Assessment of Technological Capabilities for Forming Al-C-B System Coatings on Steel Surfaces by Electrospark Alloying Method
Abstract
:1. Introduction
2. Methodology
3. Results and Discussions
4. Kinetics of the Formation of Al-C-B Coatings by the ESA Method
- εn, εT—concentration fluctuation amplitude and temperature fluctuation amplitude;
- ξ, η—independent white noise;
- τT—temperature relaxation time;
- τσ—stress relaxation time;
- τ—concentration relaxation time;
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayer, E. Mechanical Seals; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Tarel’nik, V.B.; Martsinkovskii, V.S.; Zhukov, A.N. Increase in the Reliability and Durability of Metal Impulse End Seals. Chem. Pet. Eng. 2017, 53, 114–120. [Google Scholar] [CrossRef]
- Keddam, M.; Chegroune, R.; Kulka, M.; Makuch, N.; Panfil, D.; Siwak, P.; Taktak, S. Characterization, Tribological and Mechanical Properties of Plasma Paste Borided AISI 316 Steel. Trans. Indian Inst. Met. 2018, 71, 79–90. [Google Scholar] [CrossRef]
- Pischov, M.N.; Belskiy, S.E.; Andreykovets, E.P.; Zhishkevich, M.M. Effect of complex borating on the characteristics of hardening gears in bench tests. Cast. Metall. 2015, 107–112. [Google Scholar]
- Jain, V.; Sundararajan, G. Influence of the pack thickness of the boronizing mixture on the boriding of steel. Surf. Coat. Technol. 2002, 149, 21–26. [Google Scholar] [CrossRef]
- Joshi, A.A.; Hosmani, S.S. Pack-Boronizing of AISI 4140 Steel: Boronizing Mechanism and the Role of Container Design. Mater. Manuf. Process. 2014, 29, 1062–1072. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Ortiz-Domínguez, M.; Tapia-Quintero, C.; Rodríguez-Castro, G.; Jiménez-Reyes, M.Y.; Chávez-Gutiérrez, E. Kinetics and Boron Diffusion in the FeB/Fe2B Layers Formed at the Surface of Borided High-Alloy Steel. J. Mater. Eng. Perform. 2012, 21, 1714–1723. [Google Scholar] [CrossRef]
- Gunes, I.; Yıldız, I. Investigation of Adhesion and Tribological Behavior of Borided AISI 310 Stainless Steel. Matéria 2016, 21, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Builov, V.N.; Lyulyakov, I.V. Investigation into the Process of Electrolysis Borating of Steel Parts. Surf. Eng. Appl. Electrochem. 2018, 54, 338–344. [Google Scholar] [CrossRef]
- Carrera-Espinoza, R.; Figueroa-Lopez, U.; Martínez-Trinidad, J.; Campos-Silva, I.; Hernandez-Sanchez, E.; Motallebzadeh, A. Tribological behavior of borided AISI 1018 steel under linear reciprocating sliding conditions. Wear 2016, 362, 1–7. [Google Scholar] [CrossRef]
- Sizov, I.G. Otsenka khrupkosti boridnykh sloyev posle elektronno-luchevogo borirovaniya (Evaluation of fragility of bored layers after electronic beam boring). Sovrem. Nauk. Tekhnologii Mod. High Technol. 2005, 11. Available online: https://elibrary.ru/item.asp?id=11524022 (accessed on 20 January 2021). (In Russian).
