Detection and Imaging of Damages and Defects in Fibre-Reinforced Composites by Magnetic Resonance Technique
Abstract
1. Introduction
2. Composite Laminates and Test Coupons
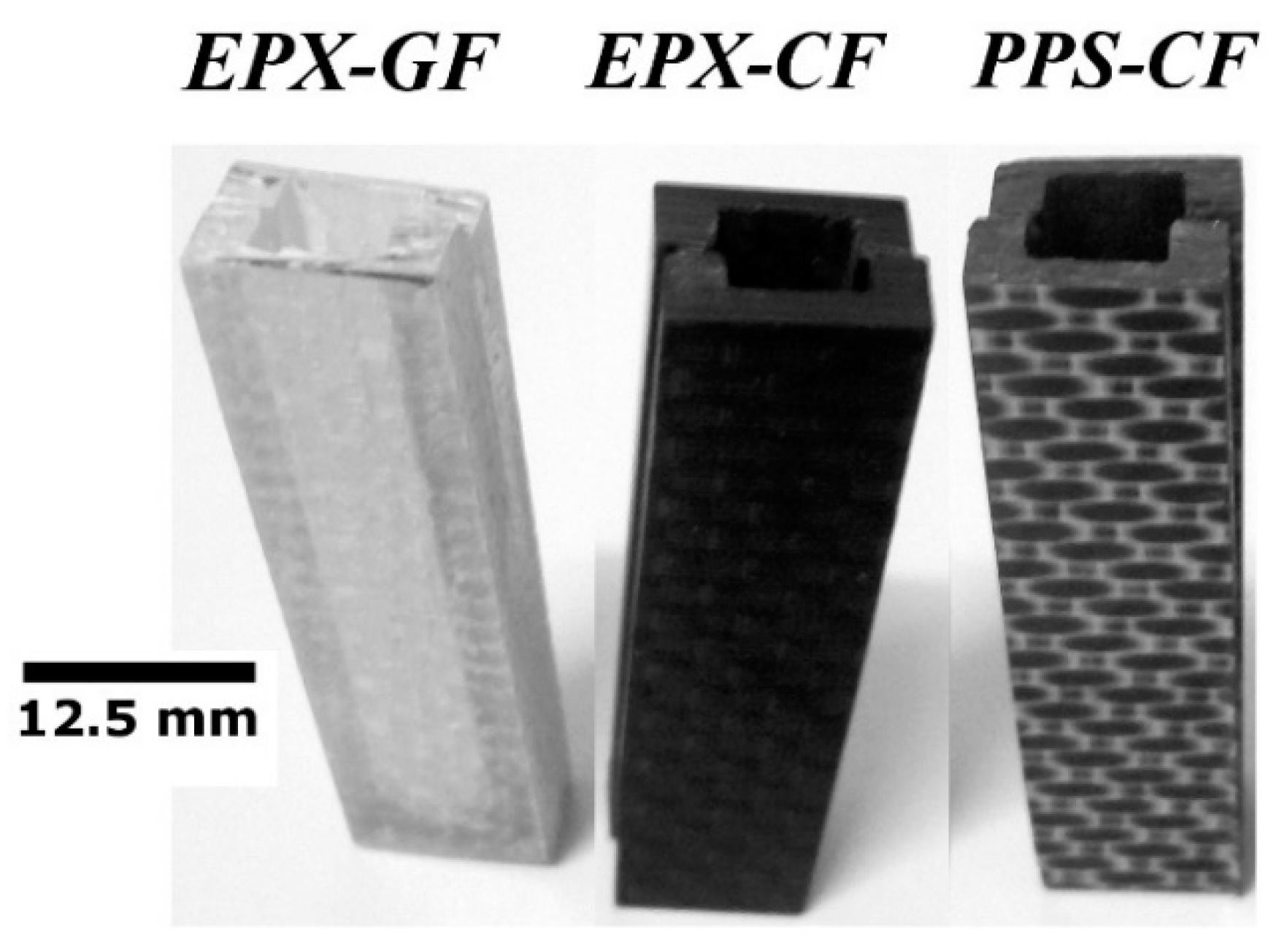
2.1. FRP Laminates
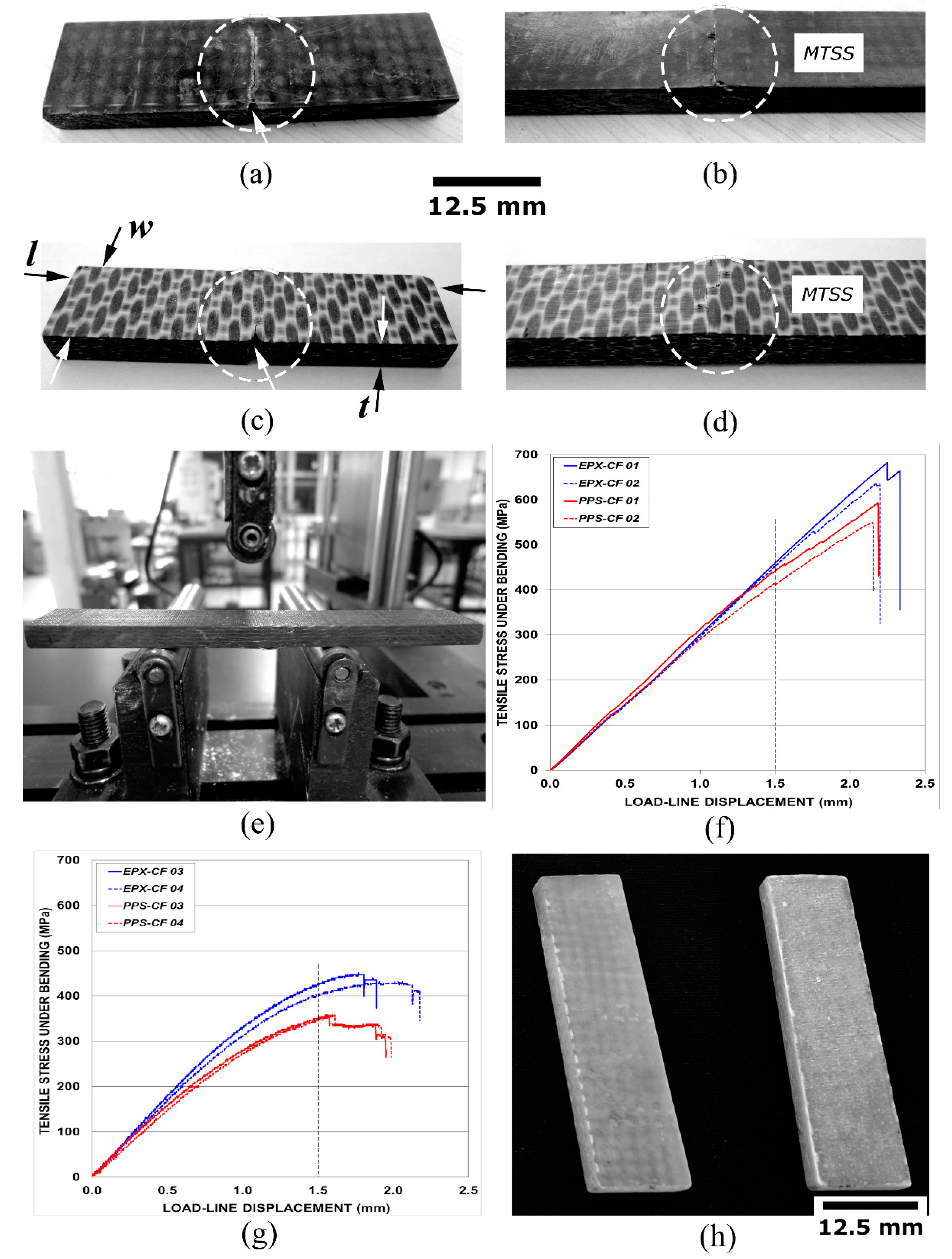
2.2. Purposely Damaged Test Specimens
2.3. Defectively Manufactured Test Coupons
3. MRI Equipment and Protocols
4. Experimental Procedures
4.1. Electrical Conductivity Measurements
4.2. Void and Fibre Contents
