Effect of Iron (III) Oxide Powder on Thermal Conductivity and Diffusivity of Lime Mortar
Abstract
1. Introduction
2. Materials and Methods
2.1. Components
2.2. Components’ Dimensional Characterization
2.3. Mortar Preparation and Curing
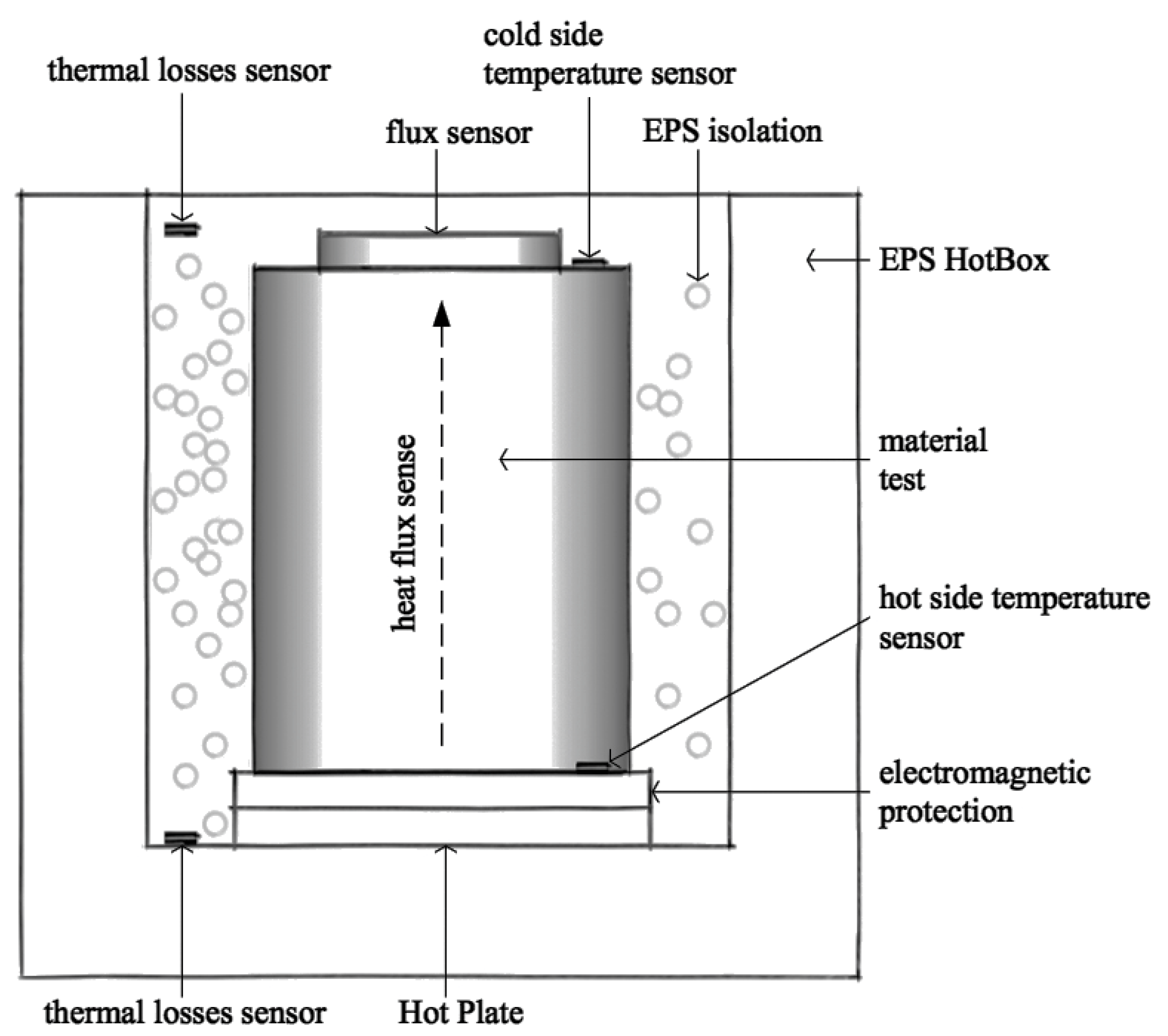
2.4. Experimental Method
2.4.1. Apparent Density
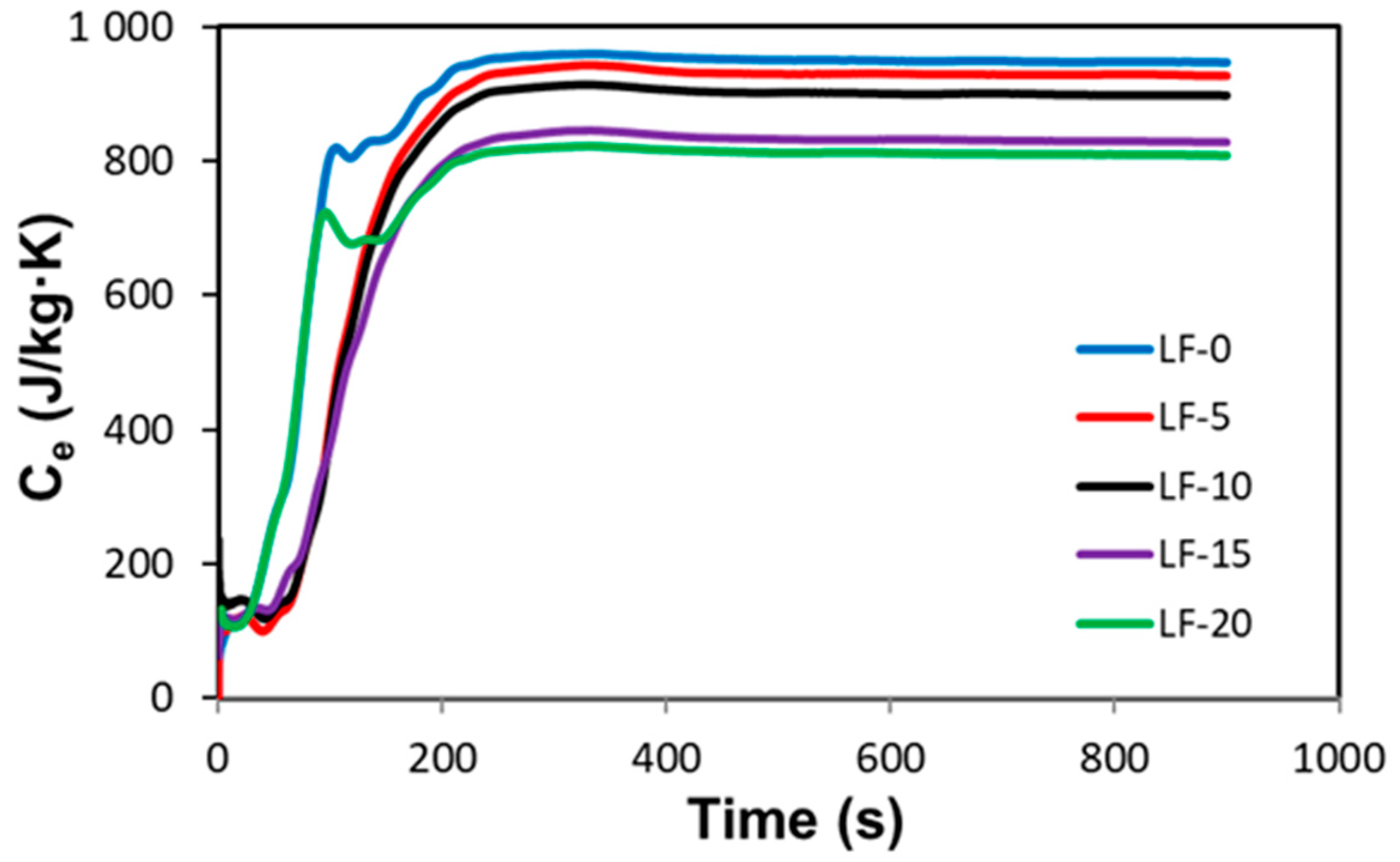