- Rotshtein, V.P.; Proskurovsky, D.I.; Ozur, G.E.; Ivanov, Y.F.; Markov, A.B. Surface modification and alloying of metallic materials with low-energy high-current electron beams. Surf. Coat. Technol. 2004, 180, 377–381. [Google Scholar] [CrossRef]
- Yun, E.; Lee, S. Improvement of hardness and wear resistance in stainless-steelbased surface composites fabricated by high-energy electron beam irradiation. Surf. Coat. Technol. 2006, 200, 3478–3485. [Google Scholar] [CrossRef]
- Santana, D.A.; Koga, G.Y.; Wolf, W.; Bataev, I.A.; Ruktuev, A.A.; Bolfarini, C.; Kiminami, C.S.; Botta, W.J.; Jorge, A.M., Jr. Wear-resistant boride reinforced steel coatings produced by non-vacuum electron beam cladding. Surf. Coat. Technol. 2020, 386, 125466. [Google Scholar] [CrossRef]
- Chernega, S.M.; Zaulichnyi, Y.V. Effect of Chromium on the Structure and Properties of Boride Diffusion Coatings. Powder Metall. Met. Ceram. 2000, 39, 594–598. [Google Scholar] [CrossRef]
- Chernega, S.M.; Poliakov, I.A.; Krasovskiy, M.O. Formation of Complex Diffusion Boride Layers on Metastable Austenitic Nitrogen-Containing Chromium—Manganese Steels in Conditions of Action of an External Magnetic Field. Metallofiz. Noveishie Tekhnol. 2016, 38, 1479–1495. [Google Scholar] [CrossRef] [Green Version]
- Kulka, M.; Makuch, N.; Pertek, A. Microstructure and properties of laser-borided 41Cr4 steel. Opt. Laser Technol. 2013, 45, 308–318. [Google Scholar] [CrossRef]
- Sashank, S.; Dinesh Babu, P.; Marimuthu, P. Experimental studies of laser borided low alloy steel and optimization of parameters using response surface methodology. Surf. Coat. Technol. 2019, 363, 255–264. [Google Scholar] [CrossRef]
