Polyurethane Foams for Domestic Sewage Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. PUR Foams Used in the Study
2.2. Determination of PUR Foam Properties
- flotation test
- water absorption (WA)
- apparent density as per ISO 845 standard [38].
- open porosity by hydrostatic method
2.3. Microscopic Examination
2.4. Content of the Adsorbed Biomass and SRT Sludge Retention Time
2.5. Lab-Scale Model
3. Results and Discussion
3.1. Hydrophobic Properties of the Foams
3.2. Physical Properties of the Foams
3.3. Microscopic Evaluation of the Foams
3.3.1. Cell Morphology of PUR Foams
3.3.2. Pore Size, Porosity and Specific Surface Area
3.3.3. Content of the Adsorbed Biomass and Sludge Retention Time (SRT)
3.3.4. Structural Properties of the Foams with Biomass
3.3.5. Regeneration Process
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havens, P.L.; Rase, H.F. Reusable immobilized enzyme/polyurethane sponge for removal and detoxification of localized organophosphate pesticide spills. Ind. Eng. Chem. Res. 1993, 32, 2254–2258. [Google Scholar] [CrossRef]
- Moe, W.M.; Irvine, R.L. Polyurethane Foam Medium for Biofiltration. I: Characterization. J. Environ. Eng. 2000, 126, 815–825. [Google Scholar] [CrossRef]
- Wang, J.; Van Tittelboom, K.; De Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Partovinia, A.; Rasekh, B. Review of the immobilized microbial cell systems for bioremediation of petroleum hy-drocarbons polluted environments. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1–38. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Sehar, S.; Naz, I. Role of the Biofilms in Wastewater Treatment. In Microbial Biofilms—Importance and Applications; IntechOpen: London, UK, 2016; pp. 121–144. [Google Scholar]
- Romaškevič, T.; Budrienė, S.; Pielichowski, K.; Pielichowski, J. Application of polyurethane-based materials for immobilization of enzymes and cells: A review. Chemija 2006, 17, 74–89. [Google Scholar]
- Oyarzun, P.; Alarcón, L.; Calabriano, G.; Bejarano, J.; Nuñez, D.; Ruiz-Tagle, N.; Urrutia, H. Trickling filter tech-nology for biotreatment of nitrogenous compounds emitted in exhaust gases from fishmeal plants. J. Environ. Manag. 2019, 232, 165–170. [Google Scholar] [CrossRef]
- Mousavi, S.A.R.; Ibrahim, S.; Aroua, M.K.; Ghafari, S. Bioelectrochemical denitrification: A review. Int. J. Chem. Environ. Eng. 2011, 2, 140–146. [Google Scholar]
- Chu, L.; Wang, J. Comparison of polyurethane foam and biodegradable polymer as carriers in moving bed biofilm reactor for treating wastewater with a low C/N ratio. Chemosphere 2011, 83, 63–68. [Google Scholar] [CrossRef]
- Varesche, M.B.; Zaiat, M.; Vieira, L.G.T.; Vazoller, R.F.; Foresti, E. Microbial colonization of polyurethane foam matrices in horizontal-flow anaerobic immobilized-sludge reactor. Appl. Microbiol. Biotechnol. 1997, 48, 534–538. [Google Scholar] [CrossRef]
- Shareefdeen, Z.; Baltzis, B.C.; Oh, Y.-S.; Bartha, R. Biofiltration of methanol vapor. Biotechnol. Bioeng. 1993, 41, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, N.; Hewawasam, C.; Hatamoto, M.; Yamaguchi, T.; Harada, H.; Araki, N. Downflow Hanging Sponge System: A Self-Sustaining Option for Wastewater Treatment. In Wastewater Treatment; IntechOpen: London, UK, 2020; pp. 1–20. [Google Scholar] [CrossRef]
