Effect of Ni on the Suppression of Sn Whisker Formation in Sn-0.7Cu Solder Joint
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
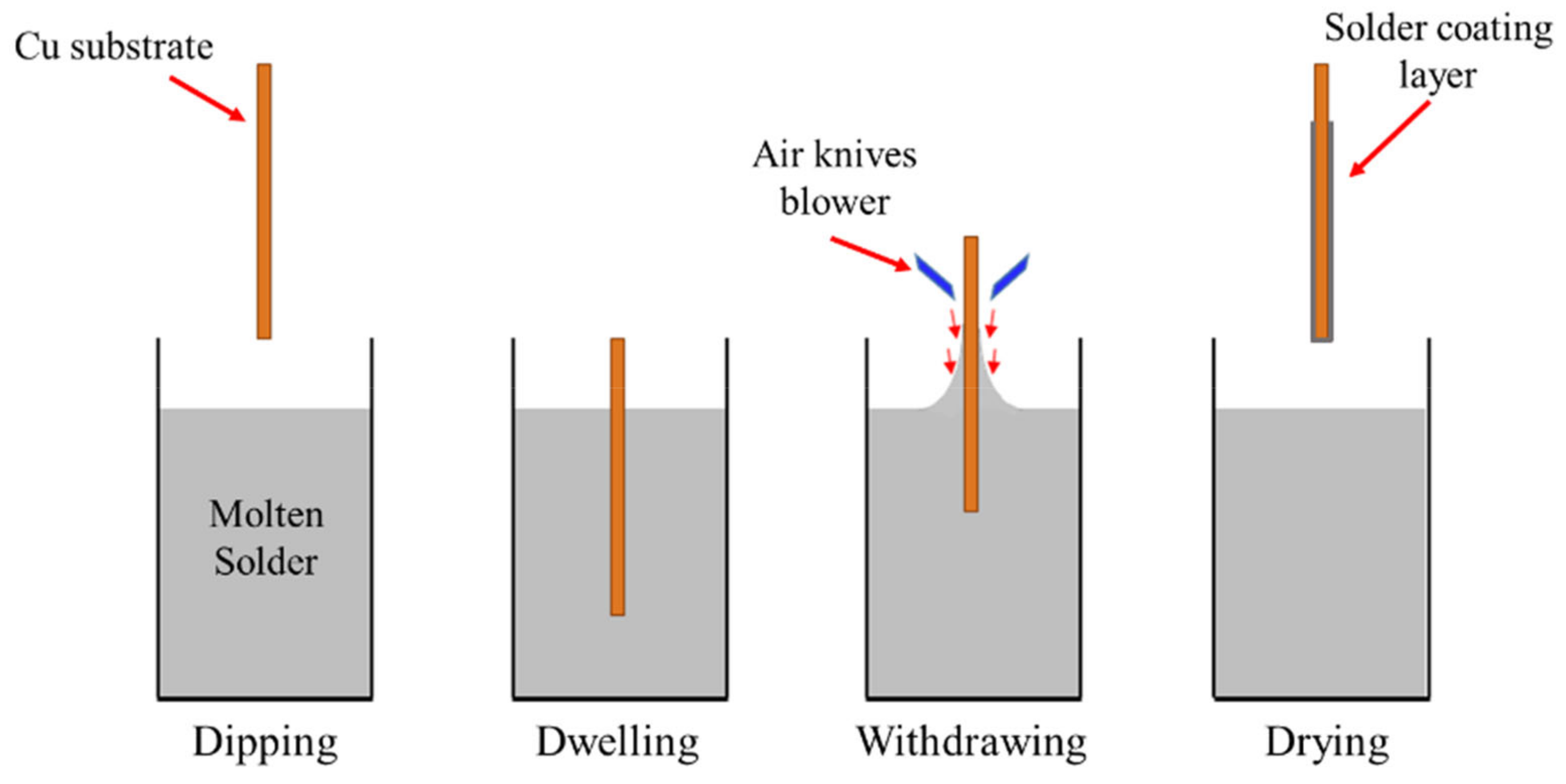
2.2. Sample Preparation
2.3. Testing and Characterization Method
3. Results and Discussion
4. Conclusions
- There was a noticeable correlation between the Sn whisker growth and the composition of the lead-free solder alloy. The Sn-0.7Cu-0.05Ni solder joint had a great suppression effect on the nucleation and growth of Sn whiskers of the Pb-free solder joint.
- The small amount of Ni addition (~500 ppm) was able to alter the microstructure of Cu6Sn5 to form a (Cu,Ni)6Sn5 IMC intermetallic layer, and it is very significant to the nucleation and growth of Sn whiskers.