- Bataev, I.A.; Bataev, A.A.; Golkovsky, M.G.; Teplykh, A.Y.; Burov, V.G.; Veselov, S.V. Non-vacuum electron-beam boriding of low-carbon steel. Surf. Coat. Technol. 2012, 207, 245–253. [Google Scholar] [CrossRef]
- Kukhar, V.V.; Grushko, A.V.; Vishtak, I.V. Shape Indexes for Dieless Forming of the Elongated Forgings with Sharpened End by Tensile Drawing with Rupture. Solid State Phenom. 2018, 284, 408–415. [Google Scholar] [CrossRef]
- Astafiev, G.I.; Fainshmidt, E.M.; Pegashkin, V.F.; Pilipenko, V.V.; Andriyanov, A.V.; Pilipenko, V.F.; Khomenko, A.Y. Sposob Elektroerozionnogo Borirovaniya Poverkhnosti Detail iz Stali i Chuguna (Method of Electroerosive Boriding of a Surface of a Part Made of Steel and Cast Iron). Russian Patent 2421307 C2 RU, 8 October 2008. (In Russian). Available online: https://elibrary.ru/item.asp?id=37747807 (accessed on 20 January 2021).
- Tarelnyk, V.B.; Gaponova, O.P.; Konoplyanchenko, Y.V.; Yevtushenko, N.S.; Herasymenko, V.O. The Analysis of a Structural State of Surface Layer after Electroerosive Alloying. II. Features of Formation of Electroerosive Coatings on Special Steels and Alloys by Hard Wear-Resistant and Soft Antifriction Materials. Metallofiz. Noveishie Tekhnol. 2018, 40, 795–815. [Google Scholar] [CrossRef]
- Tarelnyk, V.; Martsynkovskyy, V.; Gaponova, O.; Konoplianchenko, I.; Belous, A.; Gerasimenko, V.; Zakharov, M. New method for strengthening surfaces of heat treated steel parts. In Proceedings of the 15th International Scientific and Engineering Conference Hermetic Sealing, Vibration Reliability and Ecological Safety of Pump and Compressor Machinery (HERVICON + PUMPS 2017), Sumy, Ukraine, 5–8 September 2017; Volume 233, p. 012048. [Google Scholar]
