Manganese and Nickel Acetylacetonates as Curatives for Chloroprene Rubber Based on Heck’s Reaction
Abstract
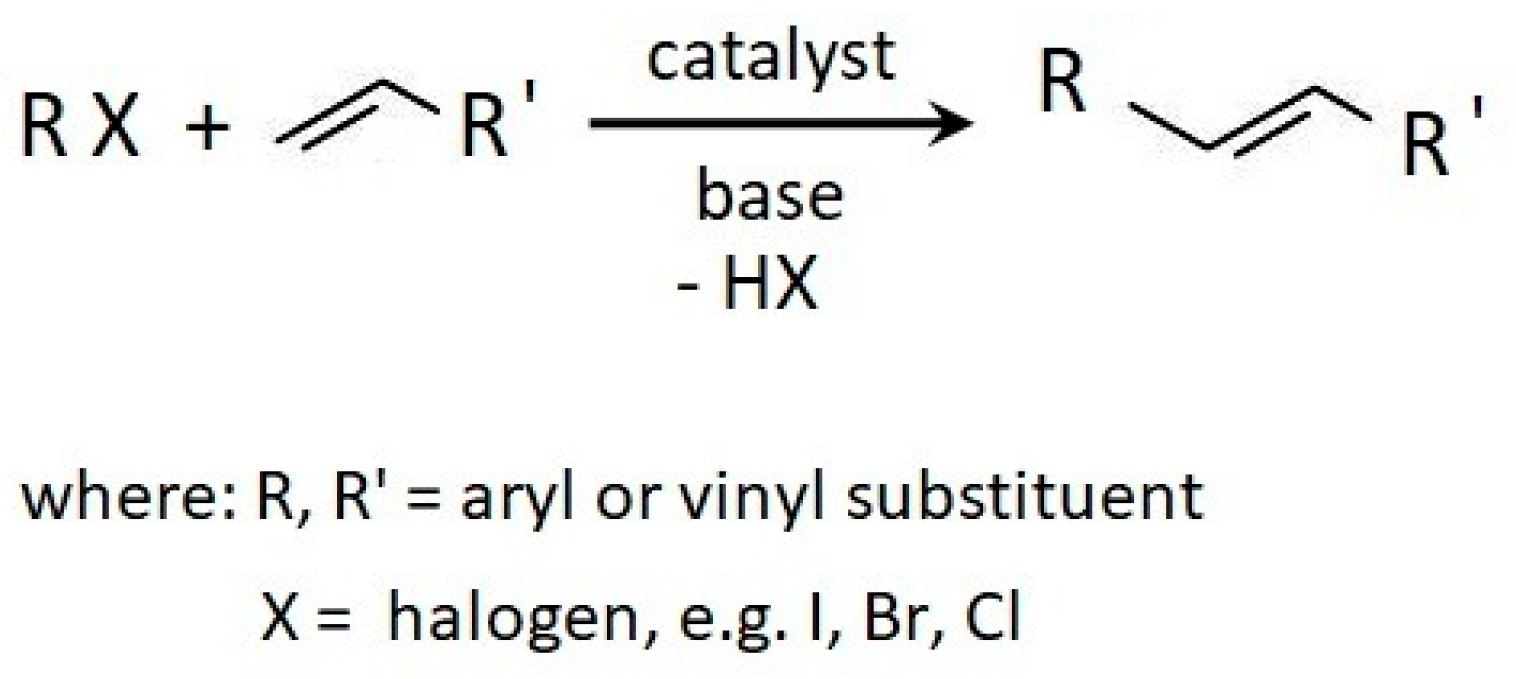
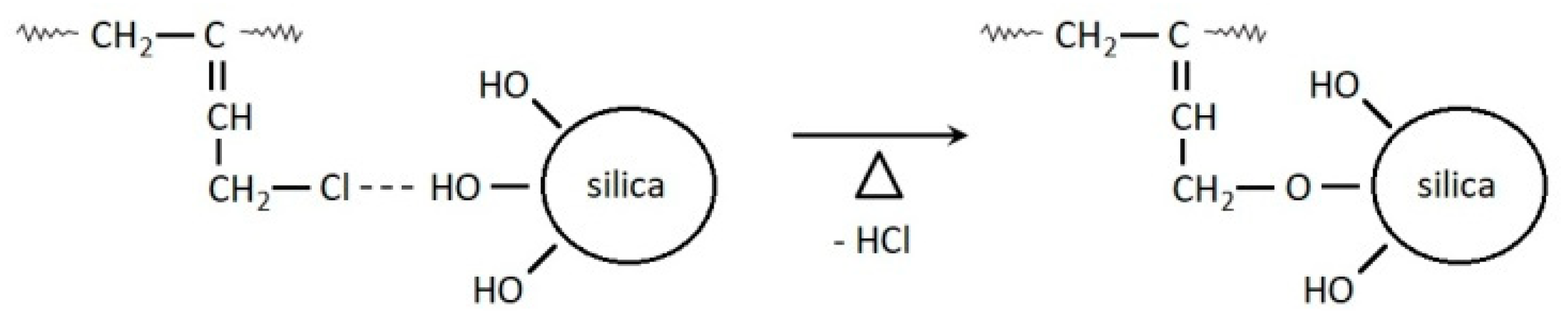
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
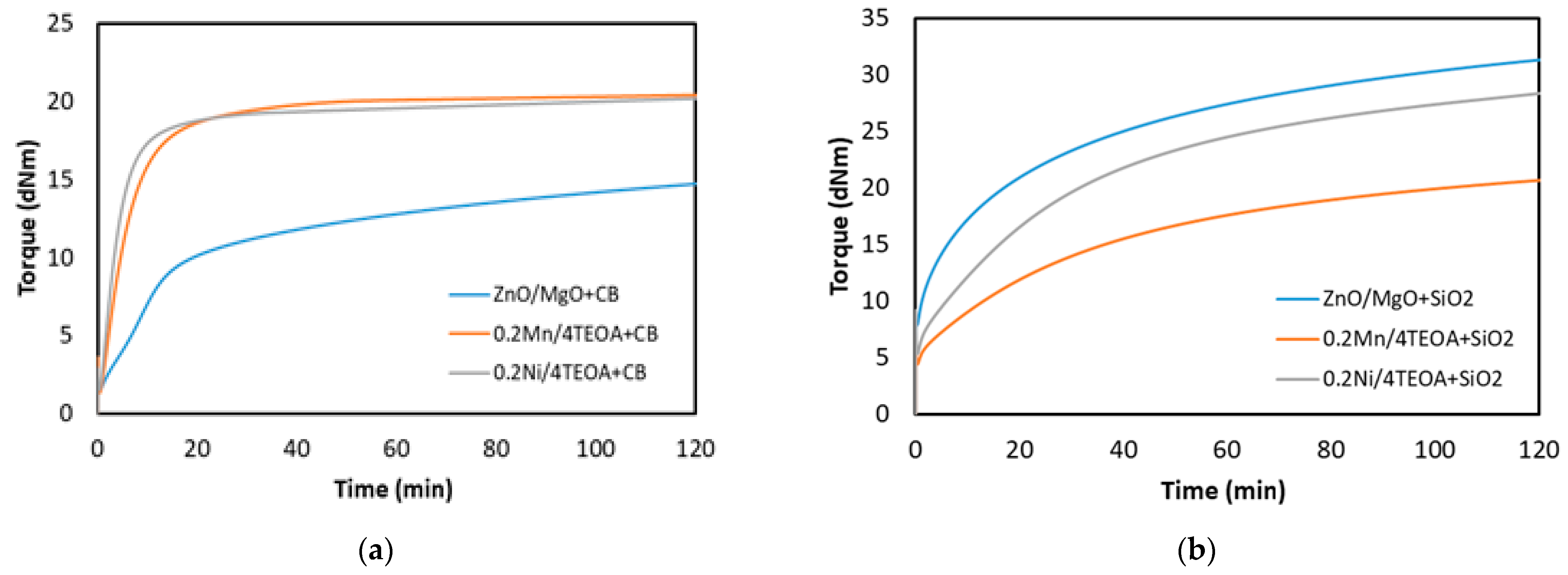
3.1. Cure Characteristic of CR Compounds and Crosslinking Degree of the Vulcanizates
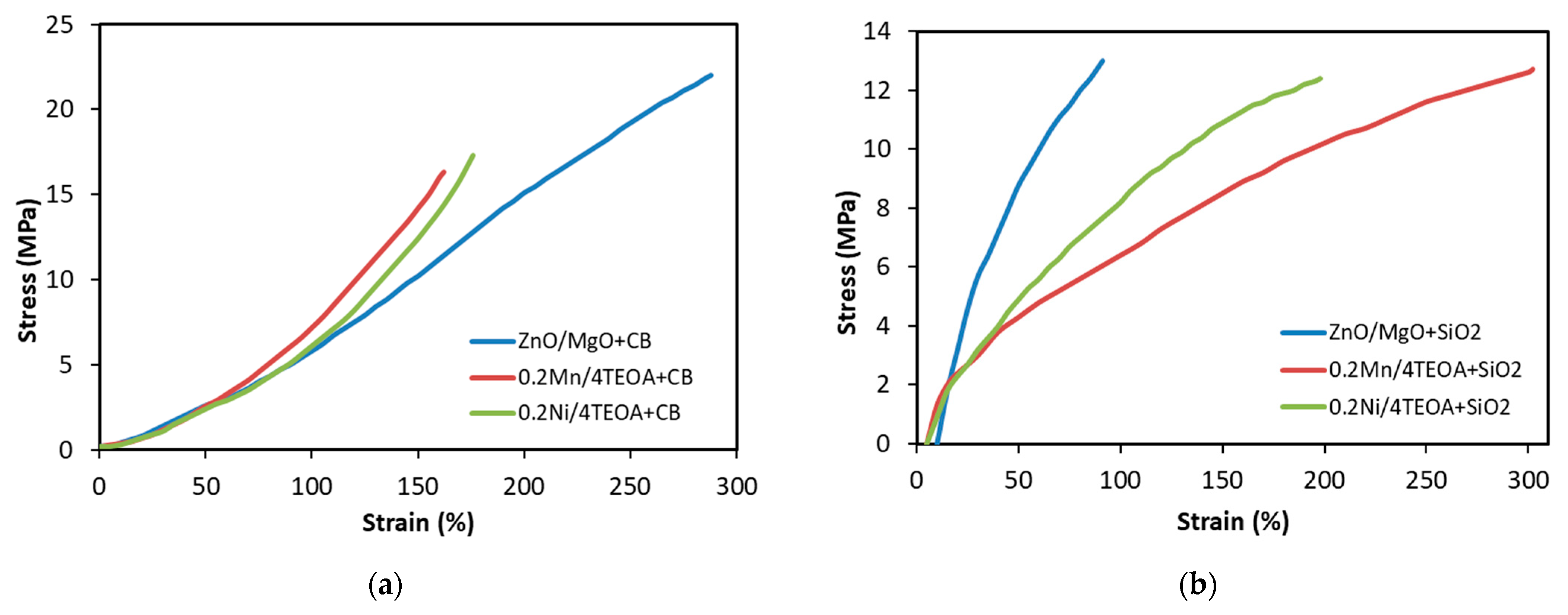
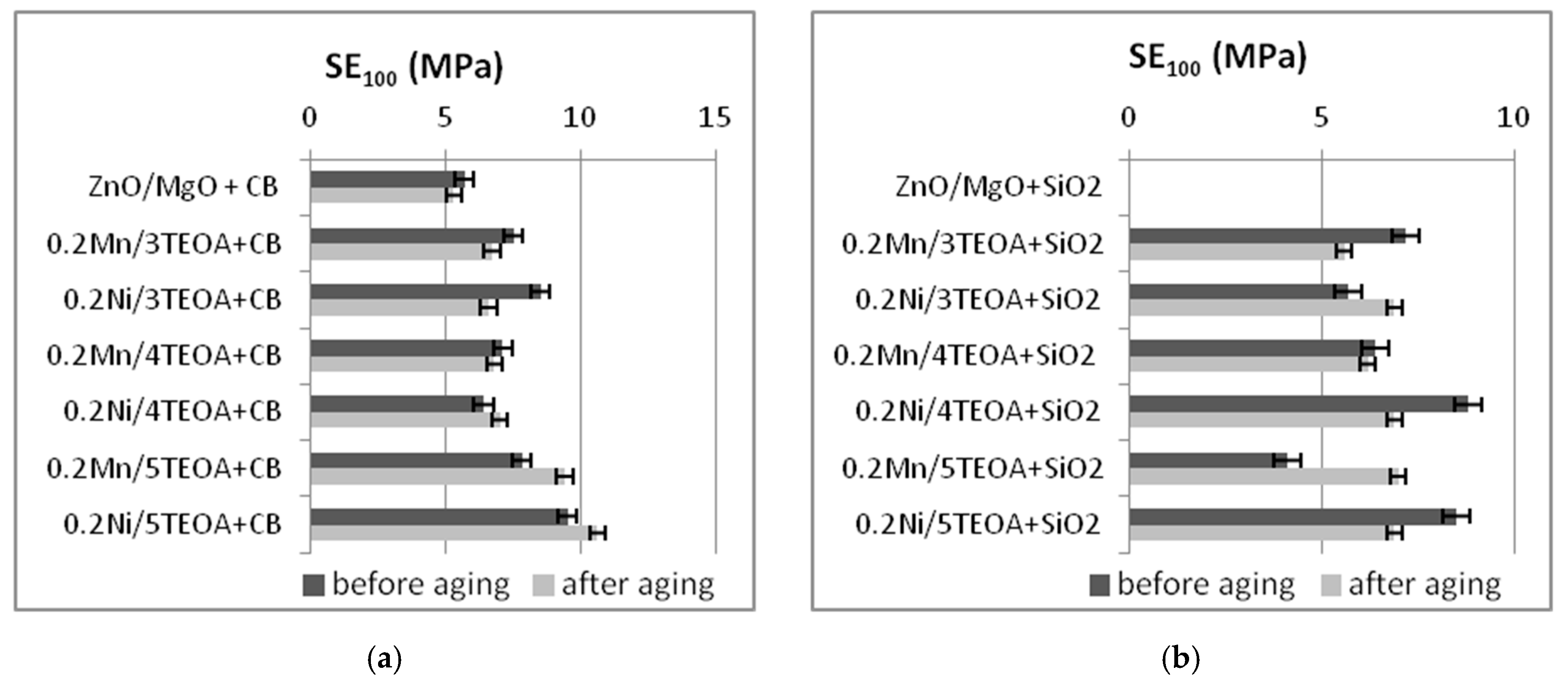
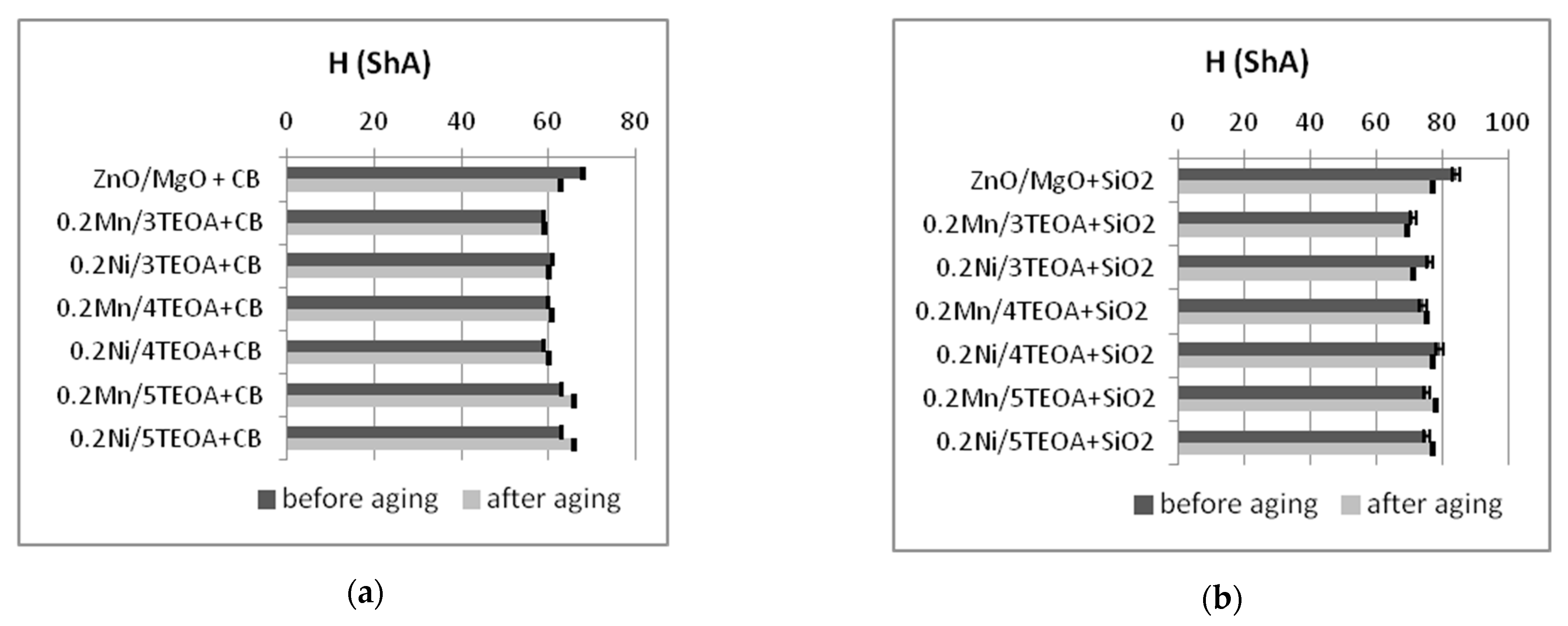
3.2. Mechanical Properties and Resistance to Thermo-Oxidative Aging of Vulcanizates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Musch, R.; Magg, H. Polychloroprene Rubber. In Handbook of Specialty Elastomers; CRC Press: Boca Raton, FL, USA, 2008; pp. 2–36. [Google Scholar]
- Datta, S. Special Purpose Elastomers: Synthesis, Structure-Property Relationship, Compounding, Processing and Applications. In Advances in Elastomers; Springer: Berlin, Germany, 2004; pp. 118–120. [Google Scholar]
- Das, A.; Costa, F.R.; Wagenknecht, U.; Heinrich, G. Nanocomposites based on chloroprene rubber: Effect of chemical nature and organic modification of nanoclay on the vulcanizate properties. Eur. Polym. J. 2008, 44, 3456–3465. [Google Scholar] [CrossRef]
- Zheng, J.; Tan, J.; Gao, H.; Wang, C.; Dong, Z. Preparation of low temperature resistant and high electrical insulation chloroprene rubber–butadiene rubber blends. Rubber Chem. Technol. 2014, 87, 360–369. [Google Scholar] [CrossRef]
- Gilbert, M. Plastics Materials: Introduction and Historical. In Brydson’s Plastics Materials; Elsevier: Oxford, UK, 2017; pp. 1–18. [Google Scholar] [CrossRef]
- Glenn, F.E. Chloroprene Polymers. In Encyclopedia of Polymer Science and Technology; John Wiley&Sons: Hoboken, NJ, USA, 2005; pp. 189–234. [Google Scholar]
- Berry, K.I. The Quest for a Safer Accelerator for Polychloroprene Rubber. Ph.D. Thesis, Aston University, Birmingham, UK, April 2014. [Google Scholar]