4.3. Immersion in Proton-Rich Liquid Environment
4.4. MRI Scanning
4.4.1. Electromagnetic Interference (EMI)
4.4.2. Defective and Damaged Specimens
4.5. Stereoscopic Analysis
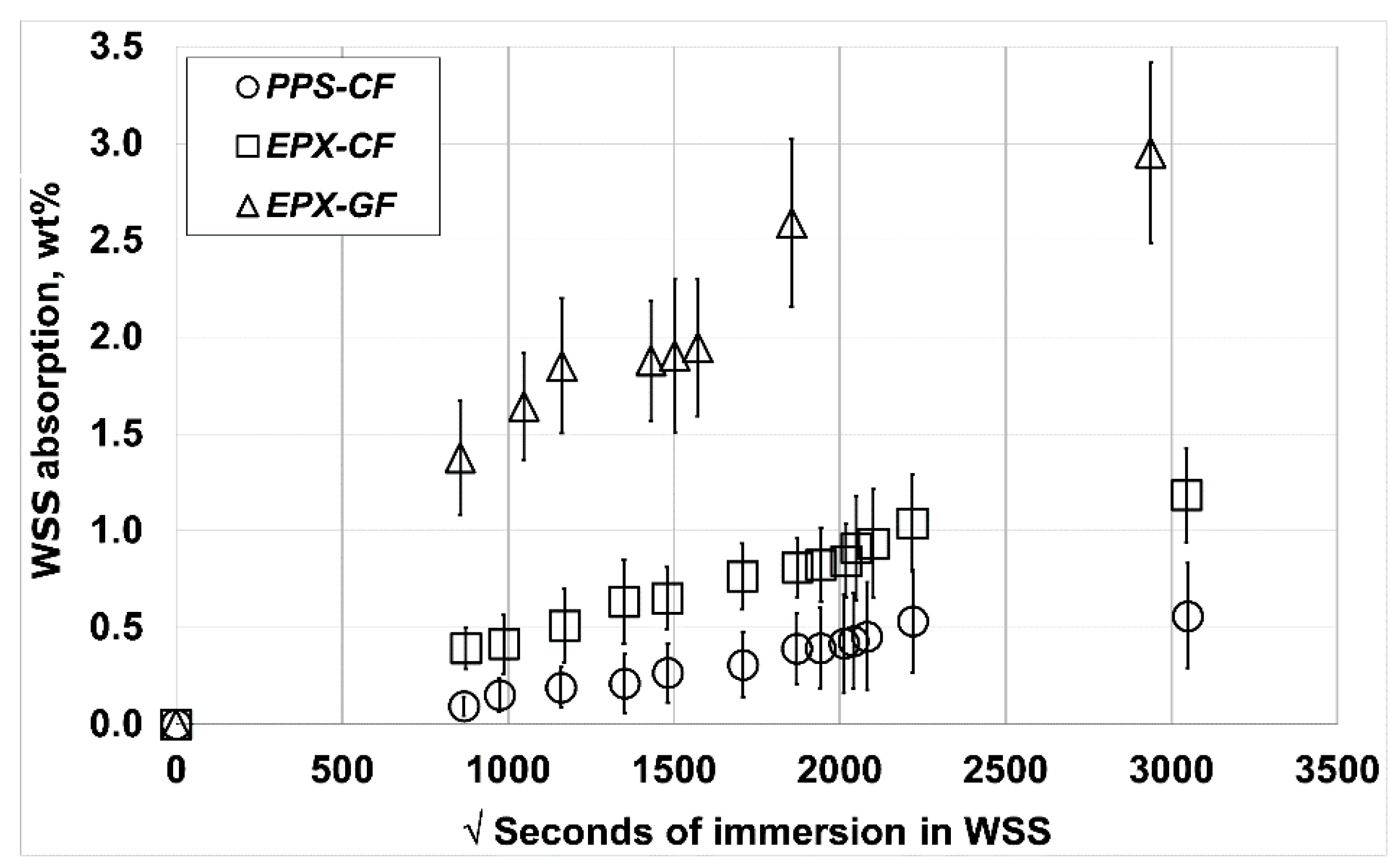
4.6. WSS Absorption
5. Results and Discussion
5.1. Electrical Conductivity Measurements
5.2. Absorbed Liquid by Immersion
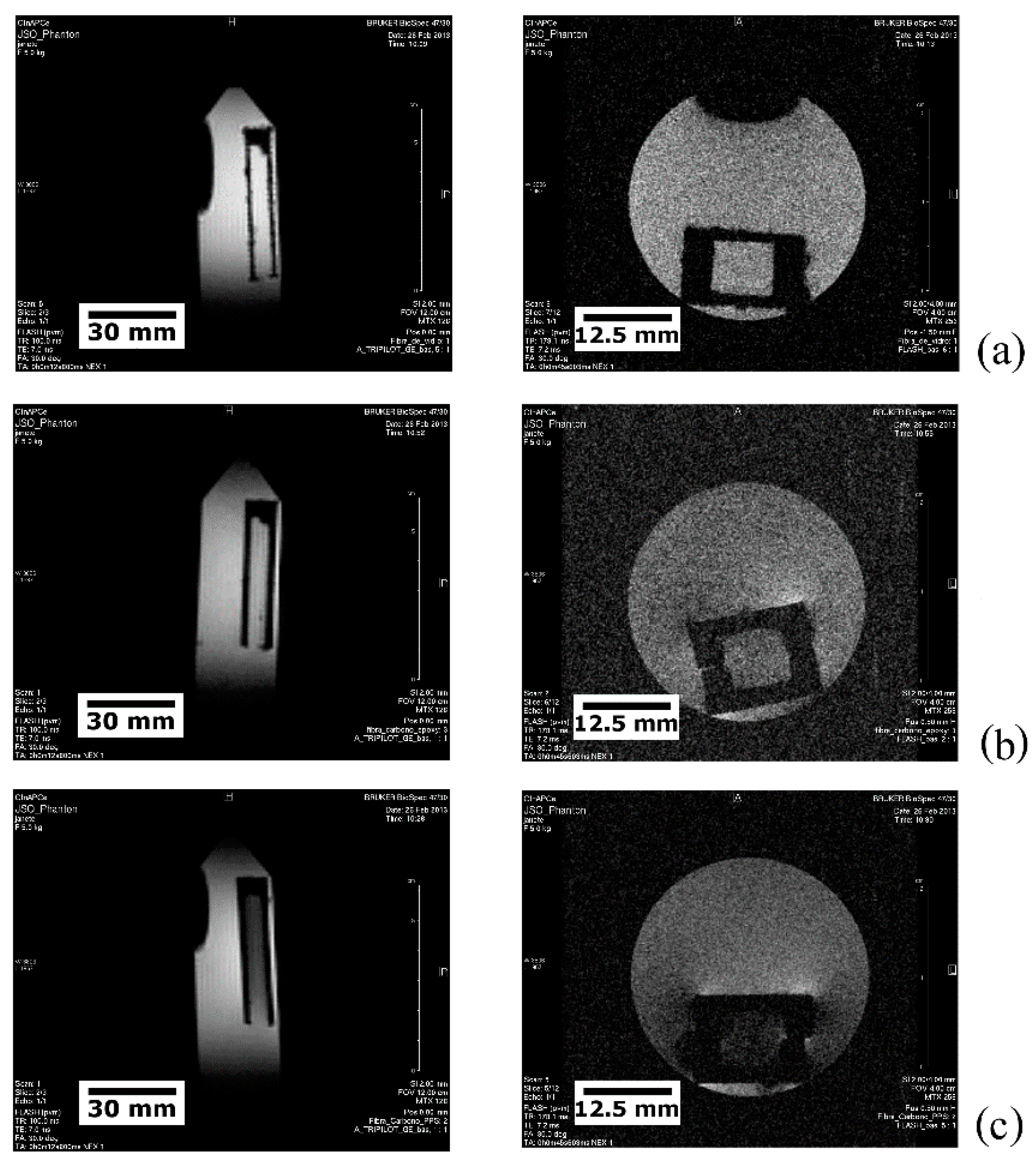
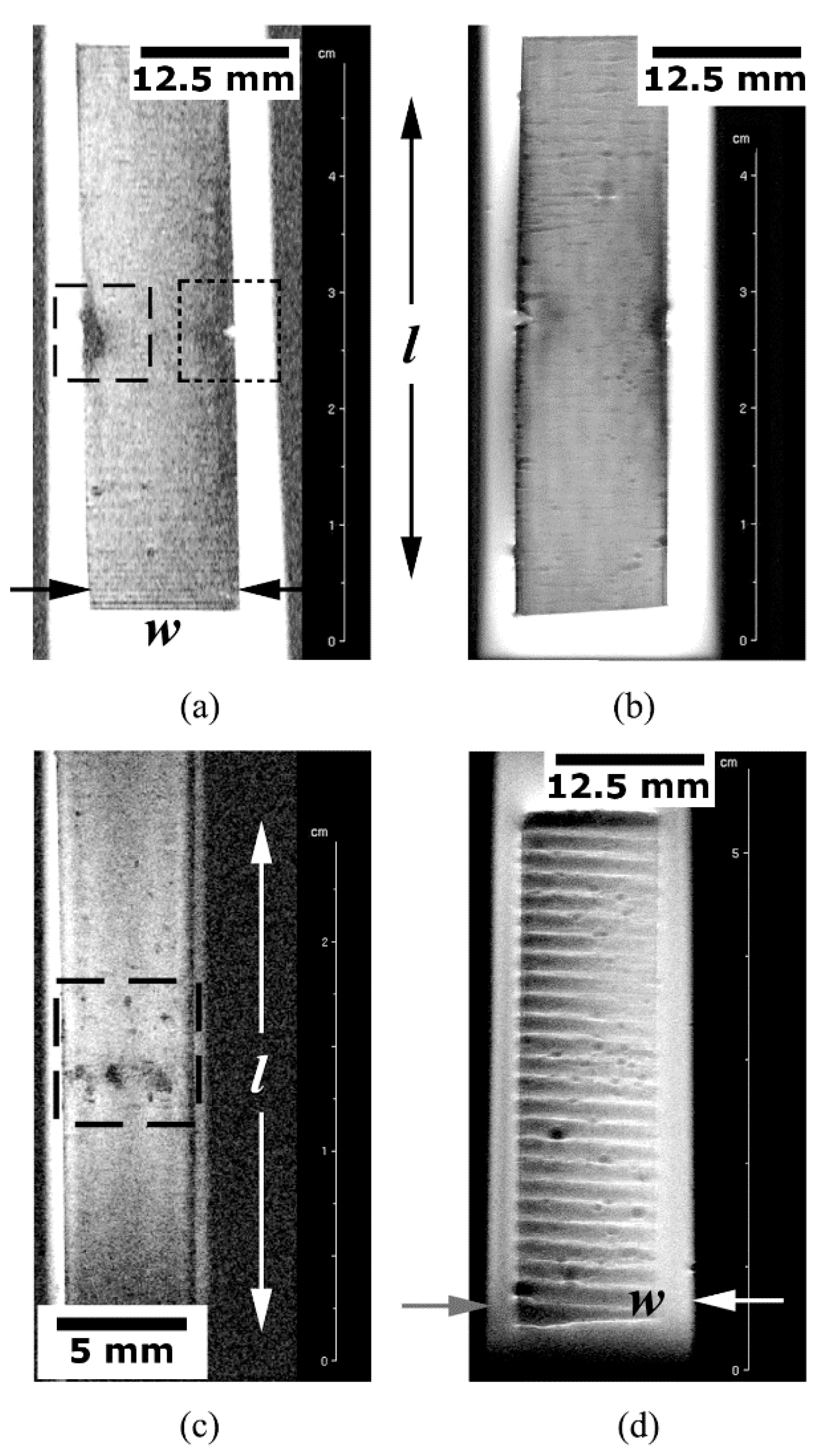
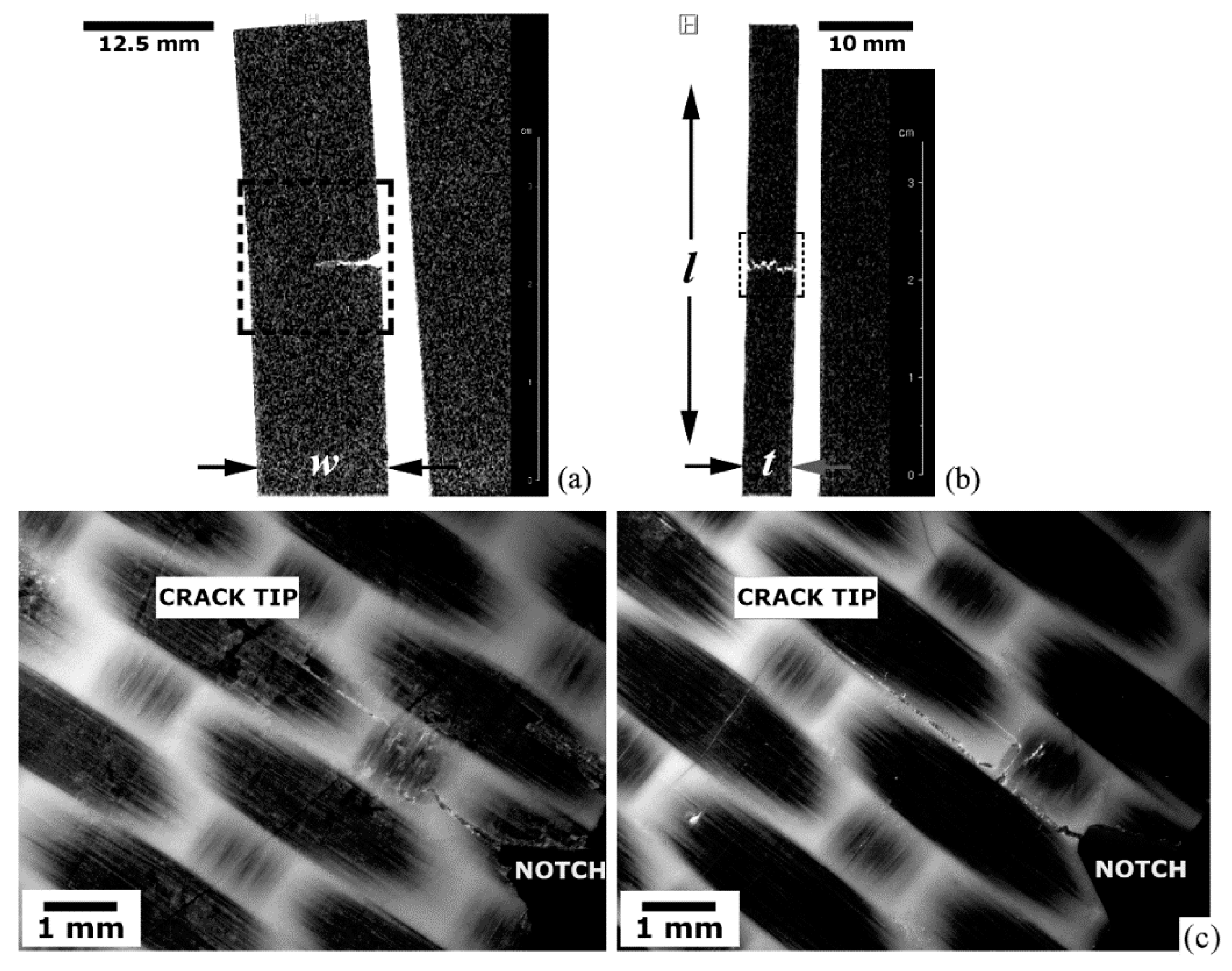
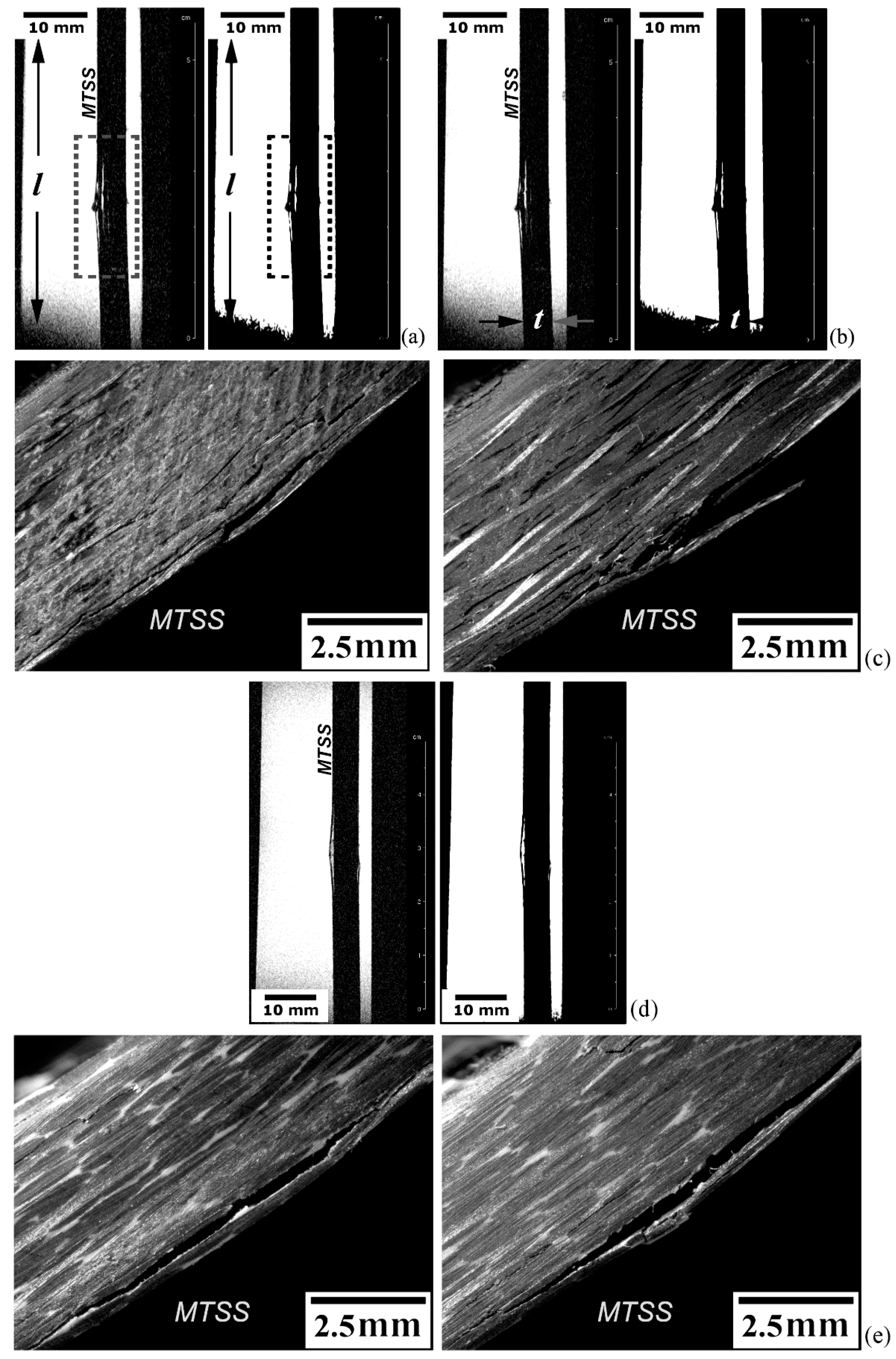