- Tarelnyk, V.; Martsynkovskyy, V.; Gaponova, O.; Konoplianchenko, I.; Dovzyk, M.; Tarelnyk, N.; Gorovoy, S. New sulphiding method for steel and cast iron parts. In Proceedings of the 15th International Scientific and Engineering Conference Hermetic Sealing, Vibration Reliability and Ecological Safety of Pump and Compressor Machinery (HERVICON + PUMPS 2017), Sumy, Ukraine, 5–8 September 2017; Volume 233, p. 012049. [Google Scholar]
- Martsynkovskyy, V.; Tarelnyk, V.; Konoplianchenko, I.; Gaponova, O.; Dumanchuk, M. Technology Support for Protecting Contacting Surfaces of Half-Coupling—Shaft Press Joints Against Fretting Wear. In Advances in Design, Simulation and Manufacturing II, Proceedings of the 2nd International Conference on Design, Simulation, Manufacturing: The Innovation Exchange, DSMIE 2019, Lutsk, Ukraine, 11–14 June 2019; Springer: Cham, Switzerland, 2019; pp. 216–225. [Google Scholar]
- Myslyvchenko, O.M.; Gaponova, O.P.; Tarelnyk, V.B.; Krapivka, M.O. The Structure Formation and Hardness of High-Entropy Alloy Coatings Obtained by Electrospark Deposition. Powder Metall. Met. Ceram. 2020, 59, 201–208. [Google Scholar] [CrossRef]
- Korotaev, D.N. Tekhnologicheskiye Vozmozhnosti Formirovaniya Iznosostoykikh Nanostruktur Elektroiskrovym Legirovaniyem (Technological Possibilities of Forming Wear-Resistant Nanostructures by Electrospark Alloying); SibADI: Omsk, Russia, 2009. (In Russian) [Google Scholar]
- Kheifets, M.L. Puti povysheniya effektivnosti protsessov formirovaniya poverkhnostey s pozitsiyey sinergetiki. (Ways to improve the efficiency of the processes of forming surfaces with a synergetic position). Vestn. Mashinostroyeniya Mech. Eng. Bull. 1994, 2. Available online: https://www.elibrary.ru/item.asp?id=37187249 (accessed on 20 January 2021). (In Russian).