- Shanks, R.A.; Kong, I. General purpose elastomers. In Advances in Elastomers II; Visakh, P.M., Thomas, S.A.K., Chandra Mathew, A.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 11–46. [Google Scholar]
- Chokanandsombat, Y.; Sirisinha, C. MgO and ZnO as reinforcing fillers in cured polychloroprene rubber. J. Appl. Polym. Sci. 2012, 128, 2533–2540. [Google Scholar] [CrossRef]
- Akovali, G. Plastic materials: Chlorinated polyethylene (CPE), chlorinated polyvinylchloride (CPVC), chlorosulfonated polyethylene (CSPE) and polychloroprene rubber (CR). In Toxicity of Building Materials; Woodhead Publishing Limited: Cambrige, UK, 2012; pp. 54–75. [Google Scholar]
- Johnson, P.R. Polychloroprene Rubber. Rubber Chem. Technol. 1976, 49, 650–702. [Google Scholar] [CrossRef]
- Berry, K.; Liu, M.; Chakraborty, K.; Pullan, N.; West, A.; Sammon, C.; Topham, P.D. mechanism for cross-linking polychloroprene with ethylene thiourea and zinc oxide. Rubber Chem. Technol. 2015, 88, 80–97. [Google Scholar] [CrossRef] [Green Version]
- Siriwong, C.; Sae-Oui, P.; Sirisinha, C. Performance comparison of various surface modifying agents on properties of silica-filled chloroprene rubber. Rubber Chem. Technol. 2017, 90, 146–158. [Google Scholar] [CrossRef]
- Geer, W.C. Rubber Technology. Chem. Eng. News Arch. 1942, 20, 1358–1361. [Google Scholar] [CrossRef]
- Desai, H.; Hendrikse, K.G.; Woolard, C.D. Vulcanization of polychloroprene rubber. I. A revised cationic mechanism for ZnO crosslinking. J. Appl. Polym. Sci. 2007, 105, 865–876. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V.; Ballabio, D.; Mauri, A.; Cassotti, M.; Lee, S.; West, A.J.; Cartlidge, D. Qspr study of rheological and mechanical properties of chloroprene rubber accelerators. Rubber Chem. Technol. 2014, 87, 219–238. [Google Scholar] [CrossRef]
- The European Parliament; The Council of The European Union. Stabilisation, Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labeling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and1999/45/EC, and Amending Regulation (EC) No, n.d; Official Journal of the European Union: Brussels, Belgium, 2008. [Google Scholar]