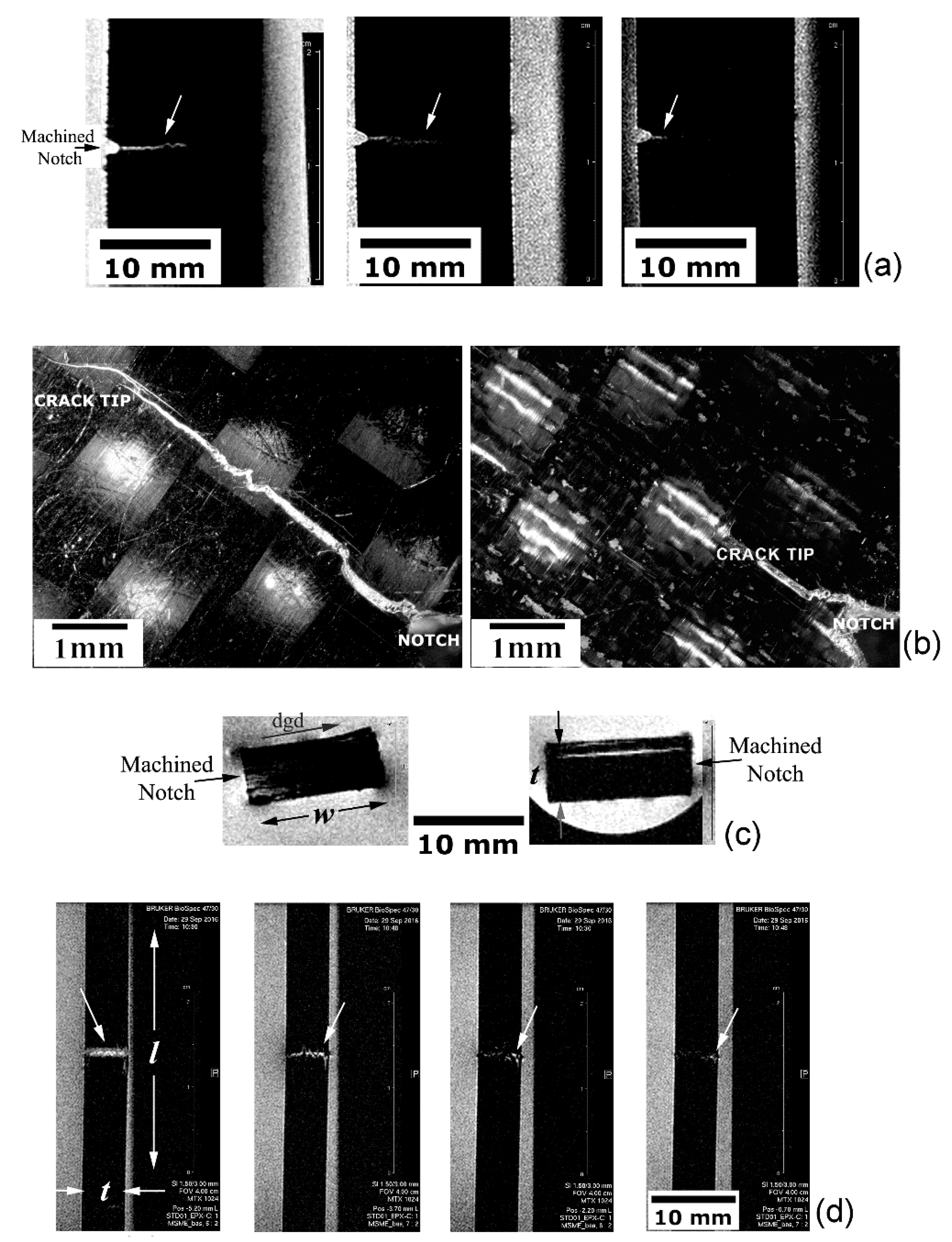
5.3. Qualitative and Quantitative Analyses of MRI Data
5.3.1. Electromagnetic Interference (EMI)
5.3.2. Defective and Damaged Specimens
Water Absorption Analysis
Surface Finish Analysis
Translaminar Fracture Analysis
Delamination Fracture Analysis
Analysis of Asymmetrical Translaminar Fracture
6. Conclusions
- The orientation, positioning, shape, and particularly the size of translaminar and delamination fractures were determined according to rigorous structural integrity assessment criteria. For example, the crack-like damage length was measured within 5% of accuracy regarding the most representative specimen dimension.
- The spatial distribution, shape, and contours of the water-filled voids were satisfactory delineated to estimate the amount of absorbed water if thinner imaging slices than this study are used.
- The surface texture of composite specimens featuring roughness, waviness, indentation, crushing, and scratches was fully outlined, with occasional artefacts not prejudicing image quality and interpretation.
- The 2D-RARE image acquisition protocol was judged preferable than the 2D-FLASH, since the latter algorithm favoured non-conservative damage assessment.
- Low electromagnetic shielding glass fibres rendered the highest, while electrically conductive carbon fibre provide the poorest quality images, particularly when the latter reinforcing fibres were mixed to thermoplastic polymer, though trustworthy image interpretation was still attainable.