- Tawfik, A.; El-Gohary, F.; Ohashi, A.; Harada, H. The influence of physical–chemical and biological factors on the removal of faecal coliform through down-flow hanging sponge (DHS) system treating UASB reactor effluent. Water Res. 2006, 40, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, A.; Wahab, R.A.; Al-Asmer, A.; Matary, F. Effect of hydraulic retention time on the performance of down-flow hanging sponge system treating grey wastewater. Bioprocess Biosyst. Eng. 2011, 34, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, N.; Hatamoto, M.; Ali, M.; Jayaswal, K.; Iguchi, A.; Okubo, T.; Takahashi, M.; Kubota, K.; Tagawa, T.; Uemura, S.; et al. Characterization of sludge properties for sewage treatment in a practical-scale down-flow hanging sponge reactor: Oxygen consumption and removal of organic matter, ammonium, and sulfur. Water Sci. Technol. 2018, 77, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Oshiki, M.; Ito, T.; Segawa, T.; Hatamoto, M.; Kato, T.; Yamaguchi, T.; Kubota, K.; Takahashi, M.; Iguchi, A.; et al. Removal of human pathogenic viruses in a down-flow hanging sponge (DHS) reactor treating municipal wastewater and health risks associated with utilization of the effluent for agricultural irrigation. Water Res. 2017, 110, 389–398. [Google Scholar] [CrossRef]
- Onodera, T.; Matsunaga, K.; Kubota, K.; Taniguchi, R.; Harada, H.; Syutsubo, K.; Okubo, T.; Uemura, S.; Araki, N.; Yamada, M.; et al. Characterization of the retained sludge in a down-flow hanging sponge (DHS) reactor with emphasis on its low excess sludge production. Bioresour. Technol. 2013, 136, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Onodera, T.; Tandukar, M.; Sugiyana, D.; Uemura, S.; Ohashi, A.; Harada, H. Development of a sixth generation down-flow hanging sponge (DHS) reactor using rigid sponge media. Bioresour. Technol. 2014, 152, 93. [Google Scholar] [CrossRef]
- Onodera, T.; Yoochatchaval, W.; Sumino, H.; Mizuochi, M.; Okadera, T.; Fujita, T.; Banjongproo, P.; Syutsubo, K. Pilot-scale experiment of down-flow hanging sponge for direct treatment of low-strength municipal wastewater in Bangkok, Thailand. Bioprocess Biosyst. Eng. 2014, 37, 2281. [Google Scholar] [CrossRef]
- Okubo, T.; Onodera, T.; Uemura, S.; Yamaguchi, T.; Ohashi, A.; Harada, H. On-site evaluation of the performance of a full-scale down-flow hanging sponge reactor as a post-treatment process of an up-flow anaerobic sludge blanket reactor for treating sewage in India. Bioresour. Technol. 2015, 194, 156–164. [Google Scholar] [CrossRef]
- Maharjan, N.; Kuroda, K.; Dehama, K.; Hatamoto, M.; Yamaguchi, T. Development of slow sponge sand filter (SpSF) as a post-treatment of UASB-DHS reactor effluent treating municipal wastewater. Water Sci. Technol. 2016, 74, 65–72. [Google Scholar] [CrossRef]
- Guillén, J.A.S. Autotrophic Nitrogen Removal from Low Concentrated Effluents: Study of System Configurations and Operational Features for Post-Treatment of Anaerobic Effluents; CRC Press: Leiden, The Netherlands, 2017. [Google Scholar]
- Miyaoka, Y.; Yoochatchaval, W.; Sumino, H.; Banjongproo, P.; Yamaguchi, T.; Onodera, T.; Okadera, T.; Syutsubo, K. Evaluation of the process performance of a down-flow hanging sponge reactor for direct treatment of domestic wastewater in Bangkok, Thailand. J. Environ. Sci. Health Part A 2017, 52, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Machdar, I.; Onodera, T.; Syutsubo, K.; Ohashi, A. Effects of sponge pore-size on the performance of a down-flow hanging sponge reactor in post-treatment of effluent from an anaerobic reactor treating domestic wastewater. Sustain. Environ. Res. 2018, 28, 282–288. [Google Scholar] [CrossRef]
- Bundy, C.A.; Wu, N.; Jong, M.-C.; Edwards, S.R.; Ahammad, Z.S.; Graham, D.W. Enhanced denitrification in Downflow Hanging Sponge reactors for decentralised domestic wastewater treatment. Bioresour. Technol. 2017, 226, 1–8. [Google Scholar] [CrossRef]
- Jowett, C. Treatment Technologies for Communal Wastewater Systems 1. In Proceedings of the WEAO Small Community Wastewater Systems: Challenges &Sustainable Solutions”, WEAO, Milton, ON, Canada, 22 October 2015. [Google Scholar]
- Available online: https://www.environmental-expert.com/products/water-warriors-trickling-filter-media-728232 (accessed on 12 November 2020).
