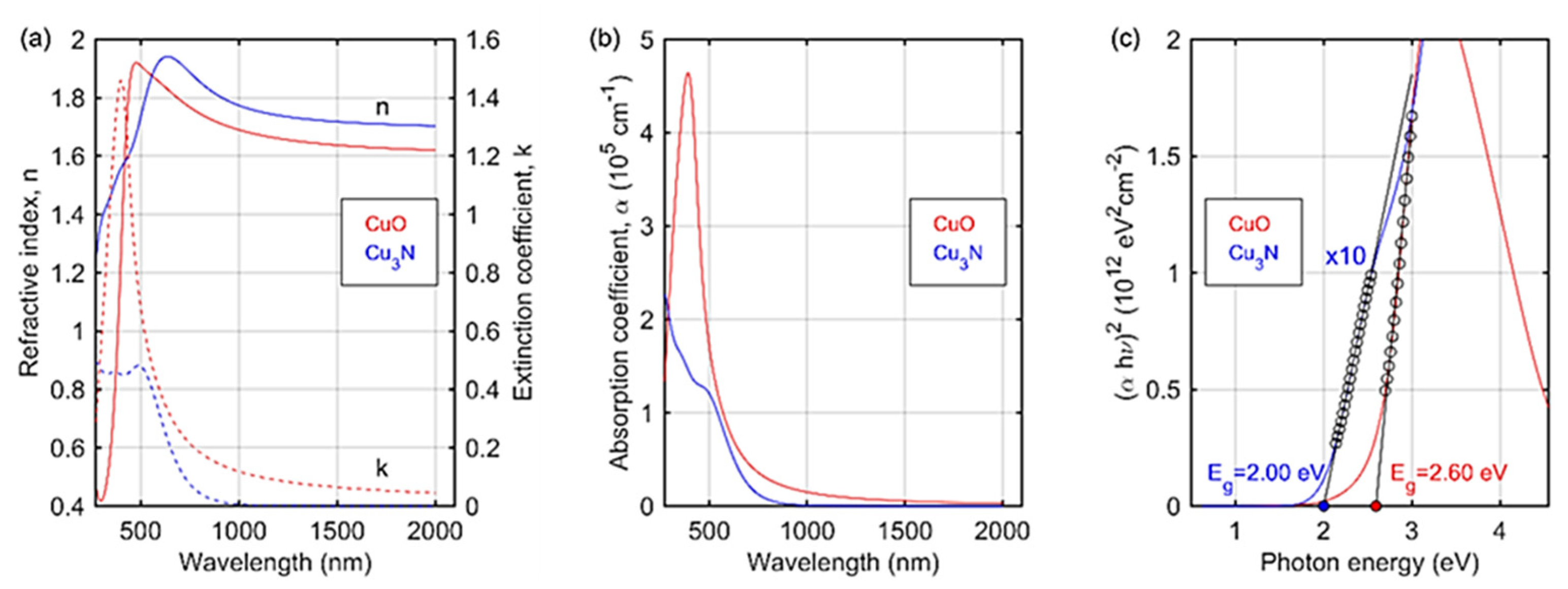
3. Results and Discussion
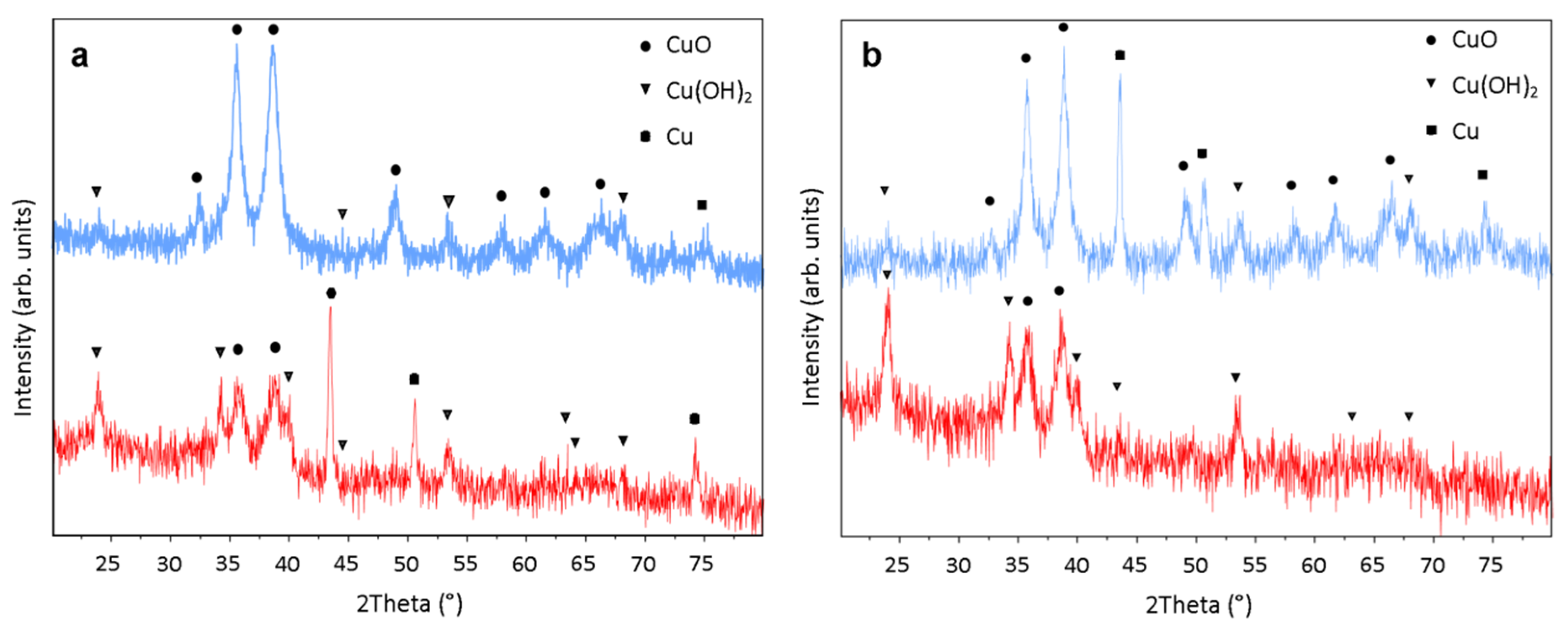
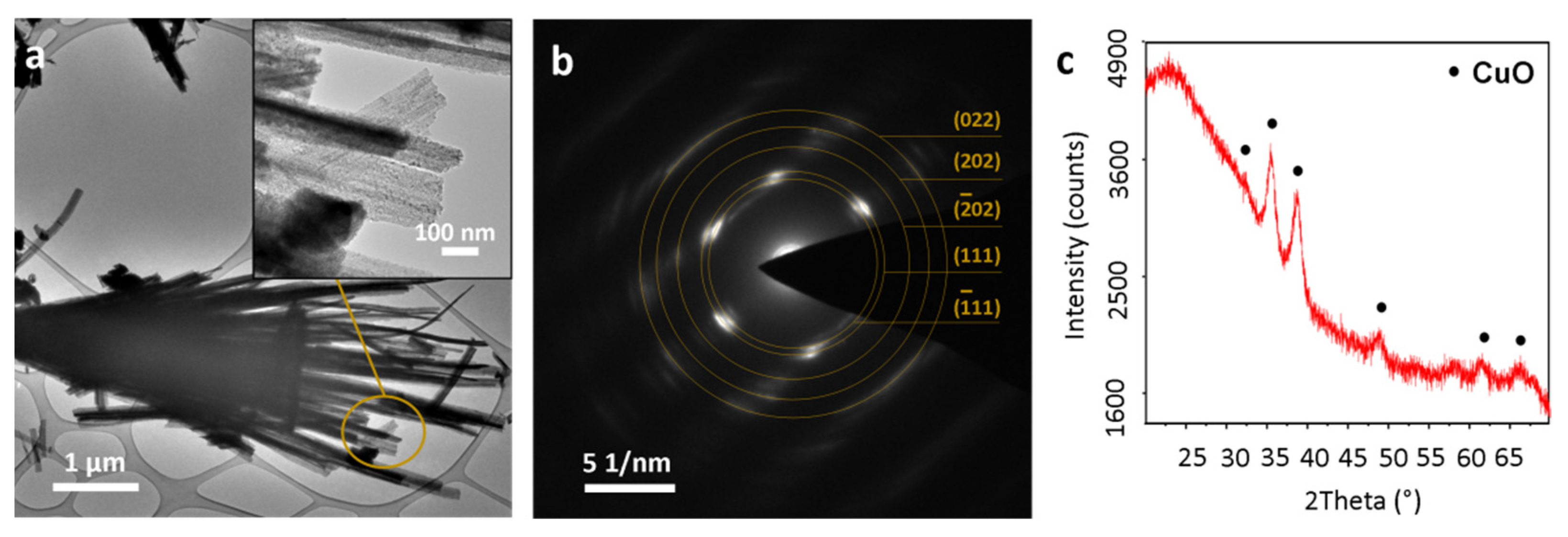
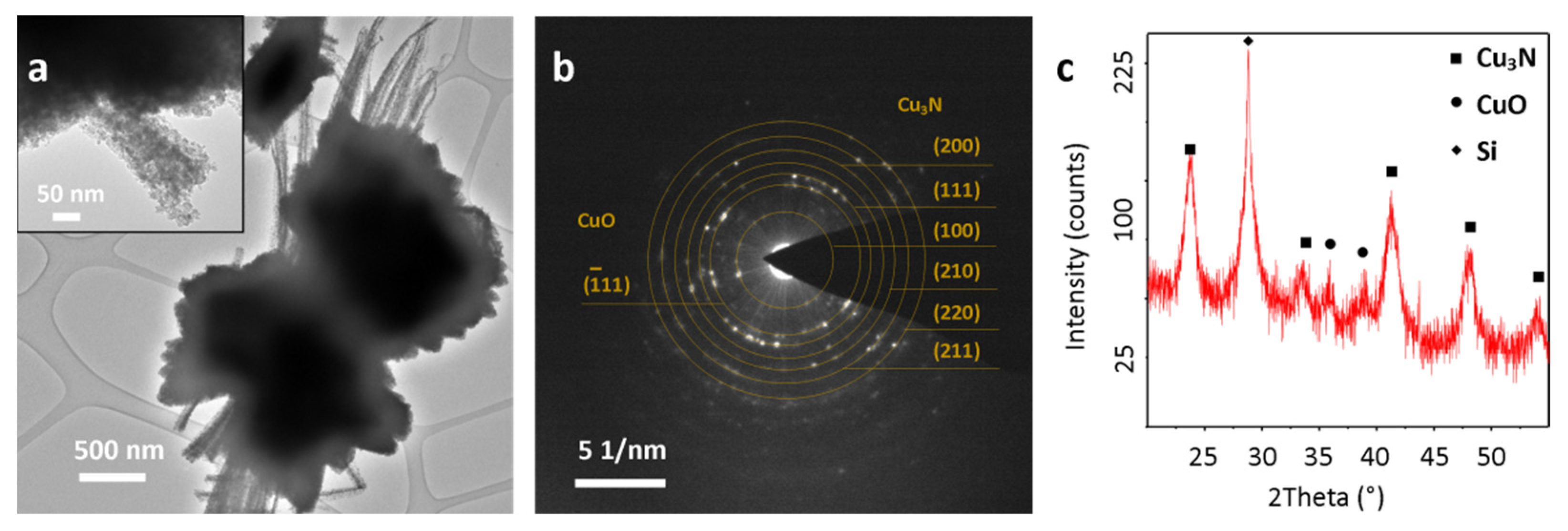
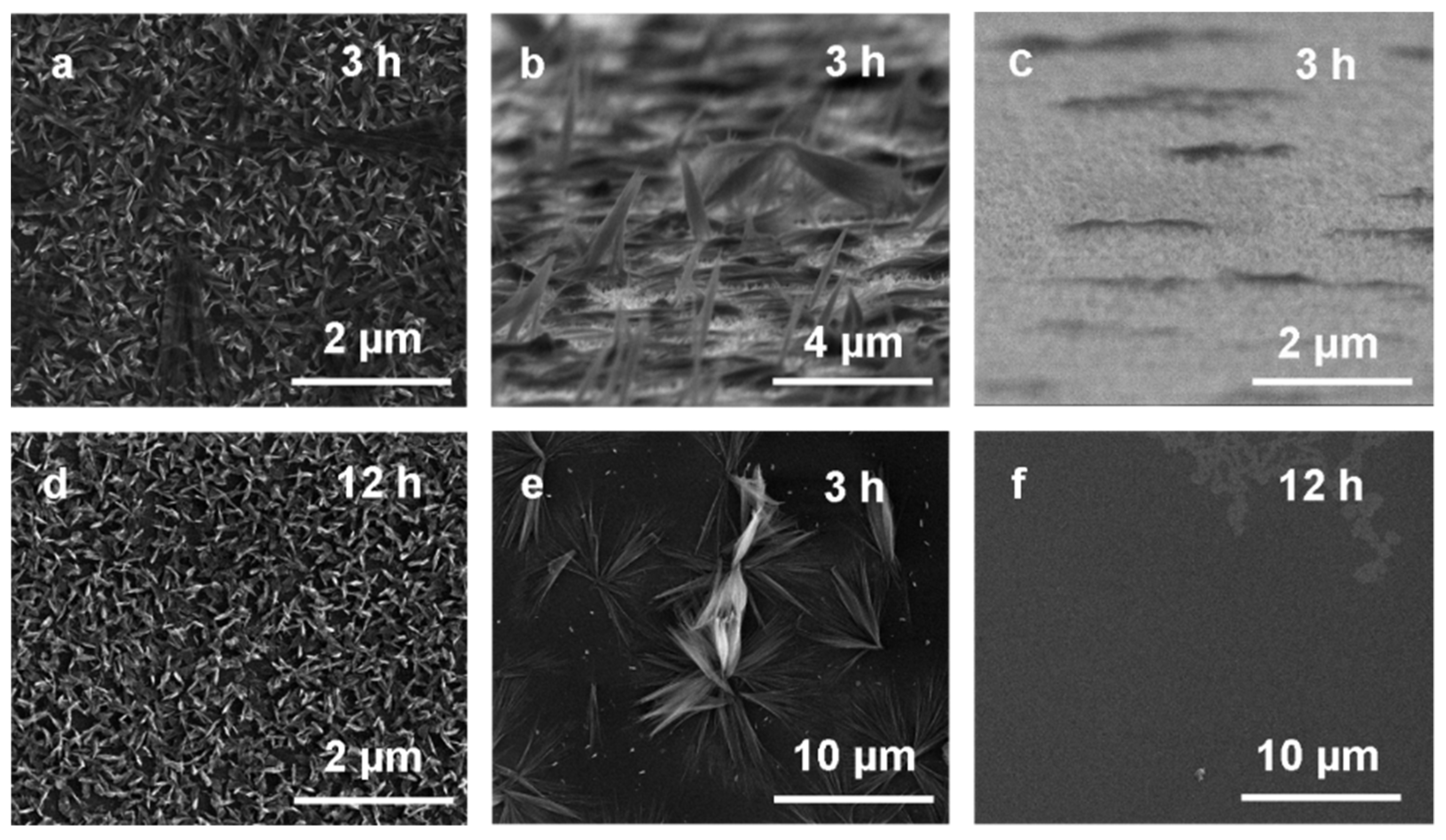
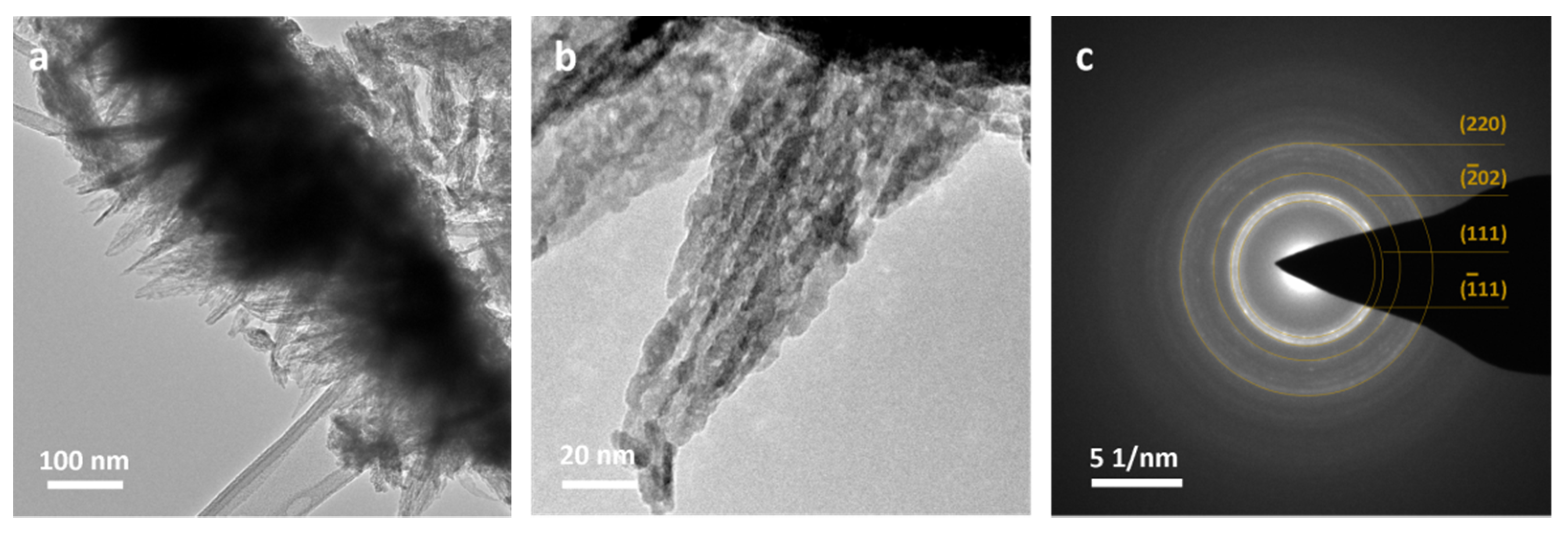
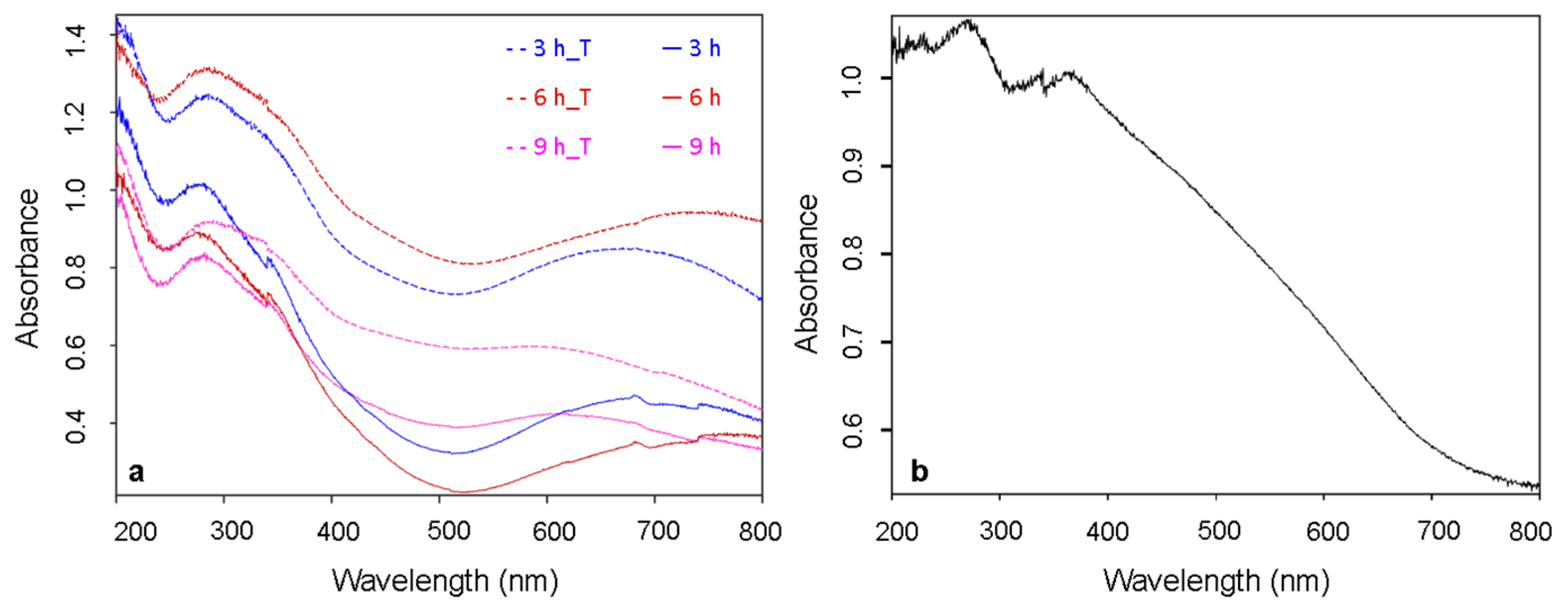
3.1. Cu3N Thin Films on Electrodeposited Copper Surfaces
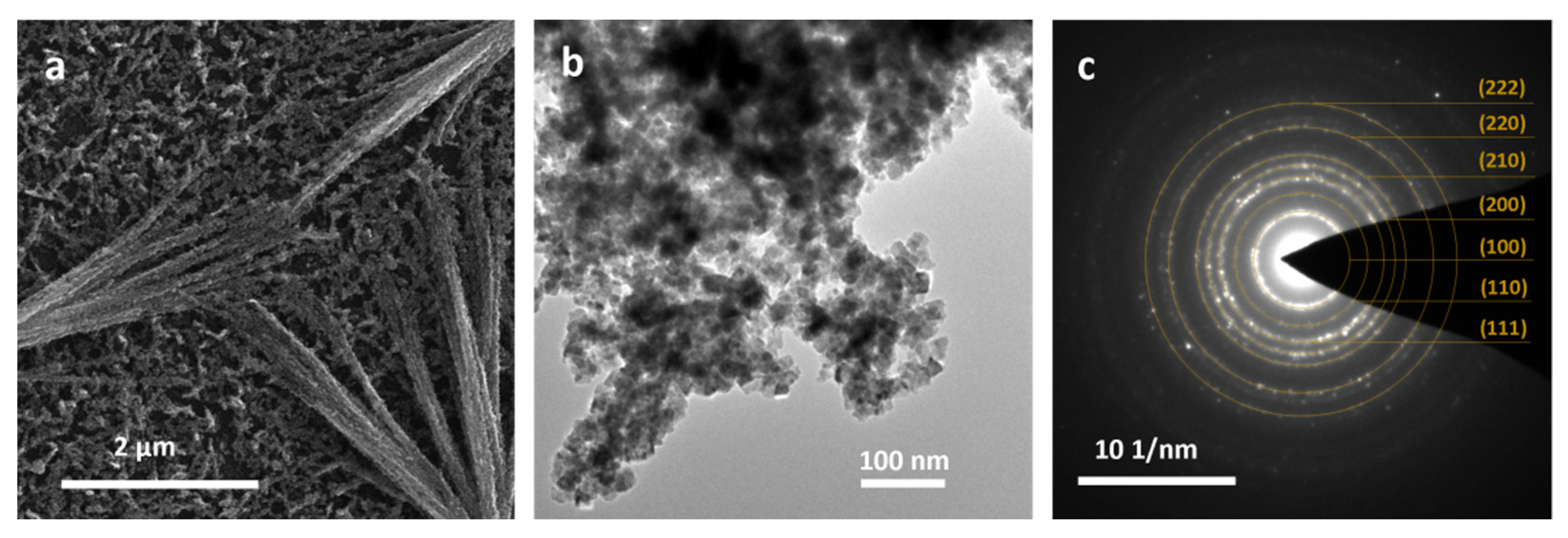
3.2. Cu3N Thin Films on Copper Surfaces Deposited by Thermal Evaporation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyama, S.T. The Chemistry of Transition Metal Carbides and Nitrides; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Mazumder, B.; Hector, A.L. Synthesis and applications of nanocrystalline nitride materials. J. Mater. Chem. 2009, 19, 4673–4686. [Google Scholar] [CrossRef]
- Xie, J.; Xie, Y. Transition Metal Nitrides for Electrocatalytic Energy Conversion: Opportunities and Challenges. Chem. Eur. J. 2016, 22, 3588–3598. [Google Scholar] [CrossRef]
- Dong, S.; Chen, X.; Zhang, X.; Cui, G. Nanostructured transition metal nitrides for energy storage and fuel cells. Coord. Chem. Rev. 2013, 257, 1946–1956. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, Y.; Yuan, Y.; Wang, C. Reactive DC magnetron deposition of copper nitride films for write-once optical recording. Mater. Lett. 2006, 60, 3758–3760. [Google Scholar] [CrossRef]
- Maruyama, T.; Morishita, T. Copper nitride and tin nitride thin films for write-once optical recording media. Appl. Phys. Lett. 1996, 69, 890–891. [Google Scholar] [CrossRef]
- Borsa, D.M.; Grachev, S.; Presura, C.; Boerma, D.O. Growth and properties of Cu3N films and Cu3N/γ′-Fe4N bilayers. Appl. Phys. Lett. 2002, 80, 1823–1825. [Google Scholar] [CrossRef]
- Liang, Z.Q.; Zhuang, T.T.; Seifitokaldani, A.; Li, J.; Huang, C.W.; Tan, C.S.; Li, Y.; De Luna, P.; Dinh, C.T.; Hu, Y.; et al. Copper-on-nitride enhances the stable electrosynthesis of multi-carbon products from CO2. Nat. Commun. 2018, 9, 3828. [Google Scholar] [CrossRef]
- Mi, Y.; Shen, S.; Peng, X.; Bao, H.; Liu, X.; Luo, J. Selective Electroreduction of CO2 to C2 Products over Cu3N-Derived Cu Nanowires. ChemElectroChem 2019, 6, 2393–2397. [Google Scholar] [CrossRef]