2.4.2. Specific Heat Capacity
2.4.3. Thermal Conductivity of Mortars
3. Results and Discussion
3.1. Density and Porosity
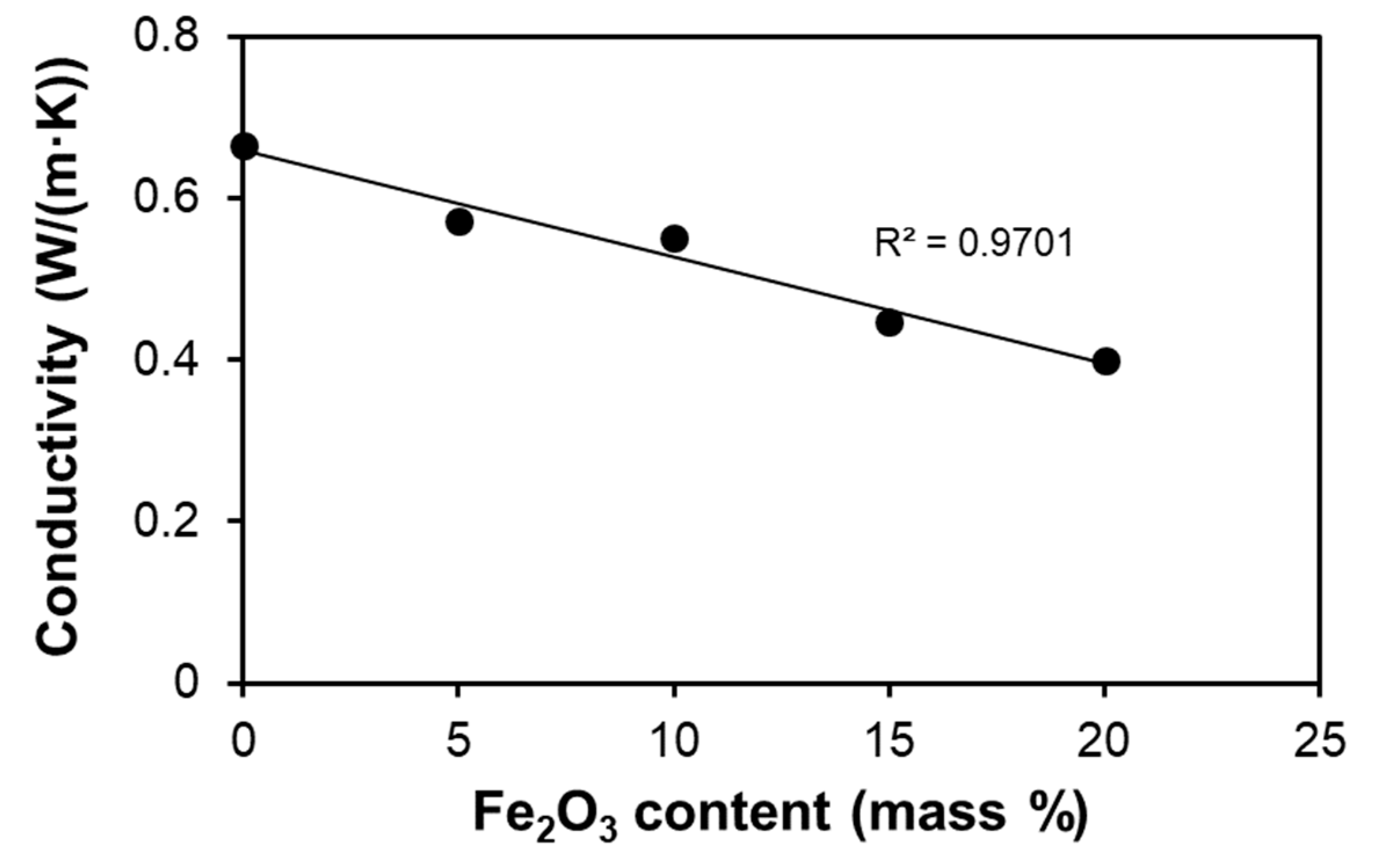
3.2. Thermal Conductivity