- Nguyen, H.T.; Le, H.T.T.; Dinh, N.T.; Phan, T.D. A research on the performance of down-flow hanging sponge (DHS) reactor treating domestic wastewater. Vietnam. J. Sci. Technol. 2018, 56, 482. [Google Scholar] [CrossRef] [Green Version]
- Dacewicz, E.; Wodnej, W.I.; Środowiska, G.K.I.S.G. Impact of the sponge structure of a multilayer sand filter on the treatment of domestic sewage with an increased content of ammonia nitrogen. Acta Sci. Pol. Form. Circumiectus 2020, 19, 53–75. [Google Scholar] [CrossRef]
- Available online: http://www.pianka.com.pl/sites/default/files/pianki_n.pdf (accessed on 23 September 2020).
- Bedla, D.; Dacewicz, E. Data clustering analysis in the assessment of wastes using in the sewage filtration. J. Water Land Dev. 2019, 41, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Dacewicz, E. The Application of Biofiltration with Polyurethane Foams for Domestic Sewage Treatment. J. Ecol. Eng. 2019, 20, 131–140. [Google Scholar] [CrossRef]
- Dacewicz, E.; Chmielowski, K. Application of multidimensional clustering for an assessment of pollutants removal from domestic wastewater using a filter with a plastic waste filling. J. Water Process. Eng. 2019, 29, 100794. [Google Scholar] [CrossRef]
- Dacewicz, E.; Jurik, L. Application of a double layer sand filter with a PUR foams layer in the treatment of domestic sewage with an increased content of ammonia nitrogen. Acta Sci. Pol. Form. Circumiectus 2019, 2, 67–81. [Google Scholar] [CrossRef]
- Chmielowski, K.; Dacewicz, E.; Bedla, D.; Mazur, R. Zastosowanie odpadów z tworzyw sztucznych w biofiltrach do oczyszczania ścieków bytowych. Przemysł Chem. 2018, 97, 1456–1459. [Google Scholar] [CrossRef]
- ISO 845:(en)—Cellular Plastics and Rubbers—Determination of Apparent Density; International Organization of Standards: Geneva, Switzerland, 2006.