- Cremer, R.; Witthaut, M.; Neuschütz, D.; Trappe, C.; Laurenzis, M.; Winkler, O.; Kurz, H. Deposition and Characterization of Metastable Cu3N Layers for Applications in Optical Data Storage. Mikrochim. Acta 2000, 133, 299–302. [Google Scholar] [CrossRef]
- Ji, A.L.; Huang, R.; Du, Y.; Li, C.R.; Wang, Y.Q.; Cao, Z.X. Growth of stoichiometric Cu3N thin films by reactive magnetron sputtering. J. Cryst. Growth 2006, 295, 79–83. [Google Scholar] [CrossRef]
- Yue, G.H.; Yan, P.X.; Liu, J.Z.; Wang, M.X.; Li, M.; Yuan, X.M. Copper nitride thin film prepared by reactive radio-frequency magnetron sputtering. J. Appl. Phys. 2005, 98, 103506. [Google Scholar] [CrossRef]
- Reichert, M.D.; White, M.A.; Thompson, M.J.; Miller, G.J.; Vela, J. Preparation and Instability of Nanocrystalline Cuprous Nitride. Inorg. Chem. 2015, 54, 6356–6362. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, T.; Yoshitake, M.; Okamoto, A.; Ogawa, S.; Nakayama, Y. Thermal decomposition of copper nitride thin films and dots formation by electron beam writing. Appl. Surf. Sci. 2001, 169–170, 358–361. [Google Scholar] [CrossRef]
- Paniconi, G.; Stoeva, Z.; Doberstein, H.; Smith, R.I.; Gallagher, B.L.; Gregory, D.H. Structural chemistry of Cu3N powders obtained by ammonolysis reactions. Solid State Sci. 2007, 9, 907–913. [Google Scholar] [CrossRef]
- Ji, A.L.; Lu, N.P.; Gao, L.; Zhang, W.B.; Liao, L.G.; Cao, Z.X. Electrical properties and thermal stability of Pd-doped copper nitride films. J. Appl. Phys. 2013, 113, 043705. [Google Scholar] [CrossRef]
- Maya, L. Deposition of crystalline binary nitride films of tin, copper, and nickel by reactive sputtering. J. Vac. Sci. Technol. A 1993, 11, 604–608. [Google Scholar] [CrossRef]
- Terada, S.; Tanaka, H.; Kubota, K. Heteroepitaxial growth of Cu3N thin films. J. Cryst. Growth 1989, 94, 567–568. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Wang, W.J.; Wang, T.M.; Chao, S.; Zheng, S.K. Thermal stability of copper nitride films prepared by rf magnetron sputtering. Thin Solid Films 1998, 325, 55–59. [Google Scholar] [CrossRef]