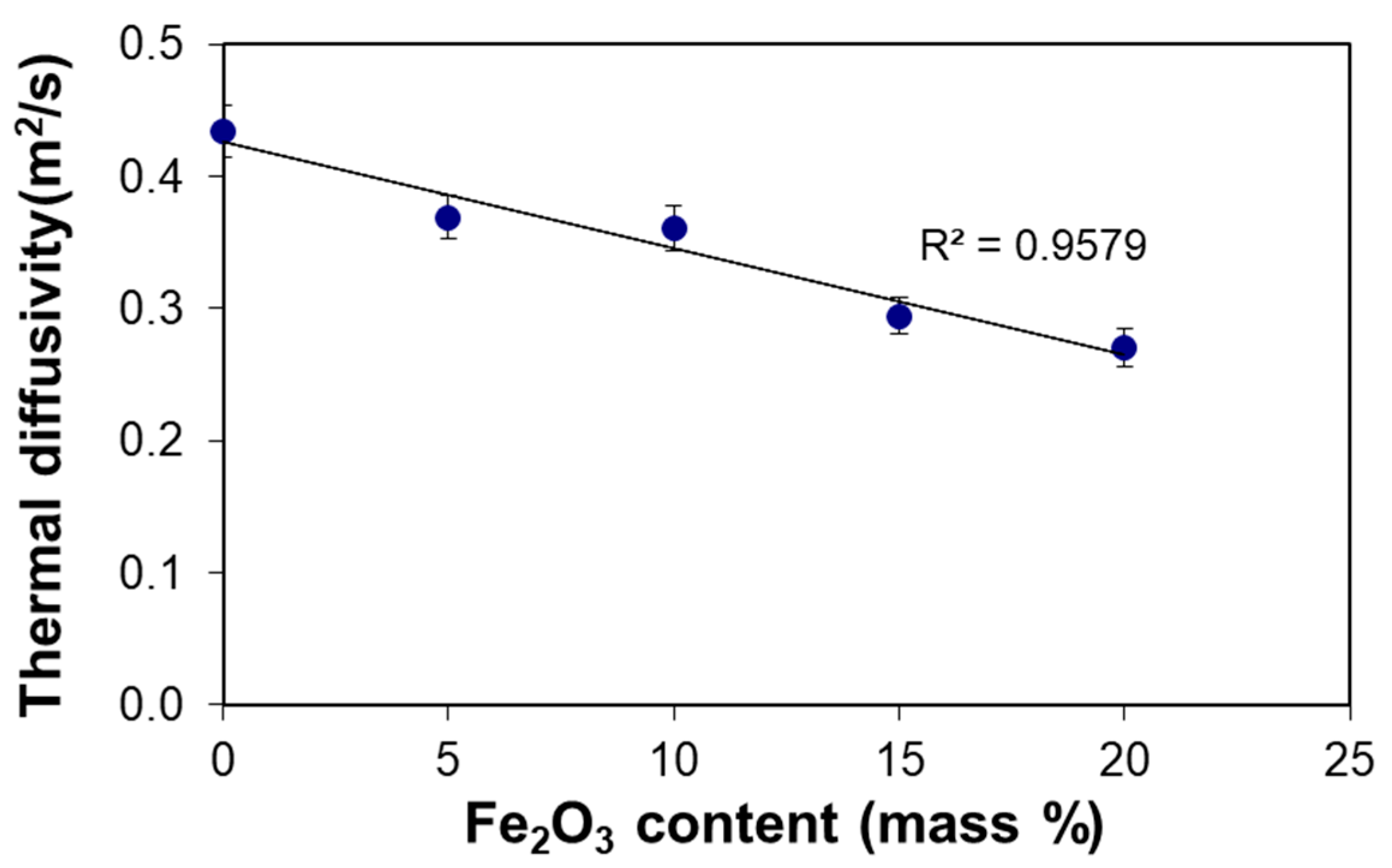
3.3. Thermal Diffusivity
4. Conclusions
- Thermal conductivity of the used additive, iron (III) oxide, is much lower than that of the lime mortar (base material).