- The obtained results indicate that magnetic resonance imaging is potentially useful to provide crucial information about flaws´ severity and criticality in fibre-reinforced polymer composite orthopaedic implanted devices for decision-making process, with potential benefits for the patient´s well-being, time and cost-savings when clinical interventions are considered unnecessary on the basis of fracture mechanics approach.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Axial |
| C | Coronal |
| CF(RP) | Carbon fibre (-reinforced polymer) |
| dgd | Damage growth direction |
| EMI | Electromagnetic interference |
| EPX-GF | Epoxy resin-glass fibre |
| EPX-CF | Epoxy resin-carbon fibre |
| FLASH | Fast low angle shot |
| FOV | Field of view |
| FRP | Fibre-reinforced polymer |
| GF(RP) | Glass fibre (-reinforced polymer) |
| l | Specimen length |
| MRI | Magnetic resonance imaging |
| PPS-CF | Poly-phenylene sulphide-carbon fibre |
| RARE | Rapid image acquisition with refocused echoes |
| S | Sagittal |
| t | Specimen thickness |
| MTSF | Maximum tensile stress surface |
| w | Specimen width |
| WSS | Water-based saline solution |
| 2D | Two-dimensional |
| 3PB | Three-point bending |
References
- Beaumont, P.W.R.; Zweben, C.H. Comprehensive Composite Materials II, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; p. 4288. ISBN 978-0-08-100534-7. [Google Scholar]
- Deng, Y.; Yang, Y.; Ma, Y.; Fan, K.; Yang, W.; Yin, G. Nano-hydroxyapatite reinforced polyphenylene sulfide biocomposite with superior cytocompatibility and in vivo osteogenesis as a novel orthopedic implant. Royal Soc. Chem. Adv. 2017, 7, 559–573. [Google Scholar] [CrossRef]
- Li, C.S.; Vannabouathong, C.; Sprague, S.; Bhandari, M. The use of carbon-fiber-reinforced (CFR) PEEK material in orthopedic implants: A systematic review. Clin. Med. Insights Arthritis Musculoskelet Disord. 2015, 8, 33–45. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Effect of TiO2 nanoparticles on the performance of polyphenylsulfone biomaterial for orthopaedic implants. J. Mater. Chem. B 2014, 2, 7502–7514. [Google Scholar] [CrossRef] [PubMed]
- Salernitano, E.; Migliaresi, C. Composite materials for biomedical applications: A review. J. App. Biomat. Biomech. 2003, 1, 3–18. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Comp. Sci. Tech. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Iqbal, H.M.N.; Keshavars, T. The challenge of biocompatibility evaluation of biocomposites. In Biomedical Composites, 2nd ed.; Ambrosio, L., Ed.; Woodhead Publishing Series in Biomaterials; Elsevier Ltd.: Sawston, UK, 2017; pp. 303–334. ISBN 978-0-08-100752-5. [Google Scholar]
- Petersen, R. Carbon fiber biocompatibility for implants. Fibers 2016, 4, 1. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Chung, D.D.L. Composite materials for biomedical applications. In Composite Materials: Functional Materials for Modern Technologies, 1st ed.; Springer: London, UK, 2003; pp. 233–243. ISBN 978-1-44-713734-4. [Google Scholar]
- Kashi, A.; Saha, S. Mechanisms of failure of medical implants during long-term use. In Biointegration of Medical Implant Materials: Science and Design, 1st ed.; Charma, C., Ed.; Woodhead Publishing Materials; Elsevier Ltd.: Sawston, UK, 2010; pp. 326–348. ISBN 978-1-84-569509-5. [Google Scholar]
- Heiner, A.D. Structural properties of fourth-generation composite femurs and tibias. J. Biomech. 2008, 4, 3282–3284. [Google Scholar] [CrossRef]
- Li, C.; Granger, C.; Del Schutte, M.S.H., Jr.; Biggers, S.B., Jr.; Kennedy, J.M.; Latour, R.A., Jr. Failure analysis of composite femoral components for hip arthroplasty. J. Rehabil. Res. Dev. 2003, 40, 131–145. [Google Scholar] [CrossRef]
- Cristofolini, L.; Viceconti, M. Mechanical validation of whole bone composite tibia models. J. Biomech. 2000, 33, 279–288. [Google Scholar] [CrossRef]
- Allcock, S.; Ali, M.A. Early failure of a carbon-fiber composite femoral component. J. Arthropast. 1997, 12, 356–358. [Google Scholar] [CrossRef]
- Cristofolini, L.; Viceconti, M.; Cappello, A.; Toni, A. Mechanical validation of whole bone composite femur models. J. Biomech. 1996, 2, 525–535. [Google Scholar] [CrossRef]
- Gavens, A.J.; Liao, K.; Maharaj, G.R.; Jamison, R.D.; Reifsnider, K.L. Evaluation of damage progression in a composite material hip implant during long-term multiaxial fatigue. In STP1128-EB Damage Detection in Composite Materials; Special Technical Publication: San Antonio, TX, USA, 1992; pp. 256–271. [Google Scholar] [CrossRef]
- Brown, R.W.; Cheng, Y.C.N.; Haacke, E.M.; Thompson, M.R.; Venkatesan, R. Magnetic Resonance Imaging: Physical Principles and Sequence Design, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; p. 914. ISBN 978-0-47-172085-0. [Google Scholar]
- Callaghan, P.T. Principles of Nuclear Magnetic Resonance Microscopy, 1st ed.; Oxford University Press Inc.: New York, NY, USA, 1991; p. 516. ISBN 0-19-853997-5. [Google Scholar]
- Morris, P.G. Nuclear Magnetic Resonance Imaging in Medicine and Biology, 1st ed.; Clarendon Press: Oxford, UK, 1986; p. 388. ISBN 0-19-855155-X. [Google Scholar]
- Rothwell, W.P. Nuclear magnetic resonance imaging. App. Opt. 1985, 24, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Craciun, H.; Mankad, K.; Lynch, J. Risk management in radiology departments. World J. Radiol. 2015, 7, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Velichko, A.; Bai, L.; Drinkwater, B.W. Ultrasonic defect characterization using parametric-manifold mapping. Proc. R. Soc. 2017, 473, 20170056. [Google Scholar] [CrossRef]
- Bougherara, H.; Rabim, E. A preliminary biomechanical assessment of a polymer composite hip implant using an infrared thermography technique validated by strain gage measurements. J. Biomech. Eng. 2011, 133, 074503. [Google Scholar] [CrossRef]
- Ishida, H. Characterization of Composite Materials, 1st ed.; Materials Characterization Series; Momentum Press: Buchanan, NY, USA, 2009; p. 277. ISBN 978-1-60-650191-7. [Google Scholar]
- Morris, R.H.; Trabi, C.L.; Spicer, A.; Langmack, K.; Boersma, W.; Weightman, J.S.; Newton, M.I. A natural fibre FRP composite material for multi-modal medical imaging and radiotherapy treatment. Mat. Lett. 2019, 252, S289–S292. [Google Scholar] [CrossRef]