- Mostoni, S.; Milana, P.; Di Credico, B.; D’Arienzo, M.; Scotti, R. Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process. Catalysist 2019, 9, 664. [Google Scholar] [CrossRef] [Green Version]
- Heideman, G. Reduced Zinc Oxide Levels in Sulphur Vulcanisation of Rubber Compounds. Ph.D. Thesis, University of Twente, Enschlede, The Netherlands, 2004. [Google Scholar]
- Sowińska, A.; Maciejewska, M. Thermal analysis applied to studying the influence of ionic liquids on the vulcanization, thermal stability and damping properties of ethylene-propylene-diene rubber. J. Therm. Anal. Calorim. 2019, 138, 2669–2681. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, E.; Reinartz, K.S. Improvement of the crosslinking of polychloroprene. Kautsch. Gummi Kunstst. 2000, 53, 419–425. [Google Scholar]
- Smejda-Krzewicka, A.; Rzymski, W.M.; Kowalski, D. Tin oxide cross-linking of chloroprene rubber. Polimers 2015, 60, 186–191. [Google Scholar] [CrossRef]
- Smejda-Krzewicka, A.; Olejnik, A.; Strzelec, K. The role of iron(III) oxide in chloroprene and butadiene rubber blends’ cross-linking, structure, thermal and mechanical characteristics. Iran. Polym. J. 2019, 28, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Smejda-Krzewicka, A.; Dmowska-Jasek, A.; Kobędza, P. Method for curing of chloroprene rubber. Int. J. Polym. Anal. Charact. 2018, 1, 475–486. [Google Scholar]
- Dziemidkiewicz, A.; Pingot, M.; Maciejewska, M. Metal complexes as new pro-ecological crosslinking agents for chloroprene rubber based on heck coupling reaction. Rubber Chem. Technol. 2019, 92, 589–597. [Google Scholar] [CrossRef]
- Dziemidkiewicz, A.; Anyszka, R.; Blume, A.; Maciejewska, M. Reaction mechanism of halogenated rubber crosslinking using a novel environmentally friendly curing system. Polym. Test. 2020, 84, 106354. [Google Scholar] [CrossRef]