- Yue, G.H.; Yan, P.X.; Wang, J. Study on the preparation and properties of copper nitride thin films. J. Cryst. Growth 2005, 274, 464–468. [Google Scholar] [CrossRef]
- Borsa, D.M.; Boerma, D.O. Growth, structural and optical properties of Cu3N films. Surf. Sci. 2004, 548, 95–105. [Google Scholar] [CrossRef]
- Soto, G.; Díaz, J.A.; De la Cruz, W. Copper nitride films produced by reactive pulsed laser deposition. Mater. Lett. 2003, 57, 4130–4133. [Google Scholar] [CrossRef]
- Gallardo-Vega, C.; de la Cruz, W. Study of the structure and electrical properties of the copper nitride thin films deposited by pulsed laser deposition. Appl. Surf. Sci. 2006, 252, 8001–8004. [Google Scholar] [CrossRef]
- Wu, H.; Chen, W. Copper Nitride Nanocubes: Size-Controlled Synthesis and Application as Cathode Catalyst in Alkaline Fuel Cells. J. Am. Chem. Soc. 2011, 133, 15236–15239. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hector, A.L.; Owen, J.R. Evaluation of Cu3N and CuO as Negative Electrode Materials for Sodium Batteries. J. Phys. Chem. C 2014, 118, 29568–29573. [Google Scholar] [CrossRef]
- Vaughn, D.D.; Araujo, J.; Meduri, P.; Callejas, J.F.; Hickner, M.A.; Schaak, R.E. Solution Synthesis of Cu3PdN Nanocrystals as Ternary Metal Nitride Electrocatalysts for the Oxygen Reduction Reaction. Chem. Mater. 2014, 26, 6226–6232. [Google Scholar] [CrossRef]
- Deshmukh, R.; Zeng, G.; Tervoort, E.; Staniuk, M.; Wood, D.; Niederberger, M. Ultrasmall Cu3N Nanoparticles: Surfactant-Free Solution-Phase Synthesis, Nitridation Mechanism, and Application for Lithium Storage. Chem. Mater. 2015, 27, 8282–8288. [Google Scholar] [CrossRef]
- Pinkas, J.; Huffman, J.C.; Baxter, D.V.; Chisholm, M.H.; Caulton, K.G. Mechanistic Role of H2O and the Ligand in the Chemical Vapor Deposition of Cu, Cu2O, CuO, and Cu3N from Bis (1,1,1,5,5,5-hexafluoropentane-2,4-dionato) copper (II). Chem. Mater. 1995, 7, 1589–1596. [Google Scholar] [CrossRef]
- Fallberg, A.; Ottosson, M.; Carlsson, J.O. CVD of Copper(I) Nitride. Chem. Vap. Depos. 2009, 15, 300–305. [Google Scholar] [CrossRef]