- The methodic structure of the (Cu,Ni)6Sn5 IMC interfacial layer was relatively thinner and more refined, with a continuous fine scallop-shaped IMC interfacial layer consequently enhanced a greater incubation period for the nucleation and growth of the Sn whisker.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.-S.; Yu, C.-H.; Yang, J.-M. Behavior of tin whisker formation and growth on lead-free solder finish. Thin Solid Film. 2006, 504, 350–354. [Google Scholar] [CrossRef]
- Lin, C.-K.; Lin, T.-H. Effects of continuously applied stress on tin whisker growth. Microelectron. Reliab. 2008, 48, 1737–1740. [Google Scholar] [CrossRef]
- Baated, A.; Kim, K.-S.; Suganuma, K. Effect of intermetallic growth rate on spontaneous whisker growth from a tin coating on copper. J. Mater. Sci. Mater. Electron. 2011, 22, 1685–1693. [Google Scholar] [CrossRef]
- Ashworth, M.A.; Wilcox, G.D.; Higginson, R.L.; Heath, R.J.; Liu, C.; Mortimer, R.J. The effect of electroplating parameters and substrate material on tin whisker formation. Microelectron. Reliab. 2015, 55, 180–191. [Google Scholar] [CrossRef]
- Salleh, M.; al Bakri, A.M.M.; Somidin, F.; Sandu, A.V.; Saud, N.; Kamaruddin, H.; McDonald, S.D.; Nogita, K. A comparative study of solder properties of Sn-0.7 Cu lead-free solder fabricated via the powder metallurgy and casting methods. Revista Chimie 2013, 64, 725–728. [Google Scholar]
- Ibrahim, I.N.A.; Salleh, M.A.A.M. Effect of Zinc Additions on Sn-0.7Cu-0.05Ni LeadFree Solder Alloy. Short review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 238, 012012. [Google Scholar] [CrossRef]
- Mahim, Z.; Salleh, M.; Saud, N. Effect on microstructural and physical properties of Sn-3.0Ag-0.5Cu lead-free solder with the addition of SiC particles. Eur. J. Mater. Sci. Eng. 2019, 4, 37–43. [Google Scholar] [CrossRef]
- Hashim, A.N.; Salleh, M.A.A.M.; Ramli, M.M.; Yee, K.C.; Mokhtar, N.Z.M. Preliminary study on the effect of Ni addition on tin (Sn) whisker growth from lead-free solder coating. IOP Conf. Ser. Mater. Sci. Eng. 2020, 957, 012062. [Google Scholar] [CrossRef]
- Jadhav, N.; Williams, M.; Pei, F.; Stafford, G.; Chason, E. Altering the mechanical properties of Sn films by alloying with Bi: Mimicking the effect of Pb to suppress whiskers. J. Electron. Mater. 2013, 42, 312–318. [Google Scholar] [CrossRef]
- Mokhtar, N.Z.M.; Salleh, M.A.A.M.; Hashim, A.N.; Nazri, S.F. Effects of Gallium addition on the thermal properties and whiskers growth under electrical current stressing. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 012001. [Google Scholar] [CrossRef]
- Chason, E.; Jadhav, N.; Pei, F. Effect of layer properties on stress evolution, intermetallic volume, and density during tin whisker formation. JOM 2011, 63, 62. [Google Scholar] [CrossRef]
- Amin, N.L.M.; Yusof, S.Z.; Kahar, T.A.A.; Bakar, A.O.; Fadil, N.A. Tin Whiskers Formation and Growth on Immersion Sn Surface Finish under External Stresses by Bending. Iop Conf. Ser. Mater. Sci. Eng. 2017, 238, 012001. [Google Scholar] [CrossRef]
- Doudrick, K.; Chinn, J.; Williams, J.; Chawla, N.; Rykaczewski, K. Rapid method for testing efficacy of nano-engineered coatings for mitigating tin whisker growth. Microelectron. Reliab. 2015, 55, 832–837. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Dong, Z.; Huang, H.; Nishimura, T.; Nogita, K. Nanoindentation characterization of intermetallic compounds formed between Sn–Cu (–Ni) ball grid arrays and Cu substrates. Mater. Sci. Eng. B 2009, 164, 44–50. [Google Scholar] [CrossRef]