- The porosity of mortar increases with the addition of Fe2O3 fine powder from 39% in the base mortar (0% Fe2O3) to 47% in the mortar containing 20% of Fe2O3. The thermal conductivity of a porous ceramics drops significantly with the porosity.
- Due to the extremely low solubility of Fe2O3 in aqueous media, the area of the neck formed between iron (III) oxide particles is small compared to the necks between lime particles. Therefore, the effective surface of a solid phase able to transfer the heat by conduction diminishes, thus, improving the thermal insulation capability.
- The use of iron (III) oxide as an additive, which causes an increase of density and a decrease of thermal conductivity compared to base mortar, leads to a significant improvement of the thermal inertia of the resulting mortar.
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A review of unconventional sustainable building insulation materials. SM&T 2015, 4, 1–17. [Google Scholar]
- European Commission. EU Energy, Transport and GHG Emissions, Trends to 2050 Reference Scenario; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Brás, A.; Faria, P. Effectiveness of mortars composition on the embodied carbon long-term impact. Energy Build. 2017, 154, 523–528. [Google Scholar] [CrossRef]
- Van Balen, K. Carbonation reaction of lime, kinetics at ambient temperature. Cem. Concr. Res. 2005, 35, 647–657. [Google Scholar] [CrossRef]
- Galván-Ruiz, M.; Velázquez-Castillo, R. Lime, an Ancient Material as a Renewed Option for Construction. Ing. Investig. Tecnol. 2011, 12, 93–102. [Google Scholar]
- Soumia, M.; Youssef, M.; Abou bakr Cherki, A. Thermal properties of the composite material clay/granular cork. Constr. Build. Mater. 2014, 70, 183–190. [Google Scholar]
- Benfratello, S.; Capitano, C.; Peri, G.; Rizzo, G.; Sorrentino, G. Thermal and structural properties of a hemp-lime biocomposite. Constr. Build. Mater. 2013, 48, 745–754. [Google Scholar] [CrossRef]
- Pochwała, S.; Makiola, D.; Anweiler, S.; Böhm, M. The Heat Conductivity Properties of Hemp–Lime Composite Material Used in Single-Family Buildings. Materials 2020, 13, 1011. [Google Scholar] [CrossRef]
- Barreca, F.; Fichera, C.R. Use of olive stone as an additive in cement lime mortar to improve thermal insulation. Energy Build. 2013, 62, 507–513. [Google Scholar] [CrossRef]
- Briga-Sá, A.; Nascimento, D.; Teixeira, N.; Pinto, J.; Caldeira, F.; Varum, H.; Paiva, A. Textile waste as an alternative thermal insulation building material solution. Constr. Build. Mater. 2013, 38, 155–160. [Google Scholar] [CrossRef]
- Ashour, T.; Georg, H.; Wu, W. Performance of straw bale wall: A case of study. Energy Build. 2011, 43, 1960–1967. [Google Scholar] [CrossRef]
- Kochhar, G.S.; Manohar, K. Use of coconut fiber as a low-cost thermal insulator. Insulation materials: Testing and applications. ASTM 1997, 3, 283–291. [Google Scholar]