- Szczȩsny, R.; Szłyk, E.; Wiśniewski, M.A.; Hoang, T.K.A.; Gregory, D.H. Facile preparation of copper nitride powders and nanostructured films. J. Mater. Chem. C 2016, 4, 5031–5037. [Google Scholar] [CrossRef]
- Juza, R.; Hahn, H. Über die Kristallstrukturen von Cu3N, GaN und InN Metallamide und Metallnitride. Z. Anorg. Allg. Chem. 1938, 239, 282–287. [Google Scholar] [CrossRef]
- Juza, R.; Hahn, H. Kupfernitrid. Z. Anorg. Allg. Chem. 1939, 241, 172–178. [Google Scholar] [CrossRef]
- Lee, B.S.; Yi, M.; Chu, S.Y.; Lee, J.Y.; Kwon, H.R.; Lee, K.R.; Kang, D.; Kim, W.S.; Lim, H.B.; Lee, J.; et al. Copper nitride nanoparticles supported on a superparamagnetic mesoporous microsphere for toxic-free click chemistry. Chem. Commun. 2010, 46, 3935–3937. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.L.; Zhong, H.X.; Zhang, Q.; Liu, K.H.; Yan, J.M.; Jiang, Q. Integrated Cu3N porous nanowire array electrode for high-performance supercapacitors. J. Mater. Chem. A 2017, 5, 18972–18976. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, X.; Liu, D.; Hao, S.; Kong, R.; Du, G.; Asiri, A.M.; Sun, X. Copper-Nitride Nanowires Array: An Efficient Dual-Functional Catalyst Electrode for Sensitive and Selective Non-Enzymatic Glucose and Hydrogen Peroxide Sensing. Chem. Eur. J. 2017, 23, 4986–4989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Guo, T.; Yao, M.S.; Lin, Y.H.; Nan, C.W. A comprehensive review on synthesis methods for transition-metal oxide nanostructures. CrystEngComm 2015, 17, 3551–3585. [Google Scholar] [CrossRef]
- Ghadiri, A.M.; Rabiee, N.; Bagherzadeh, M.; Kiani, M.; Fatahi, Y.; Di Bartolomeo, A.; Dinarvand, R.; Webster, T.J. Green synthesis of CuO- And Cu2O-NPs in assistance with high-gravity- And flowering of nanobiotechnology. Nanotechnology 2020, 31, 15. [Google Scholar] [CrossRef]
- Xu, C.H.; Woo, C.H.; Shi, S.Q. Formation of CuO nanowires on Cu foil. Chem. Phys. Lett. 2004, 399, 62–66. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, S.; Yang, Z.; Liu, A.; Qian, Y.; Tang, S.; Yang, S. Growth of novel nanostructured copper oxide (CuO) films on copper foil. J. Cryst. Growth 2006, 291, 479–484. [Google Scholar] [CrossRef]