- Dziemidkiewicz, A.; Maciejewska, M. CR composites with improved processing safety crosslinked via Heck’s reaction. J. Appl. Polym. Sci. 2021, 138, 1–13. [Google Scholar] [CrossRef]
- Dziemidkiewicz, A.; Maciejewska, M.; Pingot, M. Thermal analysis of halogenated rubber cured with a new cross-linking system. J. Therm. Anal. Calorim. 2019, 138, 4395–4405. [Google Scholar] [CrossRef] [Green Version]
- Bhanage, B.M.; Fujita, S.-I.; Arai, M. Heck reactions with various types of palladium complex catalysts: Application of multiphase catalysis and supercritical carbon dioxide. J. Organomet. Chem. 2003, 687, 211–218. [Google Scholar] [CrossRef]
- Gholivand, K.; Salami, R.; Farshadfar, K.; Butcher, R.J. Synthesis and structural characterization of Pd(II) and Cu(I) complexes containing dithiophosphorus ligand and their catalytic activities for Heck reaction. Polyhedron 2016, 119, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Kinney, E.P.; Yang, Z. Ligand-Free Heck Reaction: Pd(OAc)2 as an Active Catalyst Revisited. J. Org. Chem. 2003, 68, 7528–7531. [Google Scholar] [CrossRef] [PubMed]
- Jutand, A. Mechanisms of the Mizoroki-Heck Reaction. In The Mizoroki-Heck Reaction, 1st ed.; Oestreich, M., Ed.; John Wiley & Sons: Chichester, UK, 2009; pp. 1–50. [Google Scholar]
- Jagtap, S.V. Heck Reaction—State of the Art. Catalyst 2017, 7, 267. [Google Scholar] [CrossRef]
- Waghmode, S.B.; Arbuj, S.S.; Wani, B.N.; Gopinath, C. Palladium chloride catalyzed photochemical Heck reaction. Can. J. Chem. 2013, 91, 348–351. [Google Scholar] [CrossRef]
- Wang, S.-S.; Yang, G.-Y. Recent developments in low-cost TM-catalyzed Heck-type reactions (TM = transition metal, Ni, Co, Cu, and Fe). Catal. Sci. Technol. 2016, 6, 2862–2876. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Azizi, G. Iron-catalyzed cross-coupling reaction: Recyclable heterogeneous iron catalyst for selective olefination of aryl iodides in poly(ethylene glycol) medium. Green Chem. 2013, 15, 1030–1034. [Google Scholar] [CrossRef]