- Moropoulou, A.; Bakolas, A.; Aggelakopoulou, E. The effects of limestone characteristics and calcination temperature to the reactivity of the quicklime. Cem. Concr. Res. 2001, 31, 633–639. [Google Scholar] [CrossRef]
- Schumacher, G.; Juniper, L. Coal utilisation in the cement and concrete industries. Coal Handb. Towards Clean. Prod. 2013, 2, 387–426. [Google Scholar]
- Liu, K.; Wang, Z.; Jin, C.; Wang, F.; Lu, X. An experimental study on thermal conductivity of iron ore sand cement mortar. Constr. Build. Mater. 2015, 101, 932–941. [Google Scholar] [CrossRef]
- Martínez-Ramírez, S.; Puertas, F.; Blanco-Varela, M.T.; Thompson, G.E.; Almendros, P. Behaviour of Repair Lime Mortars by Wet Deposition Process. Cem. Concr. Res. 1998, 28, 221–229. [Google Scholar] [CrossRef]
- Robador, M.D.; Arroyo, F. Characterization of Roman coatings from the Roman house in Mérida (Spain). J. Cult. Herit. 2013, 14, s52–s58. [Google Scholar] [CrossRef]
- Boyton, R.S. Chemistry and Technology of Lime and Limestone; John Wiley & Sons: New York, NY, USA, 1966. [Google Scholar]
- Banfill, P.F.G.; Forrester, A.M. A Relationship between Hydraulicity and Permeability of Hydraulic Lime. In Proceedings of the International RILEM Workshop ‘Historic Mortars: Characteristics and Tests’, RILEM, Paisley, Scotland, 12–14 May 1999; pp. 173–183. [Google Scholar]
- Available online: http://www.engineeringtoolbox.com/thermal-conductivity-d_429.html (accessed on 27 November 2020).
- Shakelford, J.; Alexander, W. Material Science and Engineering Handbook, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Krebs, R.E. The History and Use of Our Earth’s Chemical Elements, 2nd ed.; Greenwood Publishing Group: Westport, CT, USA, 2006; pp. 102–105. [Google Scholar]
- Acevedo-Sandoval, O.; Ortiz-Hernández, E.; Cruz-Sánchez, M.; Cruz-Chávez, E. El papel de óxidos de hierro en suelos. Terra Latinoam. 2004, 22, 485–497. [Google Scholar]
- Kishar, E.A.; Alasqalani, M.Y.; Sarraj, Y.R.; Ahmed, D.A. The Effect of Using Commercial Red and Black Iron Oxides as a Concrete Admixtures on its Physiochemical and Mechanical Properties. Int. J. Sci. Res. 2015, 4, 1389–1393. [Google Scholar]
- Largeau, M.A.; Mutuku, R.; Thuo, J. Effect of Iron Powder (Fe2O3) on Strength, Workability, and Porosity of the Binary Blended Concrete. Open J. Civ. Eng. 2018, 8, 411–425. [Google Scholar] [CrossRef]
- Masdeu, F.; Muñoz, J.; Carmona, C.; Horrach, G. Mortero de cal Termoaislante y su uso en Edificación. Spanish Utility Model Patent ES1222024 U, 18 December 2018. [Google Scholar]
- ISO 12439. Mixing Water for Concrete; International Organization for Standarization: Geneva, Switzerland, 2010. [Google Scholar]
- ASTM C1363-11, ASTM 90 (reapproved), Standard Test Method for Thermal Performance of Building Materials and Envelope Assemblies by Means of a Hot-Box Method Apparatus; ASTM: West Conshohocken, PA, USA, 2014.