- Członka, S.; Strąkowska, A.; Strzelec, K.; KAIRYTĖ, A.; Kremensas, A. Bio-Based Polyurethane Composite Foams with Improved Mechanical, Thermal, and Antibacterial Properties. Materials 2020, 13, 1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dacewicz, E. Application of the filtration bed with a foam-sand filling for treatment of sewage with an elevated concentration of ammonia nitrogen. Infrastruct. Ecol. Rural Areas 2019, 1, 165–180. [Google Scholar]
- Dacewicz, E. Waste assessment decision support systems used for domestic sewage treatment. J. Water Process. Eng. 2019, 31, 100885. [Google Scholar] [CrossRef]
- Dang-Vu, T.; Hupka, J.; Drzymala, J. Impact of roughness on hydrophobicity of particles measured by the Washburn method. Physicochem. Probl. Miner. Process. 2006, 40, 45–52. [Google Scholar]
- Szczypka, A. Wastewater Treatment Process with the Use of Unconventional Filling of a Biological Reactor (Dacewicz E. Supervisor). Master’s Thesis, University of Agriculture, Kraków, Poland, 2018. [Google Scholar]
- Beas, R.E.Y.; Kujawa-Roeleveld, K.; Van Lier, J.B.; Zeeman, G. A downflow hanging sponge (DHS) reactor for faecal coliform removal from an upflow anaerobic sludge blanket (UASB) effluent. Water Sci. Technol. 2015, 72, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.; Tummala, A.; Kapadia, C.; Dandamudi, M.; Belovich, J.M. Procedure to Quantify Biofilm Activity on Carriers Used in Wastewater Treatment Systems. J. Environ. Eng. 2006, 132, 1422–1430. [Google Scholar] [CrossRef] [Green Version]
- Uemura, S.; Suzuki, S.; Maruyama, Y.; Harada, H. Direct treatment of settled sewage by DHS reactors with different sizes of sponge support media. Int. J. Environ. Res. 2012, 6, 25–32. [Google Scholar]