- Hashim, A.N.; Salleh, M.A.A.M.; Mokhtar, N.Z.M.; Idris, S. Tin (Sn) whisker growth from electroplated Sn finished. IOP Conf. Ser. Mater. Sci. Eng. 2019, 701, 012005. [Google Scholar] [CrossRef]
- Cheng, J.; Vianco, P.T.; Zhang, B.; Li, J.C. Nucleation and growth of tin whiskers. Appl. Phys. Lett. 2011, 98, 241910. [Google Scholar] [CrossRef]
- Zhang, W.; Egli, A.; Schwager, F.; Brown, N. Investigation of Sn-Cu intermetallic compounds by AFM: New aspects of the role of intermetallic compounds in whisker formation. IEEE Trans. Electron. Packag. Manuf. 2005, 28, 85–93. [Google Scholar] [CrossRef]
- Illés, B.; Horváth, B. Whiskering behaviour of immersion tin surface coating. Microelectron. Reliab. 2013, 53, 755–760. [Google Scholar] [CrossRef]
- Pei, F.; Briant, C.L.; Kesari, H.; Bower, A.F.; Chason, E. Kinetics of Sn whisker nucleation using thermally induced stress. Scr. Mater. 2014, 93, 16–19. [Google Scholar] [CrossRef]
- Chason, E.; Jadhav, N.; Chan, W.; Reinbold, L.; Kumar, K. Whisker formation in Sn and Pb–Sn coatings: Role of intermetallic growth, stress evolution, and plastic deformation processes. Appl. Phys. Lett. 2008, 92, 171901. [Google Scholar] [CrossRef]
- Illés, B.; Skwarek, A.; Ratajczak, J.; Dušek, K.; Bušek, D. The influence of the crystallographic structure of the intermetallic grains on tin whisker growth. J. Alloy. Compd. 2019, 785, 774–780. [Google Scholar] [CrossRef]
- Kim, K.S.; Yu, C.H.; Han, S.W.; Yang, K.C.; Kim, J.H. Investigation of relation between intermetallic and tin whisker growths under ambient condition. Microelectron. Reliab. 2008, 48, 111–118. [Google Scholar] [CrossRef]
- Jagtap, N.J.P.; Chason, E. Whisker growth under a controlled driving force: Pressure induced whisker nucleation and growth. Scr. Mater. 2020, 182, 43–47. [Google Scholar] [CrossRef]
- Ramli, M.I.I.; Yusuf, M.S.S.; Salleh, M.A.A.M. Effect of bismuth content on microstructure, melting temperature and undercooling of Sn-0.7Cu solder alloy. Eur. J. Mater. Sci. Eng. 2018, 3, 2013–2017. [Google Scholar]
- Somidin, F.; Maeno, H.; Salleh, M.M.; Tran, X.Q.; McDonald, S.D.; Matsumura, S.; Nogita, K. Characterising the polymorphic phase transformation at a localised point on a Cu6Sn5 grain. Mater. Charact. 2018, 138, 113–119. [Google Scholar] [CrossRef]
- Pei, F.; Jadhav, N.; Chason, E. Correlation between surface morphology evolution and grain structure: Whisker/hillock formation in Sn-Cu. JOM 2012, 64, 1176–1183. [Google Scholar] [CrossRef]
- Zeng, G.; McDonald, S.D.; Gu, Q.; Terada, Y.; Uesugi, K.; Yasuda, H.; Nogita, K. The influence of Ni and Zn additions on microstructure and phase transformations in Sn–0.7 Cu/Cu solder joints. Acta Mater. 2015, 83, 357–371. [Google Scholar] [CrossRef]
- Zeng, G.; McDonald, S.D.; Mu, D.; Terada, Y.; Yasuda, H.; Gu, Q.; Salleh, M.M.; Nogita, K. The influence of ageing on the stabilisation of interfacial (Cu, Ni) 6 (Sn, Zn) 5 and (Cu, Au, Ni) 6Sn5 intermetallics in Pb-free Ball Grid Array (BGA) solder joints. J. Alloy. Compd. 2016, 685, 471–482. [Google Scholar] [CrossRef]
- Nogita, K.; Mu, D.; McDonald, S.; Read, J.; Wu, Y. Effect of Ni on phase stability and thermal expansion of Cu6−xNixSn5 (X= 0, 0.5, 1, 1.5 and 2). Intermetallics 2012, 26, 78–85. [Google Scholar] [CrossRef]