- Weast, R.; Lide, D. Handbook of Chemistry and Physics, 70th ed.; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Kamseu, E.; Nait-Ali, B.; Bignozzi, M.C.; Leonelli, C.; Rossignol, S.; Smith, D.S. Bulk composition and microstructure dependence of effective thermal conductivity of porous inorganic polymer cements. J. Eur. Ceram. Soc. 2012, 32, 1593–1603. [Google Scholar] [CrossRef]
- Dos Santos, W.N. Effect of moisture and porosity on the thermal properties of a conventional refractory concrete. J. Eur. Ceram. Soc. 2003, 23, 745–755. [Google Scholar] [CrossRef]
- Niubó, M.; Formosa, J.; Maldonado-Alameda, A.; del Valle-Zermeño, R.; Chimenos, J.M. Magnesium phosphate cement formulated with low grade magnesium oxide with controlled porosity and low thermal conductivity as a function of admixture. Ceram. Int. 2016, 42, 15049–15056. [Google Scholar] [CrossRef]
- Bergman, T.L.; Lavine, A.S.; Incropera, F.P.; DeWitt, D.P. Fundamentals of Heat and Mass Transfer, 7th ed.; John Wiley & Sons: Jefferson City, MI, USA, 2011; pp. 116–117. [Google Scholar]
- Rhee, S.K. Porosity—Thermal conductivity correlations for ceramic materials. Mat. Sci. Eng. 1975, 20, 89–93. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 3rd ed.; Pearson: London, UK, 2008; pp. 78–89. [Google Scholar]
- Marshall, A.L. Thermal properties of concrete. Build. Sci. 1972, 7, 167–174. [Google Scholar] [CrossRef]
- Gulbea, L.; Vitinab, I.; Setinac, J. The influence of cement on properties of lime mortars. Procedia Eng. 2017, 172, 325–332. [Google Scholar] [CrossRef]
- Ng, S.; Low, K.; Tioh, N. Newspaper sandwiched aerated lightweight concrete wall panels—thermal inertia, transient thermal behavior and surface temperature prediction. Energy Build. 2011, 43, 1636–1645. [Google Scholar] [CrossRef]