- Uemura, S.; Okubo, T.; Maeno, K.; Takahashi, M.; Kubota, K.; Harada, H. Evaluation of water distribution and oxygen mass transfer in sponge support media for a down-flow hanging sponge reactor. Int. J. Environ. Res. 2016, 6, 25–32. [Google Scholar]
- Devinny, J.S.; Deshusses, M.A.; Webster, T.S. Biofiltration for Air Pollution Control; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Chmielowski, K.; Pawełek, J.; Dacewicz, E. Treatment of high strength domestic sewage on filters filled with polyurethane foam with addition of effective microorganisms. Arch. Environ. Prot. 2020, 46, 21–32. [Google Scholar]
- Uemura, S.; Harada, H.; Fang, H.H.P. Application of UASB Technology for Sewage Treatment with a Novel Post-treatment Process. In Environmental Anaerobic Technology; World Scientific: Singapore, 2010; pp. 91–112. [Google Scholar] [CrossRef]
- Tandukar, M.; Machdar, I.; Uemura, S.; Ohashi, A.; Harada, H. Potential of a Combination of UASB and DHS Reactor as a Novel Sewage Treatment System for Developing Countries: Long-Term Evaluation. J. Environ. Eng. 2006, 132, 166–172. [Google Scholar] [CrossRef]
- Tawfik, A.; El-Zamel, T.; Herrawy, A.; El-Taweel, G. Fate of parasites and pathogenic bacteria in an anaerobic hybrid reactor followed by downflow hanging sponge system treating domestic wastewater. Environ. Sci. Pollut. Res. 2015, 22, 12235–12245. [Google Scholar] [CrossRef] [PubMed]
- Araki, N.; Ohashi, A.; Machdar, I.; Harada, H. Behaviors of nitrifiers in a novel biofilm reactor employing hanging sponge-cubes as attachment site. Water Sci. Technol. 1999, 39, 23. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Dharmawan, F.; Palmer, C.G. Roles of polyurethane foam in aerobic moving and fixed bed bioreactors. Bioresour. Technol. 2010, 101, 1435–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]