- Salleh, M.M.; McDonald, S.; Gourlay, C.; Belyakov, S.; Yasuda, H.; Nogita, K. Effect of Ni on the Formation and Growth of Primary Cu 6 Sn 5 Intermetallics in Sn-0.7 wt.% Cu Solder Pastes on Cu Substrates During the Soldering Process. J. Electron. Mater. 2016, 45, 154–163. [Google Scholar] [CrossRef]
- Jiang, B.; Xian, A.-P. Whisker growth on tin finishes of different electrolytes. Microelectron. Reliab. 2008, 48, 105–110. [Google Scholar] [CrossRef]
- Jadhav, N.; Buchovecky, E.J.; Reinbold, L.; Kumar, S.; Bower, A.F.; Chason, E. Understanding the correlation between intermetallic growth, stress evolution, and Sn whisker nucleation. IEEE Trans. Electron. Packag. Manuf. 2010, 33, 183–192. [Google Scholar] [CrossRef]
- Skwarek, A.; Pluska, M.; Ratajczak, J.; Czerwinski, A.; Witek, K.; Szwagierczak, D. Analysis of tin whisker growth on lead-free alloys with Ni presence under thermal shock stress. Mater. Sci. Eng. B 2011, 176, 352–357. [Google Scholar] [CrossRef]
- Nogita, K.; Gourlay, C.; Nishimura, T. Cracking and phase stability in reaction layers between Sn-Cu-Ni solders and Cu substrates. JOM 2009, 61, 45–51. [Google Scholar] [CrossRef]
- Nogita, K.; Yasuda, H.; Gourlay, C.M.; Suenaga, S.; Tsukamoto, H.; Mcdonald, S.D.; Takeuchi, A.; Uesugi, K.; Suzuki, Y. Synchrotron micro-XRF measurements of trace element distributions in BGA type solders and solder joints. Trans. Jpn. Inst. Electron. Packag. 2010, 3, 40–46. [Google Scholar] [CrossRef][Green Version]
- Nogita, K. Stabilisation of Cu6Sn5 by Ni in Sn-0.7 Cu-0.05 Ni lead-free solder alloys. Intermetallics 2010, 18, 145–149. [Google Scholar] [CrossRef]










| Composition | Sn-0.7Cu (wt.%) | Sn-0.7Cu-0.05Ni (wt.%) | Cu Substrate (wt.%) |
|---|---|---|---|
| Sn | 99.2276 | 99.2184 | – |
| Cu | 0.7683 | 0.6563 | 99.9426 |
| Ni | – | 0.0528 | – |
| Sb | 0.0024 | 0.0064 | – |
| Pb | 0.0013 | 0.0162 | 0.0053 |
| Zn | <0.0002 | <0.0002 | 0.0142 |
| Fe | 0.0042 | 0.0041 | – |
| Al | 0.0003 | 0.0004 | 0.0148 |
| In | 0.0012 | 0.0034 | – |
| Hot-Dip Soldering Parameters | Value |
|---|---|
| Preheat Temperature (°C) | 200 |
| Preheat Time (s) | 60 |
| Preheat Rate (°C/s) | 2.5 |
| Peak Temperature (°C) | 265 |
| Immersion Withdrawal Speed (mm/s) | 10 |
| Immersion Dwell Time (s) | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashim, A.N.; Salleh, M.A.A.M.; Sandu, A.V.; Ramli, M.M.; Yee, K.C.; Mohd Mokhtar, N.Z.; Chaiprapa, J. Effect of Ni on the Suppression of Sn Whisker Formation in Sn-0.7Cu Solder Joint. Materials 2021, 14, 738. https://doi.org/10.3390/ma14040738
Hashim AN, Salleh MAAM, Sandu AV, Ramli MM, Yee KC, Mohd Mokhtar NZ, Chaiprapa J. Effect of Ni on the Suppression of Sn Whisker Formation in Sn-0.7Cu Solder Joint. Materials. 2021; 14(4):738. https://doi.org/10.3390/ma14040738
Chicago/Turabian StyleHashim, Aimi Noorliyana, Mohd Arif Anuar Mohd Salleh, Andrei Victor Sandu, Muhammad Mahyiddin Ramli, Khor Chu Yee, Noor Zaimah Mohd Mokhtar, and Jitrin Chaiprapa. 2021. "Effect of Ni on the Suppression of Sn Whisker Formation in Sn-0.7Cu Solder Joint" Materials 14, no. 4: 738. https://doi.org/10.3390/ma14040738
APA StyleHashim, A. N., Salleh, M. A. A. M., Sandu, A. V., Ramli, M. M., Yee, K. C., Mohd Mokhtar, N. Z., & Chaiprapa, J. (2021). Effect of Ni on the Suppression of Sn Whisker Formation in Sn-0.7Cu Solder Joint. Materials, 14(4), 738. https://doi.org/10.3390/ma14040738