- Meng, X.; Du, Z.; Wang, Y. Feasibility of magnetic resonance imaging monitoring of postoperative total knee arthroplasty without metal artifacts: A preliminary study of a novel implant model. Biomed. Res. Int. 2018, 2018, 8194670. [Google Scholar] [CrossRef]
- Zimel, M.N.; Hwang, S.; Riedel, E.R.; Healey, J.H. Carbon fiber intramedullary nails reduce artifact in postoperative advanced imaging. Skel. Radiol. 2015, 44, 1317–1325. [Google Scholar] [CrossRef]
- Longo, J.A., III; Koeneman, J.B. Orthopedic applications of carbon fiber composites. In Biomaterials Engineering and Devices: Human Applications, 1st ed.; Wise, D.L., Trantolo, D.J., Lewandrowski, K.U., Gresser, J.D., Cattaneo, M.V., Yaszemski, M.J., Eds.; Springer-Science: New York, NY, USA, 2000; Volume 2, pp. 203–214. ISBN 978.1.59.259197.8. [Google Scholar]
- Enge, D.J., Jr.; Castro, A.A.; Fonseca, E.K.U.N.; Baptista1, E.; Padial, M.B.; Rosemberg, L.A. Main complications of hip arthroplasty: Pictorial essay. Radiol. Bras. 2020, 53, 56–62. [Google Scholar] [CrossRef]
- Garcea, S.C.; Mavrogordato, M.N.; Scott, A.E.; Sinclair, I.; Spearing, S.M. Fatigue micromechanism characterisation in carbon fibre reinforced polymers using synchrotron radiation computed tomography. Comp. Sci. Tech. 2014, 99, 23–30. [Google Scholar] [CrossRef]
- Garcea, S.C.; Wang, Y.; Withers, P.J. X-ray computed tomography of polymer composites. Compos. Sci. Technol. 2018, 156, 305–319. [Google Scholar] [CrossRef]
- Vercio, R.C.; Basmajian, H.G. Fracture of a carbon fiber reinforced intramedullary femoral nail. J. Am. Acad. Orthop. Surg. 2019, 27, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Djukic, L.P.; Herszberg, I.; Walsh, W.R.; Schoeppner, G.A.; Prusty, B.G.; Kelly, D.W. Contrast enhancement in visualisation of woven composite tow architecture using a MicroCT Scanner. Part 1: Fabric coating and resin additives. Compos. Part A 2009, 40, 553–565. [Google Scholar] [CrossRef]
- Lex, T.R.; Brummel, B.R.; Attia, M.F.; Giambalvo, L.N.; Lee, K.G.; Van Horn, B.A.; Whitehead, D.C.; Alexis, F. Iodinated polyesters with enhanced X-ray contrast properties for biomedical imaging. Sci. Rep. 2020, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Vien, B.S.; Chiu, W.K.; Russ, M.; Fitzgerald, M. A Quantitative approach for the bone-implant osseointegration assessment based on ultrasonic elastic guided waves. Sensors 2019, 19, 454. [Google Scholar] [CrossRef]
- Tarpani, J.R.; Portela, A.M.A. Magnetic resonance imaging of mechanically damaged and liquid-contaminated core cells in polymer composite sandwich panels. J. Sandw. Struct. Mat. 2017, 20, 30. [Google Scholar] [CrossRef]
- Hozack, W.; Parvizi, J.; Bender, B. Surgical Treatment of Hip Arthritis: Reconstruction, Replacement, and Revision, 1st ed.; Elsevier: Philadelphia, PA, USA, 2019; p. 544. ISBN 978-1-41-605898-4. [Google Scholar]
- Mehboo, L.L.H.; Chang, S.H. Application of composites to orthopedic prostheses for effective bone healing: A review. Comp. Struct. 2014, 118, 328–341. [Google Scholar] [CrossRef]
- Garcia, F.G.; Leyva, M.E.; Queiroz, A.A.A.; Higa, O.Z. Epoxy networks for medicine applications: Mechanical properties and in-vitro biological properties. J. Appl. Pol. Sci. 2009, 112, 1215–1225. [Google Scholar] [CrossRef]
- Hastings, G.W. Carbon and plastic materials for orthopaedic implants. In Materials Sciences and Implant Orthopedic Surgery, 1st ed.; Kossowsky, R., Kossovsky, N., Eds.; Series E: Applied Sciences; Martinus Nijhoff Publishers: Dordrecht, The Netherlands, 1986; Volume 116, pp. 263–284. ISBN 978-9-40-108492-5. [Google Scholar]
- Hastings, G.W. Carbon fibre composites for orthopaedic implants composites. Composites 1978, 9, 193–197. [Google Scholar] [CrossRef]
- Lotz, J.C.; Cheal, E.J.; Hayes, W.C. Stress distributions within the proximal femur during gait and falls: Implications for osteoporotic fracture. Osteop. Inter. 1995, 5, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Hennig, J.; Nauerth, A.; Friedburg, H. RARE imaging: A fast imaging method for clinical MR. Magn. Reson. Med. 1986, 3, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Block, K.T.; Frahm, J. Magnetic resonance imaging in real time: Advances using radial FLASH. J. Magn. Reson. Imag. 2010, 31, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Sood, B.; Pecht, M. The effect of epoxy/glass interfaces on CAF failures in printed circuit boards. Microel. Reliab. 2018, 82, 235–243. [Google Scholar] [CrossRef]
- Valdes, L.B. Resistivity measurements on germanium for transistors. Proc. IRE 1954, 42, 420–427. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in-vivo surface structure changes in bioactive glass ceramic A-W. J. Biom. Mat. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- What Helps with Hip Replacement Recovery? Available online: https://www.healthline.com/health/hip-replacement-recovery (accessed on 3 March 2020).
- Redpath, T.R. Signal-to-noise ratio in MRI. Brit. J. Radiol. 1998, 71, 704–707. [Google Scholar] [CrossRef]
- Ibrahim, M.E. Nondestructive evaluation of thick-section composites and sandwich structures: A review. Compos. Part A 2014, 64, 36–48. [Google Scholar] [CrossRef]
- Grancarić, A.M.; Jerković, I.; Koncar, V.; Cochrane, C.; Kelly, F.M.; Soulat, D.; Legrand, X. Conductive polymers for smart textile applications. J. Ind. Text. 2017, 48, 612–642. [Google Scholar] [CrossRef]
- Abdel-Fattah, W.I.; Sallam, A.M.; Ibrahim, I.H.; Ibrahim, H. AC electric conductivity and biochemical analyses of physiologic solutions to follow biomimetic coatings on corals impregnated with Ag or Zn or Sr ions. Biomaterials 2009, 1, 1–9. [Google Scholar] [CrossRef]
- Costa, M.L.; Rezende, M.C.; Almeida, S.F.M. Effect of void content on the moisture absorption in polymeric composites. Polym-Plast. Technol. 2007, 45, 691–698. [Google Scholar] [CrossRef]
- Choi, H.; Ahn, K.; Nam, J.D.; Chun, H. Hygroscopic aspects of epoxy/carbon fiber composite laminates in aircraft environments. Compos. Part A 2001, 32, 709–720. [Google Scholar] [CrossRef]
- Powers, D.A. Interaction of Water with Epoxy. Sandia National Laboratories Report 2009—SAND2009-4405. 2009; p. 57. Available online: https://prod-ng.sandia.gov/techlib-noauth/access-control.cgi/2009/094405.pdf (accessed on 20 September 2019).