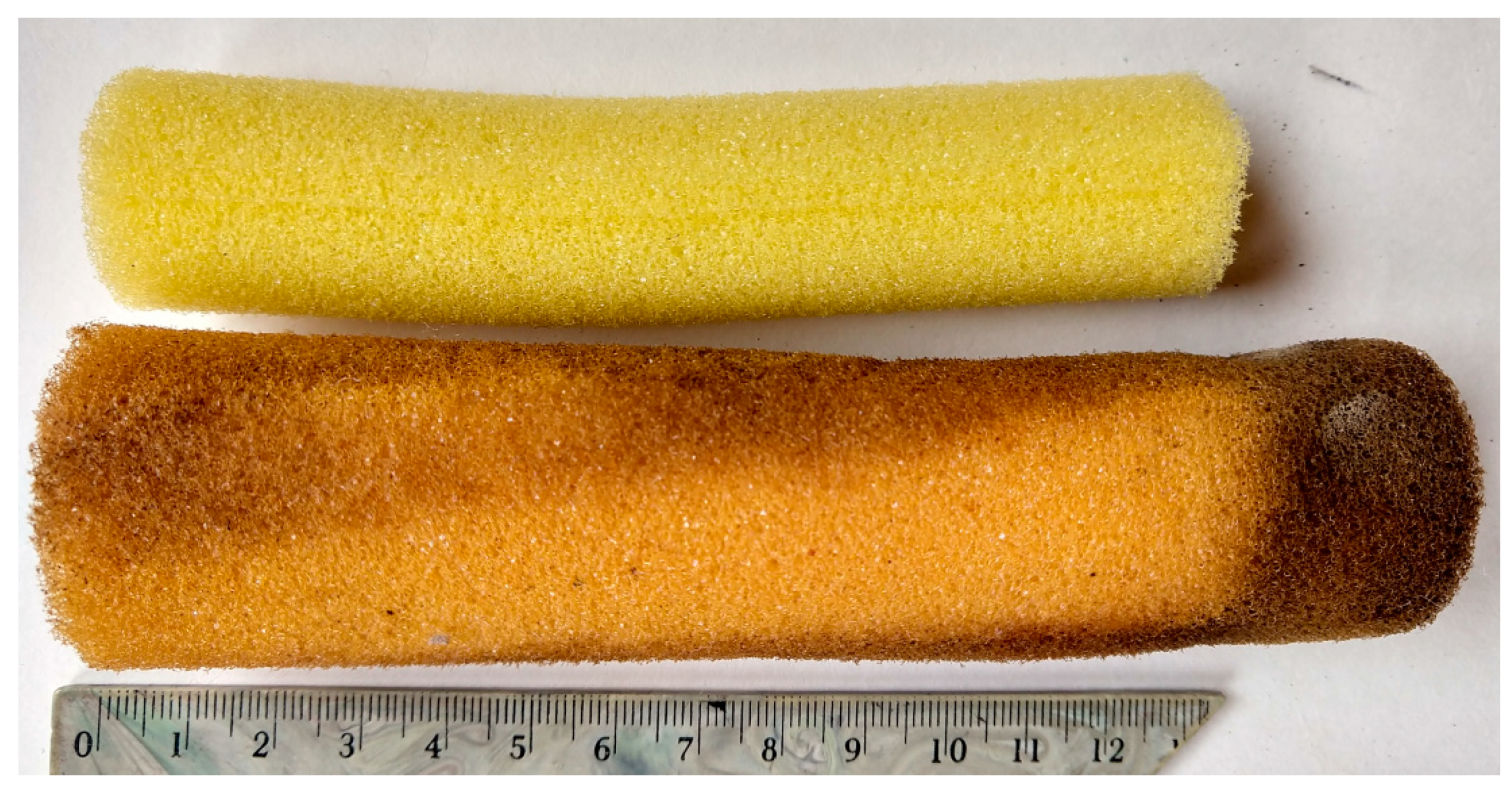

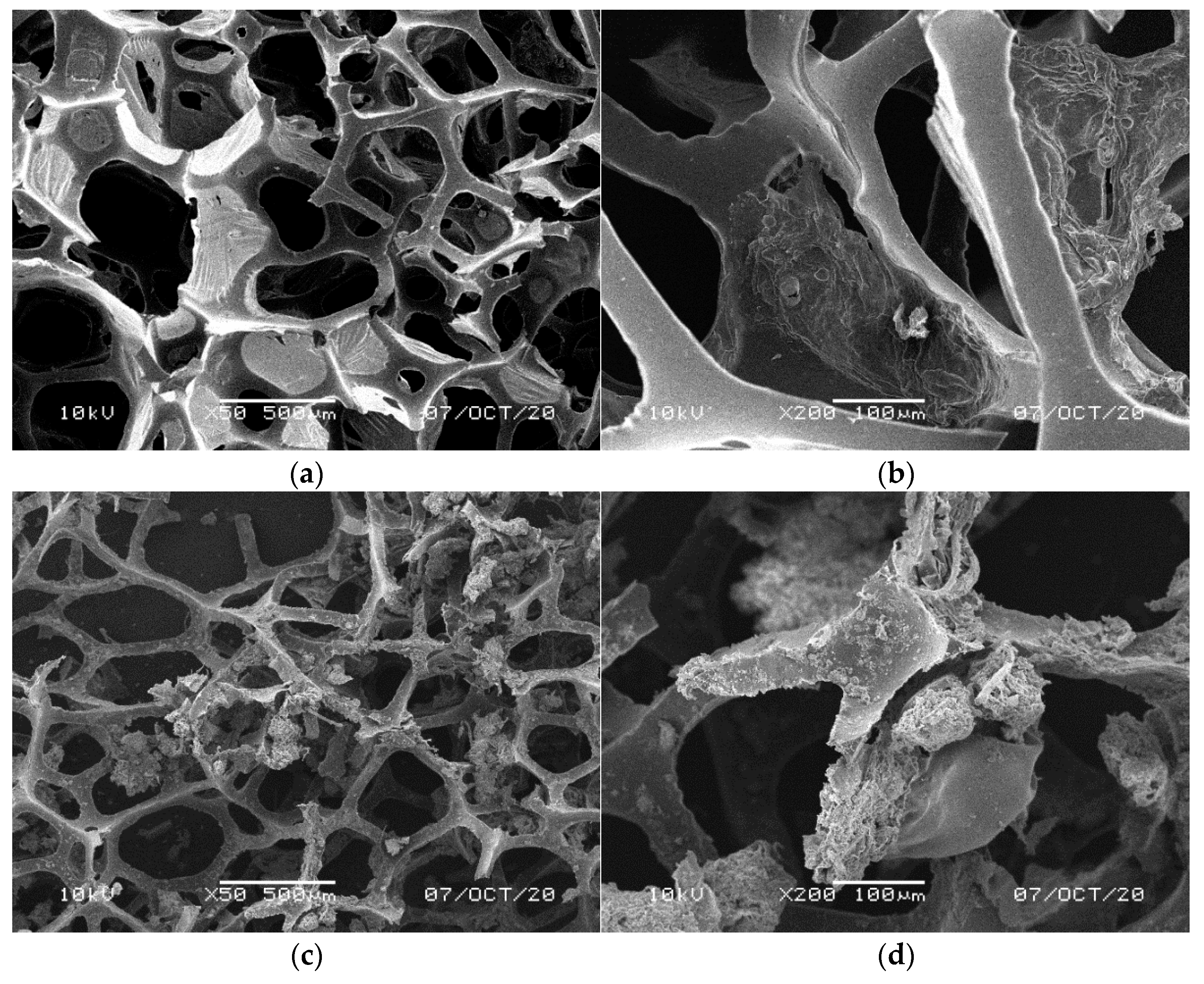








| Type | Content in the Mixture [%] | Apparent Density [kg m−3] | Compressive Strength * [kPa] | Basic Color |
|---|---|---|---|---|
| N 1418 | 3.8 | 13.5–15.5 | 1.6–2.3 | white |
| N 1819 | 3.7 | 16.0–19.0 | 1.2–2.0 | white |
| N 2030 | 16.5 | 17.0–21.0 | 2.7–3.6 | white |
| N 2121 | 27.5 | 19.5–22.5 | 1.8–2.6 | yellow-green |
| N 2538 | 8.6 | 22.0–26.0 | 3.3–4.6 | lilac |
| N 2838 | 4.2 | 24.0–28.0 | 3.5–4.5 | orange |
| N 3030 | 21.6 | 27.5–31.0 | 2.7–3.7 | bluish-green |
| N 3543 | 8.5 | 32.0–36.0 | 3.8–5.0 | bluish-green |
| N 3050 | 3.0 | 27.0–31.0 | 4.4–5.8 | bluish-green |
| N 3843 | 2.6 | 34.0–38.0 | 4.0–5.3 | blue |
| Foam Color | Time After Which the Foam Is Fully Immersed [h] | Time After Which the Foam Sinks [h] |
|---|---|---|
| Brand new cylindrical foams (NF) | ||
| Green | >144 | >144 |
| Waste foams of random shapes (WF) | ||
| Bluish-green | 16 | 72 |
| Yellow-green | 16 | 56 |
| Lilac | 80 | 130 |
| Orange | 24 | 104 |
| Foam Color | Water Absorption [%] | Apparent Density [g/L] | Open Porosity [%] |
|---|---|---|---|
| Brand new cylindrical foams (NF) | |||
| Green | 55.0 ± 1.16 | 10.0 ± 0.22 | 79.5 ± 1.83 |
| Waste foams of random shapes (WF) | |||
| Bluish-green | 95.5 ± 2.24 | 13.62 ± 0.27 | 95.00 ± 2.64 |
| Yellow-green | 95.4 ± 2.25 | 15.89 ± 0.33 | 95.05 ± 2.66 |
| Lilac | 89.0 ± 2.23 | 15.44 ± 0.36 | 93.38 ± 2.80 |
| Orange | 95.6 ± 2.29 | 9.73 ± 0.21 | 94.91 ± 2.75 |