- Shinde, S.K.; Dubal, D.P.; Ghodake, G.S.; Fulari, V.J. Hierarchical 3D-flower-like CuO nanostructure on copper foil for supercapacitors. RSC Adv. 2015, 5, 4443–4447. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Fox-Robichaud, A.; Selvaganapathy, P.R. Electroplating of Multiple Materials in Parallel Using Patterned Gels with Applications in Electrochemical Sensing. Sensors 2020, 20, 886. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Zhang, M.; Chang, Y.H.; Zhai, P.; Hau, N.Y.; Huang, Y.T.; Liu, C.; Soh, A.K.; Feng, S.P. Highly Conductive and Low Cost Ni-PET Flexible Substrate for Efficient Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 5577–5584. [Google Scholar] [CrossRef] [PubMed]
- Ojo, A.A.; Dharmadasa, I.M. Electroplating of Semiconductor Materials for Applications in Large Area Electronics: A Review. Coatings 2018, 8, 262. [Google Scholar] [CrossRef]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering Physical Vapour Deposition (PVD) Coatings: A Critical Review on Process Improvement and Market Trend Demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, W.; Yang, S. Synthesis of Cu(OH)2 and CuO Nanoribbon Arrays on a Copper Surface. Langmuir 2003, 19, 5898–5903. [Google Scholar] [CrossRef]
- Luo, Q.; Mackay, R.A.; Babu, S.V. Copper Dissolution in Aqueous Ammonia-Containing Media during Chemical Mechanical Polishing. Chem. Mater. 1997, 9, 2101–2106. [Google Scholar] [CrossRef]
- Habashi, F. Kinetics and Mechanism of Copper Dissolution in Aqueous Ammonia. Ber. Bunsenges. Phys. Chem. 1963, 67, 402–406. [Google Scholar] [CrossRef]
- Halpern, J. Kinetics of the Dissolution of Copper in Aqueous Ammonia. J. Electrochem. Soc. 1953, 100, 421. [Google Scholar] [CrossRef]
- Nowakowska-Langier, K.; Skowronski, L.; Chodun, R.; Okrasa, S.; Strzelecki, G.W.; Wilczopolska, M.; Wicher, B.; Mirowski, R.; Zdunek, K. Influence of generation control of the magnetron plasma on structure and properties of copper nitride layers. Thin Solid Films 2020, 694, 137731. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Li, Y.; Ma, D.; Hou, S.; Li, L.; Hao, X.; Wang, Z. Room temperature synthesis of 2D CuO nanoleaves in aqueous solution. Nanotechnology 2011, 22, 115604–115613. [Google Scholar] [CrossRef]