- Kabaldin, Y.G. Sinergetika. Informatsionnyye Modeli Samosborki Nanosistem i Nanostrukturirovaniya Materialov pri Vneshnem Mekhanicheskom Vozdeystvii (Synergetics. Information Models of Self-Assembly of Nanosystems and Nanostructuring of Materials under External Mechanical Stress); KnAGTU: Komsomolsk-on-Amur, Russia, 2007. (In Russian) [Google Scholar]
- Brower, A.V.; Dyachenko, L.D. Uprochnyayushchiye Tekhnologi i Pokrytiya Hardening Technologies and Coatings. 2007. Available online: http://www.mashin.ru/files/u_307.pdf (accessed on 20 January 2021). (In Russian).
- Kim, V.A. Samoorganizatsiya v Protsessakh Uprochneniya, Treniya i Iznashivaniya Rezhushchego Instrumenta (Self-Organization in the Processes of Strengthening, Friction and Wear of Cutting Tool); Dalnauka: Vladivostok, Russia, 2001. (In Russian) [Google Scholar]
- Tsallis, C. Lecture Notes in Physics; Abe, S., Okamoto, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Lorenz, E.N. Deterministic nonperiodic flow. J. Atmos. Sci. 1963, 20, 130–141. [Google Scholar] [CrossRef] [Green Version]
- Zhilenko, T.I.; Yushchenko, O.V. Static description of a stochastic condensation system. Tech. Phys. 2013, 58, 1558–1562. [Google Scholar] [CrossRef]



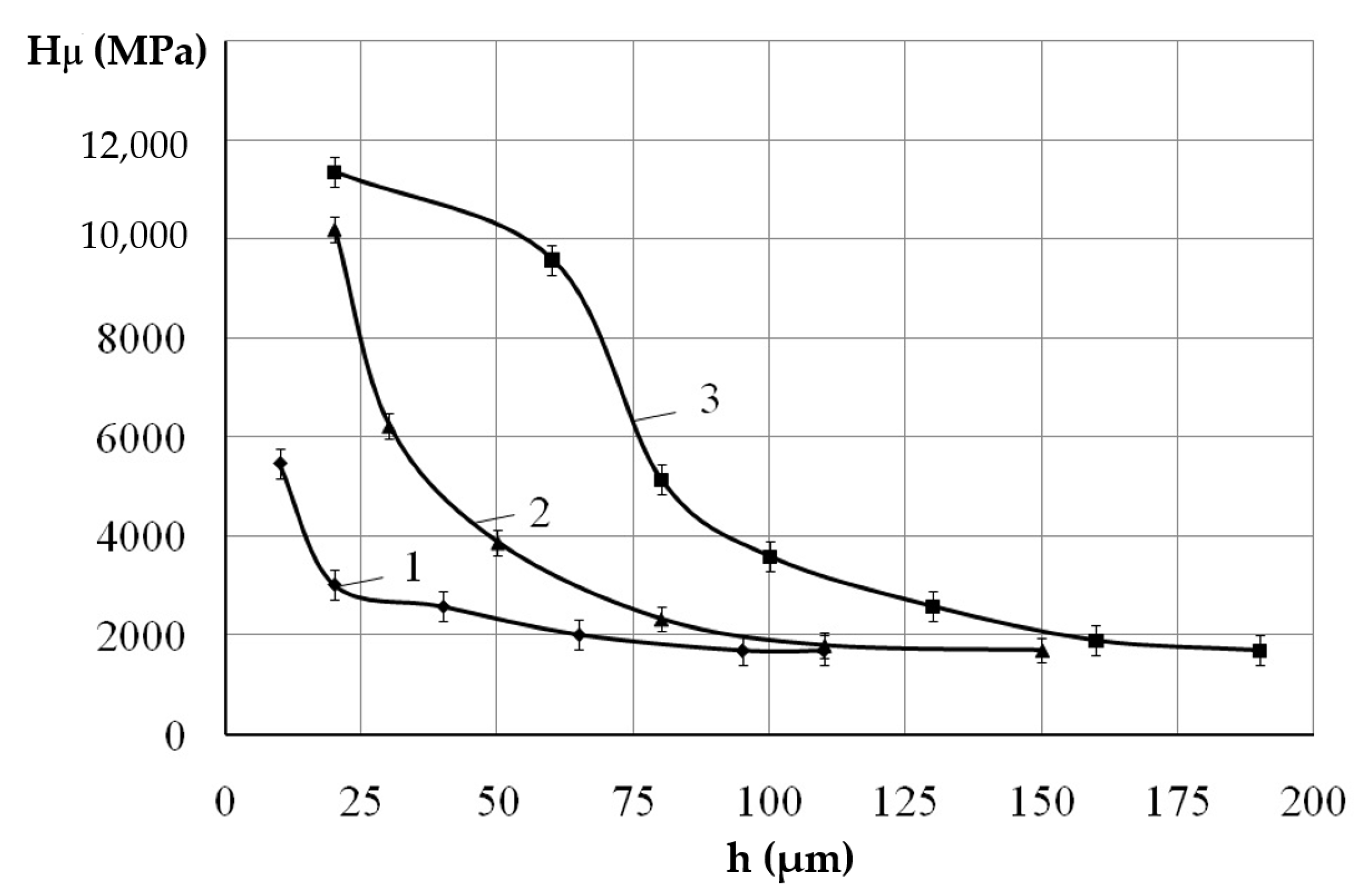
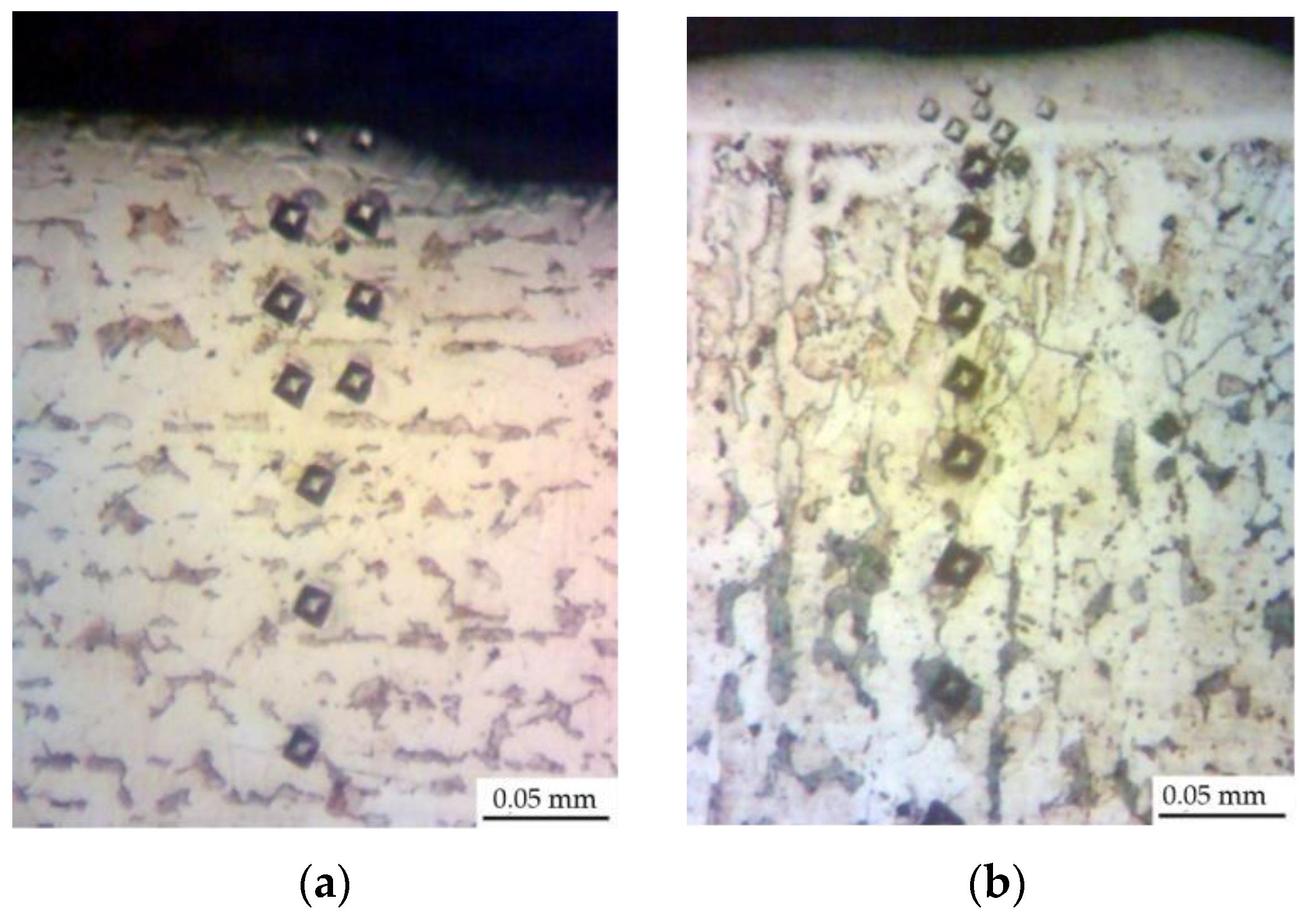
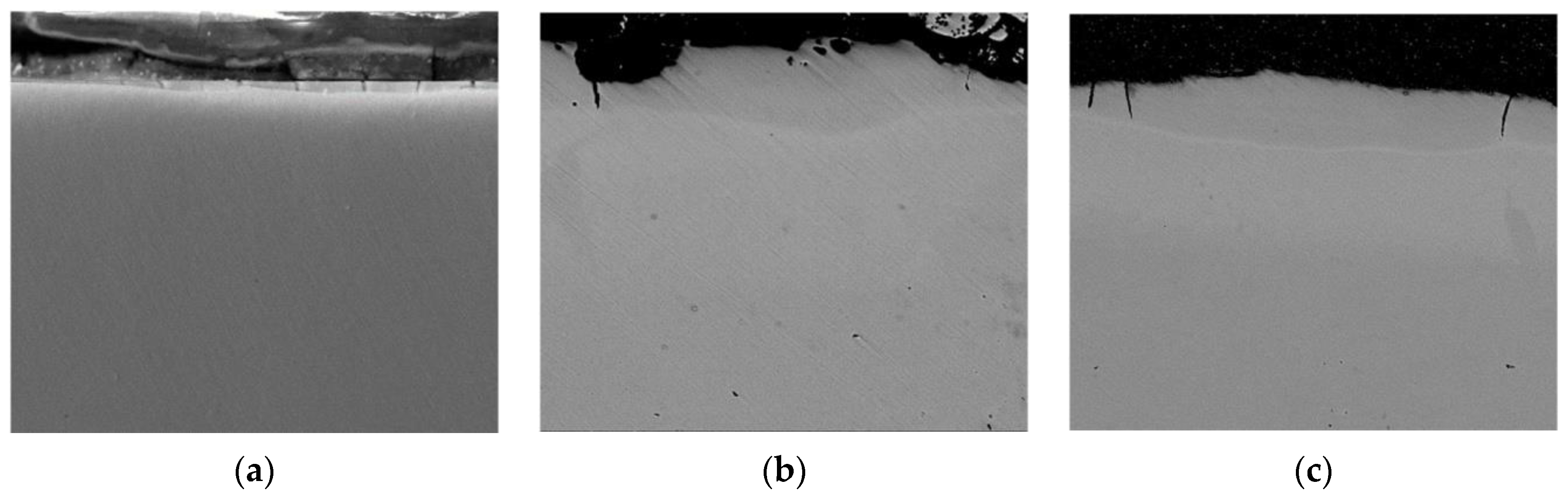




| Discharge Energy, J | Roughness, μm | Strengthened Layer | ||||
|---|---|---|---|---|---|---|
| Ra | Rz | Rmax | Hµ, MPa | h, μm | S, % | |
| Steel C40 | ||||||
| 0.13 | 1.2 | 2.9 | 7.4 | 6487 | 15 | 55 |
| 0.55 | 2.9 | 4.5 | 17.3 | 10351 | 20 | 75 |