- Loska, R.; Volla, C.M.R.; Vogel, P. Iron-Catalyzed Mizoroki-Heck Cross-Coupling Reaction with Styrenes. Adv. Synth. Catal. 2008, 350, 2859–2864. [Google Scholar] [CrossRef]
- Chuayjuljit, S.; Imvittaya, A.; Na-ranong, N.; Potiyaraj, P. Effects of Particle Size and Amount of Carbon Black and Calcium Carbonate on Curing Characteristics and Dynamic Mechanical Properties of Natural Rubber. J. Met. Mater. Miner. 2002, 12, 51–57. [Google Scholar]
- International Organization for Standardization. ISO 1817:2015, Rubber, Vulcanized or Thermoplastic—Determination of Effect of Liquids; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- International Organization for Standardization. ISO 37:2017, Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- International Organization for Standardization. ISO 188:2011, Rubber, Vulcanized or Thermoplastic—Accelerated Ageing and Heat Resistance Tests; International Organization for Standardization: Geneva, Switzerland, 2011. [Google Scholar]
- Ntumba, Y.-H.T.; Prochoń, M. The effect of modified keratin on the thermal properties of a cellulosic–elastomeric material. J. Therm. Anal. Calorim. 2016, 125, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. ISO 868:2003, Plastics and Ebonite—Determination of Indentation Hardness by Means of a durometer (Shore Hardness); International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- Hamed, G.R. Materials and Compounds. In Rubber. How to Design Rubber Components, 3rd ed.; Gent, A.N., Ed.; Hanser Publishers: Munich, Germany, 2012; pp. 11–35. [Google Scholar]
- Sae-Oui, P.; Sirisinha, C.; Thepsuwan, U.; Hatthapanit, K. Dependence of mechanical and aging properties of chloroprene rubber on silica and ethylene thiourea loadings. Eur. Polym. J. 2007, 43, 185–193. [Google Scholar] [CrossRef]
- Kosmalska, A.; Zaborski, M.; Ślusarski, L. Adsorption of curatives and activity of silica toward elastomers. Macromol. Symp. 2003, 194, 269–276. [Google Scholar] [CrossRef]