- Sithole, R.K.; MacHogo, L.F.E.; Airo, M.A.; Gqoba, S.S.; Moloto, M.J.; Shumbula, P.; Van Wyk, J.; Moloto, N. Synthesis and characterization of Cu3N nanoparticles using pyrrole-2-carbaldpropyliminato Cu(II) complex and Cu(NO3)2 as single-source precursors: The search for an ideal precursor. New J. Chem. 2018, 42, 3042–3049. [Google Scholar] [CrossRef]
- Feifel, S.C.; Lisdat, F. Silica nanoparticles for the layer-by-layer assembly of fully electro-active cytochrome c multilayers. J. Nanobiotechnology 2011, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Hozák, P.; Vorokhta, M.; Khalakhan, I.; Jarkovská, K.; Cibulková, J.; Fitl, P.; Vlček, J.; Fara, J.; Tomeček, D.; Novotný, M.; et al. New Insight into the Gas-Sensing Properties of CuOx Nanowires by Near-Ambient Pressure XPS. J. Phys. Chem. C 2019, 123, 29739–29749. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- J.A. Woolam Co. Inc. Guide to Using WVASE32®; Wextech Syst. Inc.: New York, NY, USA, 2010. [Google Scholar]
- Fujiwara, H. Spectroscopic Ellipsometry: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 9780470016084. [Google Scholar]
- Derin, H.; Kantarli, K. Optical characterization of thin thermal oxide films on copper by ellipsometry. Appl. Phys. A Mater. Sci. Process. 2002, 75, 391–395. [Google Scholar] [CrossRef]
- Skowroński, Ł.; Zdunek, K.; Chodun, R.; Skowronski, L.; Zdunek, K.; Nowakowska-Langier, K.; Chodun, R.; Trzcinski, M.; Kobierski, M.; Kustra, M.K.; et al. Characterisation of microstructural, mechanical and optical properties of TiO2 layers deposited by GIMS and PMS methods. Surf. Coat. Technol. 2015, 282, 16–23. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Plenum: New York, NY, USA, 1974; ISBN 978-1-4615-8707-1. [Google Scholar]
- Gordillo, N.; Gonzalez-Arrabal, R.; Martin-Gonzalez, M.S.; Olivares, J.; Rivera, A.; Briones, F.; Agulló-López, F.; Boerma, D.O. DC triode sputtering deposition and characterization of N-rich copper nitride thin films: Role of chemical composition. J. Cryst. Growth 2008, 310, 4362–4367. [Google Scholar] [CrossRef]