| 4.9 | 9.3 | 19.5 | 48.2 | 12350 | 60 | 95 |
| Steel C22 | ||||||
| 0.13 | 1.1 | 2.7 | 7.2 | 5474 | 20 | 60 |
| 0.55 | 2.9 | 4.1 | 16.3 | 10196 | 30 | 80 |
| 4.9 | 8.9 | 18.7 | 46.1 | 11345 | 75 | 95 |
| Discharge Energy, J | Phase | Parameters of Crystal Lattices, a, nm | Phase Content, % (mass.) |
|---|---|---|---|
| 0.13 | FCC solid solution | 36.189 | 8 |
| BCC solid solution | 28.651 | 92 | |
| 0.55 | BCC solid solution | 36.189 | 8 |
| BCC solid solution | 28.651 | 92 | |
| 4.9 | Fe4Al13 | a = 153.920 b = 81.779 c = 124.914 β = 107.3709 | 10 |
| Fe3(CB) | a = 50.818 b = 67.791 c = 45.170 | 43 | |
| FCC solid solution | a = 36.209 | 14 | |
| BCC solid solution | a = 28.699 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoszewski, B.; Gaponova, O.P.; Tarelnyk, V.B.; Myslyvchenko, O.M.; Kurp, P.; Zhylenko, T.I.; Konoplianchenko, I. Assessment of Technological Capabilities for Forming Al-C-B System Coatings on Steel Surfaces by Electrospark Alloying Method. Materials 2021, 14, 739. https://doi.org/10.3390/ma14040739
Antoszewski B, Gaponova OP, Tarelnyk VB, Myslyvchenko OM, Kurp P, Zhylenko TI, Konoplianchenko I. Assessment of Technological Capabilities for Forming Al-C-B System Coatings on Steel Surfaces by Electrospark Alloying Method. Materials. 2021; 14(4):739. https://doi.org/10.3390/ma14040739
Chicago/Turabian StyleAntoszewski, Bogdan, Oksana P. Gaponova, Viacheslav B. Tarelnyk, Oleksandr M. Myslyvchenko, Piotr Kurp, Tetyana I. Zhylenko, and Ievgen Konoplianchenko. 2021. "Assessment of Technological Capabilities for Forming Al-C-B System Coatings on Steel Surfaces by Electrospark Alloying Method" Materials 14, no. 4: 739. https://doi.org/10.3390/ma14040739