- Sowińska, A.; Maciejewska, M.; Guo, L.; Delebecq, E. Effect of SILPs on the Vulcanization and Properties of Ethylene–Propylene–Diene Elastomer. Polymers 2020, 12, 1220. [Google Scholar] [CrossRef]
- Choi, S.-S. Improvement of properties of silica-filled natural rubber compounds using polychloroprene. J. Appl. Polym. Sci. 2002, 83, 2609–2616. [Google Scholar] [CrossRef]
- Das, A.; Debnath, S.C.; De, D.; Basu, D.K. Evaluation of physical properties and curing characteristics of silica-filled ethylene-propylene-diene terpolymer in the presence of chloroprene rubber. J. Appl. Polym. Sci. 2004, 93, 196–200. [Google Scholar] [CrossRef]
- Wang, G.; Li, M.; Chen, X. Effects of fillers on mechanical properties of a water-swellable rubber. J. Appl. Polym. Sci. 1999, 72, 577–584. [Google Scholar] [CrossRef]
- Tsubokawa, N. Functionalization of carbon black by surface grafting of polymers. Prog. Polym. Sci. 1992, 17, 417–470. [Google Scholar] [CrossRef]



| Compound Description | CR | CB | SiO2 | TEOA | Me(acac) | ZnO/MgO |
|---|---|---|---|---|---|---|
| Rubber Compounds Filled with CB | ||||||
| ZnO/MgO+CB | 100 | 30 | - | - | - | 5ZnO+4MgO |
| 0.2Mn/3TEOA+CB | 100 | 30 | - | 3 | 0.2 Mn | |
| 0.2Ni/3TEOA+CB | 100 | 30 | - | 3 | 0.2 Ni | |
| 0.2Mn/4TEOA+CB | 100 | 30 | - | 4 | 0.2 Mn | |
| 0.2Ni/4TEOA+CB | 100 | 30 | - | 4 | 0.2 Ni | |
| 0.2Mn/5TEOA+CB | 100 | 30 | - | 5 | 0.2 Mn | |
| 0.2Ni/5TEOA+CB | 100 | 30 | - | 5 | 0.2 Ni | |
| Rubber Compounds Filled with SiO2 | ||||||
| ZnO/MgO+SiO2 | 100 | - | 30 | - | - | 5ZnO+4MgO |
| 0.2Mn/3TEOA+SiO2 | 100 | - | 30 | 3 | 0.2 Mn | |
| 0.2Ni/3TEOA+SiO2 | 100 | - | 30 | 3 | 0.2 Ni | |
| 0.2Mn/4TEOA+SiO2 | 100 | - | 30 | 4 | 0.2 Mn | |
| 0.2Ni/4TEOA+SiO2 | 100 | - | 30 | 4 | 0.2 Ni | |
| 0.2Mn/5TEOA+SiO2 | 100 | - | 30 | 5 | 0.2 Mn | |
| 0.2Ni/5TEOA +SiO2 | 100 | - | 30 | 5 | 0.2 Ni | |
| Rubber Compound | ML (dNm) | ΔM (dNm) | TS2 (min) | TC90 (min) |