| Foam Color | Content in the Mixture [%] | Foam Pore Content [%] | Surface Area [m2/m3] |
|---|---|---|---|
| Brand new cylindrical foams (NF) | |||
| Green | 38.9 | 75.3 ± 2.1 | 487 ±17.6 |
| Waste foams of random shapes (WF) | |||
| Bluish-green | 33.1 | 62.6 ± 1.9 | 619 ± 24.8 |
| Yellow-green | 27.5 | 63.5 ± 3.5 | 602 ± 34.9 |
| Lilac | 8.6 | 53.0 ± 2.9 | 266 ± 13.3 |
| Orange | 4.2 | 61.3 ± 5.5 | 552 ± 46.9 |
| Foam Color | Feret Diameter [mm] | Total Suspended Solids I/II Segment [g/L foam] | Biomass Content I/II Segment [g/L foam] | Percentage Biomass Content [%] |
|---|---|---|---|---|
| Brand new cylindrical foams | ||||
| Green | 0.44 ± 0.01 | 0.1792 | 0.1115 | 62.2 |
| Waste foams of random shapes | ||||
| Bluish-green | 0.62 ± 0.03 | 0.154/1.912 | 0.063/1.663 | 40.9/87.0 |
| Yellow-green | 0.67 ± 0.04 | 0.0929/0.305 | 0.056/0.245 | 60.3/80.3 |
| Lilac | 0.50 ± 0.02 | 0.2300/5.667 | 0.1067/4.867 | 46.4/85.9 |
| Orange | 1.53 ± 0.012 | 0.0643/0.4114 | 0.0371/0.333 | 57.7/80.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dacewicz, E.; Grzybowska-Pietras, J. Polyurethane Foams for Domestic Sewage Treatment. Materials 2021, 14, 933. https://doi.org/10.3390/ma14040933
Dacewicz E, Grzybowska-Pietras J. Polyurethane Foams for Domestic Sewage Treatment. Materials. 2021; 14(4):933. https://doi.org/10.3390/ma14040933
Chicago/Turabian StyleDacewicz, Ewa, and Joanna Grzybowska-Pietras. 2021. "Polyurethane Foams for Domestic Sewage Treatment" Materials 14, no. 4: 933. https://doi.org/10.3390/ma14040933
APA StyleDacewicz, E., & Grzybowska-Pietras, J. (2021). Polyurethane Foams for Domestic Sewage Treatment. Materials, 14(4), 933. https://doi.org/10.3390/ma14040933