- Nowakowska-Langier, K.; Chodun, R.; Minikayev, R.; Kurpaska, L.; Skowronski, L.; Strzelecki, G.W.; Okrasa, S.; Zdunek, K. Structure of Cu–N layers synthesized by pulsed magnetron sputtering with variable frequency of plasma generation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2017, 409, 167–170. [Google Scholar] [CrossRef]
- Li, X.; Bai, Q.; Yang, J.; Li, Y.; Wang, L.; Wang, H.; Ren, S.; Liu, S.; Huang, W. Effect of N2-gas flow rates on the structures and properties of copper nitride films prepared by reactive DC magnetron sputtering. Vacuum 2013, 89, 78–81. [Google Scholar] [CrossRef]
- Sahoo, G.; Meher, S.R.; Jain, M.K. Band gap variation in copper nitride thin films. In Proceedings of the International Conference on Advanced Nanomaterials & Emerging Engineering Technologies, Chennai, India, 24–26 July 2013; pp. 540–542. [Google Scholar]
- Velusamy, T.; Liguori, A.; Macias-Montero, M.; Padmanaban, D.B.; Carolan, D.; Gherardi, M.; Colombo, V.; Maguire, P.; Svrcek, V.; Mariotti, D. Ultra-small CuO nanoparticles with tailored energy-band diagram synthesized by a hybrid plasma-liquid process. Plasma Process. Polym. 2017, 14, 1600224. [Google Scholar] [CrossRef]
- Balamurugan, B.; Mehta, B.R. Optical and structural properties of nanocrystalline copper oxide thin films prepared by activated reactive evaporation. Thin Solid Films 2001, 396, 90–96. [Google Scholar] [CrossRef]
- Anu Prathap, M.U.; Kaur, B.; Srivastava, R. Hydrothermal synthesis of CuO micro-/nanostructures and their applications in the oxidative degradation of methylene blue and non-enzymatic sensing of glucose/H2O2. J. Colloid Interface Sci. 2012, 370, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, L.; Shi, H.; Liang, Y. A room temperature chemical route for large scale synthesis of sub-15 nm ultralong CuO nanowires with strong size effect and enhanced photocatalytic activity. CrystEngComm 2012, 14, 5914–5922. [Google Scholar] [CrossRef]