|---|---|---|---|---|
| Rubber Compounds Filled with CB | ||||
| ZnO/MgO+CB | 1.4 ± 0.1 | 14.1 ± 1.7 | 0.8 ± 0.1 | 96 ± 2 |
| 0.2Mn/3TEOA+CB | 2.0 ± 0.3 | 18.3 ± 2.0 | 0.6 ± 0.1 | 27 ± 2 |
| 0.2Ni/3TEOA+CB | 2.0 ± 0.4 | 17.1 ± 2.2 | 0.5 ± 0.2 | 25 ± 4 |
| 0.2Mn/4TEOA+CB | 1.3 ± 0.2 | 18.9 ± 2.1 | 0.7 ± 0.2 | 27 ± 3 |
| 0.2Ni/4TEOA+CB | 1.5 ± 0.2 | 18.0 ± 2.0 | 0.6 ± 0.1 | 23 ± 2 |
| 0.2Mn/5TEOA+CB | 1.2 ± 0.3 | 20.2 ± 2.3 | 0.7 ± 0.3 | 25 ± 4 |
| 0.2Ni/5TEOA+CB | 1.7 ± 0.4 | 21.2 ± 2.2 | 0.6 ± 0.2 | 21 ± 3 |
| Rubber Compounds Filled with SiO2 | ||||
| ZnO/MgO+SiO2 | 7.3 ± 0.3 | 24.3 ± 2.1 | 0.4 ± 0.3 | 98 ± 3 |
| 0.2Mn/3TEOA+SiO2 | 6.7 ± 0.4 | 22.5 ± 2.3 | 0.7 ± 0.4 | 96 ± 3 |
| 0.2Ni/3TEOA+SiO2 | 5.7 ± 0.3 | 23.4 ± 2.2 | 0.6 ± 0.3 | 98 ± 4 |
| 0.2Mn/4TEOA+SiO2 | 4.4 ± 0.4 | 16.3 ± 2.4 | 0.7 ± 0.5 | 99 ± 4 |
| 0.2Ni/4TEOA+SiO2 | 5.4 ± 0.5 | 22.5 ± 2.5 | 0.7 ± 0.4 | 89 ± 5 |
| 0.2Mn/5TEOA+SiO2 | 5.4 ± 0.3 | 19.6 ± 2.3 | 0.6 ± 0.3 | 89 ± 3 |
| 0.2Ni/5TEOA+SiO2 | 5.3 ± 0.4 | 20.6 ± 2.3 | 0.7 ± 0.4 | 89 ± 4 |
| Vulcanizate | SE50 (MPa) | SE100 (MPa) | SE150 (MPa) | TS (MPa) | EB (%) |
|---|---|---|---|---|---|
| Vulcanizates Filled With CB | |||||
| ZnO/MgO+CB | 2.7 ± 0.2 | 5.7 ± 0.2 | 10.2 ± 0.5 | 22 ± 0.9 | 288 ± 11 |
| 0.2Mn/3TEOA+CB | 2.9 ± 0.3 | 7.5 ± 1.0 | 13.9 ± 0.7 | 20.6 ± 1.2 | 189 ± 9 |
| 0.2Ni/3TEOA+CB | 3.2 ± 0.4 | 8.5 ± 1.0 | 15.0 ± 0.8 | 18.5 ± 0.6 | 167 ± 7 |
| 0.2Mn/4TEOA+CB | 2.6 ± 0.3 | 7.1 ± 0.4 | 14.2 ± 0.9 | 16.2 ± 1.8 | 162 ± 12 |
| 0.2Ni/4TEOA+CB | 2.4 ± 0.4 | 6.4 ± 0.7 | 12.4 ± 0.9 | 17.3 ± 0.7 | 176 ± 12 |
| 0.2Mn/5TEOA+CB | 3.2 ± 0.2 | 7.8 ± 0.7 | 15.8 ± 0.6 | 17 ± 1.8 | 156 ± 11 |
| 0.2Ni/5TEOA+CB | 3.5 ± 0.3 | 9.5 ± 0.4 | - | 16 ± 1.4 | 138 ± 9 |
| Vulcanizates Filled With SiO2 | |||||
| ZnO/MgO+SiO2 | 8.8 ± 0.5 | - | - | 13 ± 1.0 | 91 ± 13 |
| 0.2Mn/3TEOA+SiO2 | 3.8 ± 0.4 | 7.2 ± 0.3 | 9.4 ± 0.5 | 11.8 ± 0.6 | 198 ± 16 |
| 0.2Ni/3TEOA+SiO2 | 3.4 ± 0.5 | 5.7 ± 0.7 | 7.5 ± 0.6 | 10.3 ± 0.4 | 234 ± 20 |