- Ray, B.C. Temperature effect during humid ageing on interfaces of glass and carbon fibre reinforced epoxy composites. J. Colloid. Interface Sci. 2006, 298, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ballantine, D.S.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohltjen, H. Materials characterization. In Acoustic Wave Sensors: Theory, Design, and Physico-Chemical Applications; Academic Press Inc.: Cambridge, MA, USA, 1997; pp. 150–221. ISBN 978-0-12-077460-9. [Google Scholar]
- Soares-Pozzi, A.C.; Dibbern-Brunelli, D. Study of the influence of saline solutions in carbon/epoxy composite by luminescence, Raman and UATR/FT-IR spectroscopy. J. Mater. Sci. 2016, 51, 9342–9355. [Google Scholar] [CrossRef]
- Boroujeni, A.Y.; Tehrani, M.; Manteghi, M.; Zhou, Z.; Al-Haik, M. Electromagnetic Shielding Effectiveness of a Hybrid Carbon Nanotube/Glass Fiber Reinforced Polymer Composite. J. Eng. Mater-T ASME 2016, 138, 041001. [Google Scholar] [CrossRef]
- Munalli, D.; Dimitrakis, G.; Chronopoulos, D.; Greedy, S.; Long, A. Electromagnetic shielding effectiveness of carbon fibre reinforced composites. Compos. Part B 2019, 173, 106906. [Google Scholar] [CrossRef]
- Rea, S.P.; Wylie, D.; Linton, D.; Orr, E.; McConnell, J. EMI shielding of Woven Carbon Fibre Composites. In Proceedings of the High Frequency Postgraduate Student Colloquium, Manchester, UK, 6–7 September 2004; pp. 205–210. [Google Scholar] [CrossRef]
- Fernlund, G.; Wells, J.; Fahrang, L.; Kay, J.; Poursartip, A. Causes and remedies for porosity in composite manufacturing. IOP Conf. Ser. Mater. Sci. Eng. C 2016, 139, 012002. [Google Scholar] [CrossRef]
- Magnetic Resonance Imaging (MRI). Available online: https://my.clevelandclinic.org/health/diagnostics/4876-magnetic-resonance-imaging-mri (accessed on 1 February 2020).
- Oren, N.J.; Fleysher, R.; Lipton, M.L.L. Compressed sensing MRI: A review of the clinical literature. Br. J. Radiol. 2015, 88, 20150487. [Google Scholar] [CrossRef]
- Feng, L.; Benkert, T.; Block, K.T.; Sodickson, D.K.; Otazo, R.; Chandarana, H. Compressed Sensing for Body MRI. J. Magn. Reson. Imaging 2016, 45, 966–987. [Google Scholar] [CrossRef]
- Zhao, X.; Duan, G.; Wu, K.; Anderson, S.W.; Zhang, X. Intelligent Metamaterials Based on Nonlinearity for Magnetic Resonance Imaging. Adv. Mater. 2019, 31, 190546. [Google Scholar] [CrossRef]
- Duan, G.; Zhao, X.; Anderson, S.W.; Zhang, X. Boosting magnetic resonance imaging signal-to-noise ratio using magnetic metamaterials. Commun. Phys. 2019, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Talreja, R. Damage Mechanics of Composite Materials; Elsevier Science Publishing: Oxford, UK, 1994; Volume 9, p. 316. ISBN 978-0-44-488852-5. [Google Scholar]
- Friedrich, K. Application of Fracture Mechanics to Composite Materials; Elsevier Science Publishing: Amsterdam, The Netherlands, 1989; Volume 6, p. 671. ISBN 978-0-44-487286-9. [Google Scholar]
- Üstün, E. Static and fatigue failure of unidirectional curved composite laminates. In Structural and Computational Mechanics Book Series: Proceedings of the 20th International Conference on Composite Structures; Ferreira, A.J.M., Larbi, W., Deu, J.F., Tornabene, F., Fantuzzi, N., Eds.; Societa Editrice Esculapio: Bologna, Italy, 2016; p. 352. ISBN 978-8-89-385041-4. [Google Scholar]
- Vieille, B.; Casado, V.M.; Bouvet, C. About the impact behavior of woven-ply carbon fiber-reinforced thermoplastic- and thermosetting-composites: A comparative study. Compos. Struct. 2013, 101, 9–21. [Google Scholar] [CrossRef]


| Material | Electrical Conductivity, ohm−1·m−1 |
|---|---|
| EPX-CF | 1.7 × 103 |
| PPS-CF | 3.0 × 103 |
| EPX-GF | 1.0 × 10−10 |
| WSS | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, C.L.; Oliveira, J.S.; Tannus, A.; Tarpani, A.C.S.P.; Tarpani, J.R. Detection and Imaging of Damages and Defects in Fibre-Reinforced Composites by Magnetic Resonance Technique. Materials 2021, 14, 977. https://doi.org/10.3390/ma14040977
Alves CL, Oliveira JS, Tannus A, Tarpani ACSP, Tarpani JR. Detection and Imaging of Damages and Defects in Fibre-Reinforced Composites by Magnetic Resonance Technique. Materials. 2021; 14(4):977. https://doi.org/10.3390/ma14040977
Chicago/Turabian StyleAlves, Carine L., Janete S. Oliveira, Alberto Tannus, Alessandra Cristina Soares P. Tarpani, and José R. Tarpani. 2021. "Detection and Imaging of Damages and Defects in Fibre-Reinforced Composites by Magnetic Resonance Technique" Materials 14, no. 4: 977. https://doi.org/10.3390/ma14040977
APA StyleAlves, C. L., Oliveira, J. S., Tannus, A., Tarpani, A. C. S. P., & Tarpani, J. R. (2021). Detection and Imaging of Damages and Defects in Fibre-Reinforced Composites by Magnetic Resonance Technique. Materials, 14(4), 977. https://doi.org/10.3390/ma14040977