- Yang, C.; Xiao, F.; Wang, J.; Su, X. Synthesis and microwave modification of CuO nanoparticles: Crystallinity and morphological variations, catalysis, and gas sensing. J. Colloid Interface Sci. 2014, 435, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Borgohain, K.; Singh, J. Quantum size effects in CuO nanoparticles. Phys. Rev. B 2000, 61, 11093–11096. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, D.; Jiao, X. Fabrication of CuO pricky microspheres with tunable size by a simple solution route. J. Phys. Chem. B 2005, 109, 13561–13566. [Google Scholar] [CrossRef]
- Meshram, S.P.; Adhyapak, P.V.; Mulik, U.P.; Amalnerkar, D.P. Facile synthesis of CuO nanomorphs and their morphology dependent sunlight driven photocatalytic properties. Chem. Eng. J. 2012, 204–205, 158–168. [Google Scholar] [CrossRef]
- Langmar, O.; Ganivet, C.R.; Lennert, A.; Costa, R.D.; de la Torre, G.; Torres, T.; Guldi, D.M. Combining Electron-Accepting Phthalocyanines and Nanorod-like CuO Electrodes for p-Type Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 7688–7692. [Google Scholar] [CrossRef]
- Yahia, I.S.; Farag, A.A.M.; El-Faify, S.; Yakuphanoglu, F.; Al-Ghamdi, A.A. Synthesis, optical constants, optical dispersion parameters of CuO nanorods. Optik (Stuttg.) 2016, 127, 1429–1433. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scigala, A.; Szłyk, E.; Rerek, T.; Wiśniewski, M.; Skowronski, L.; Trzcinski, M.; Szczesny, R. Copper Nitride Nanowire Arrays—Comparison of Synthetic Approaches. Materials 2021, 14, 603. https://doi.org/10.3390/ma14030603
Scigala A, Szłyk E, Rerek T, Wiśniewski M, Skowronski L, Trzcinski M, Szczesny R. Copper Nitride Nanowire Arrays—Comparison of Synthetic Approaches. Materials. 2021; 14(3):603. https://doi.org/10.3390/ma14030603
Chicago/Turabian StyleScigala, Aleksandra, Edward Szłyk, Tomasz Rerek, Marek Wiśniewski, Lukasz Skowronski, Marek Trzcinski, and Robert Szczesny. 2021. "Copper Nitride Nanowire Arrays—Comparison of Synthetic Approaches" Materials 14, no. 3: 603. https://doi.org/10.3390/ma14030603
APA StyleScigala, A., Szłyk, E., Rerek, T., Wiśniewski, M., Skowronski, L., Trzcinski, M., & Szczesny, R. (2021). Copper Nitride Nanowire Arrays—Comparison of Synthetic Approaches. Materials, 14(3), 603. https://doi.org/10.3390/ma14030603