| 0.2Mn/4TEOA+SiO2 | 4.3 ± 0.3 | 6.4 ± 0.3 | 8.5 ± 0.4 | 12.7 ± 0.8 | 302 ± 38 |
| 0.2Ni/4TEOA+SiO2 | 4.9 ± 0.7 | 8.8 ± 2.3 | 10.9 ± 1.1 | 12.4 ± 0.6 | 198 ± 56 |
| 0.2Mn/5TEOA+SiO2 | 3.2 ± 0.3 | 4.1 ± 0.4 | 5.1 ± 0.4 | 11.4 ± 1.9 | 325 ± 40 |
| 0.2Ni/5TEOA+SiO2 | 5.8 ± 0.4 | 8.5 ± 0.6 | 10.1 ± 0.6 | 13.6 ± 0.4 | 220 ± 19 |
| Vulcanizate | Af (-) | H (ShA) |
|---|---|---|
| Vulcanizates Filled with CB | ||
| ZnO/MgO+CB | 1.0 ± 0.01 | 68 ± 1.7 |
| 0.2Mn/3TEOA+CB | 0.6 ± 0.02 | 59 ± 0.4 |
| 0.2Ni/3TEOA+CB | 0.8 ± 0.01 | 61 ± 0.5 |
| 0.2Mn/4TEOA+CB | 0.6 ± 0.03 | 60 ± 0.4 |
| 0.2Ni/4TEOA+CB | 0.6 ± 0.02 | 59 ± 0.9 |
| 0.2Mn/5TEOA+CB | 0.6 ± 0.01 | 63 ± 0.3 |
| 0.2Ni/5TEOA+CB | 0.5 ± 0.02 | 63 ± 0.4 |
| Vulcanizates Filled with SiO2 | ||
| ZnO/MgO+SiO2 | 0.3 ± 0.02 | 84 ± 0.7 |
| 0.2Mn/3TEOA+SiO2 | 0.6 ± 0.02 | 71 ± 1.5 |
| 0.2Ni/3TEOA+SiO2 | 0.4 ± 0.03 | 76 ± 1.6 |
| 0.2Mn/4TEOA+SiO2 | 0.2 ± 0.04 | 74 ± 0.8 |
| 0.2Ni/4TEOA+SiO2 | 0.3 ± 0.07 | 79 ± 0.75 |
| 0.2Mn/5TEOA+SiO2 | 0.3 ± 0.05 | 75 ± 0.9 |
| 0.2Ni/5TEOA+SiO2 | 0.3 ± 0.03 | 75 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziemidkiewicz, A.; Maciejewska, M. Manganese and Nickel Acetylacetonates as Curatives for Chloroprene Rubber Based on Heck’s Reaction. Materials 2021, 14, 807. https://doi.org/10.3390/ma14040807
Dziemidkiewicz A, Maciejewska M. Manganese and Nickel Acetylacetonates as Curatives for Chloroprene Rubber Based on Heck’s Reaction. Materials. 2021; 14(4):807. https://doi.org/10.3390/ma14040807
Chicago/Turabian StyleDziemidkiewicz, Anna, and Magdalena Maciejewska. 2021. "Manganese and Nickel Acetylacetonates as Curatives for Chloroprene Rubber Based on Heck’s Reaction" Materials 14, no. 4: 807. https://doi.org/10.3390/ma14040807
APA StyleDziemidkiewicz, A., & Maciejewska, M. (2021). Manganese and Nickel Acetylacetonates as Curatives for Chloroprene Rubber Based on Heck’s Reaction. Materials, 14(4), 807. https://doi.org/10.3390/ma14040807