- Ferrari, S. Building envelope and heat capacity: Re-discovering the thermal mass for winter energy saving. In Proceedings of the 2nd PALENC Conference and 28th AIVC Conference on Building Low Energy Cooling and Advanced Ventilation Technologies in the 21st Century, Crete, Greece, 27–29 September 2007. [Google Scholar]
- Demirel, Y. Nonequilibrium Thermodynamics. Transport and Rate Processes in Physical, Chemical and Biological Systems, 3rd ed.; ElSevier: Oxford, UK, 2014; pp. 87–88. [Google Scholar]
- Available online: http://www.tainstruments.com/pdf/literature/TP_006_MDSC_num_1_MDSC.pdf (accessed on 27 November 2020).
- Waples, D.W.; Jacob, S.; Waples, J.S. A Review and Evaluation of Specific Heat Capacities of Rocks, Minerals, and Subsurface Fluids. Part 1: Minerals and Nonporous Rocks. Nat. Resour. Res. 2004, 13, 97–122. [Google Scholar] [CrossRef]
- Bogas, J.A.; Gomes, A.; Pereira, M.F.C. Self-compacting lightweight concrete produced with expanded clay aggregate. Constr. Build. Mater. 2012, 35, 1013–1022. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, L.; Xu, J.; Li, Y. Mechanical properties of expanded polystyrene lightweight aggregate concrete and brick. Constr. Build. Mater. 2012, 27, 32–38. [Google Scholar] [CrossRef]
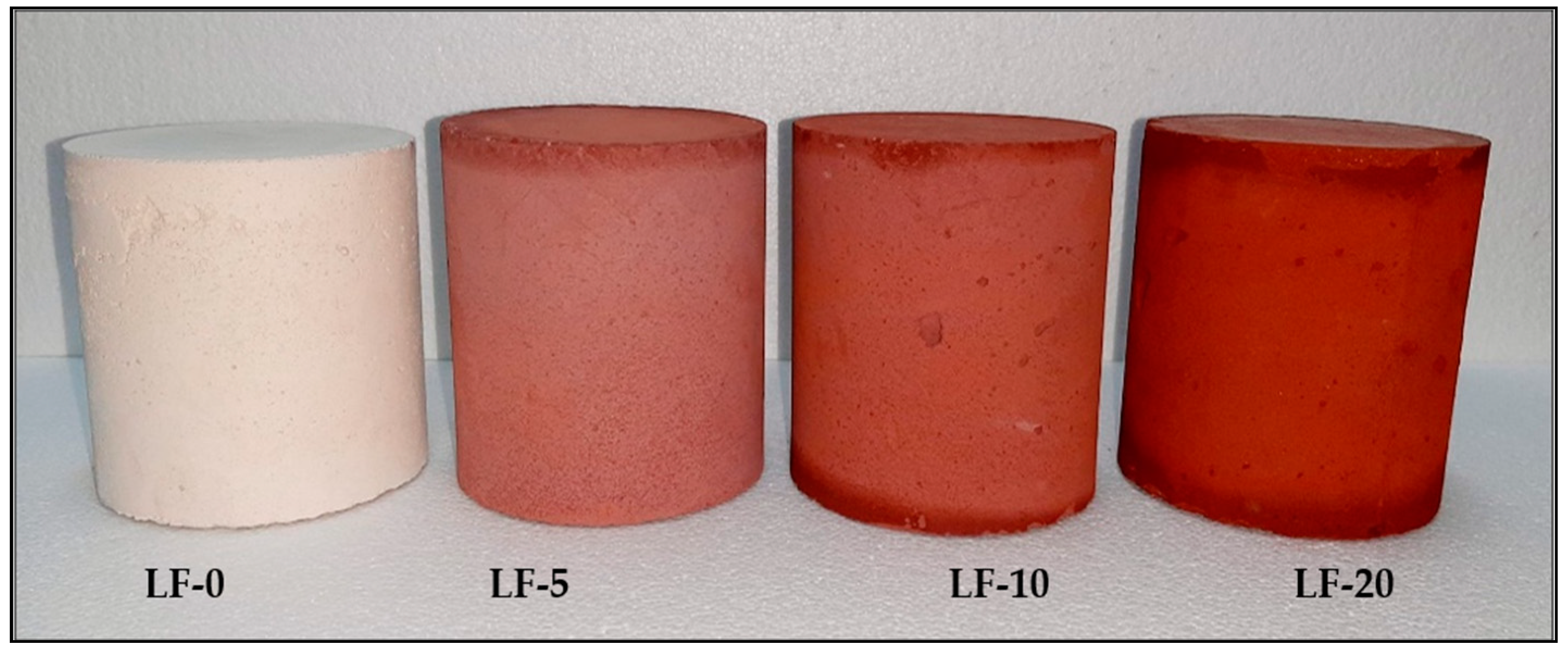



| Material | Grain Size Range (µm) | Specific Surface (cm2/g) |
|---|---|---|
| Lime | 10–2000 | 5.7 × 103 |
| Fe2O3 | 0.2–0.5 | 9.4 × 104 |
| Sample | Component Mass (g) | Fe2O3 Mass Fraction in Mortar (%) | ||
|---|---|---|---|---|
| Lime Mortar | Fe2O3 | H2O | ||
| LF-0 | 1900 | 0 | 400 | 0 |
| LF-5 | 1805 | 95 | 412 | 5 |
| LF-10 | 1710 | 190 | 436 | 10 |
| LF-15 | 1615 | 285 | 500 | 15 |
| LF-20 | 1520 | 380 | 545 | 20 |
| Sample | Fe2O3 Content (mass %) | Theoretical Bulk Density (g/cm3) | Apparent Density (g/cm3) | Porosity (%) |
|---|---|---|---|---|
| LF-0 | 0 | 2.71 ± 0.02 | 1.65 ± 0.03 | 39.2 ± 0.9 |
| LF-5 | 5 | 2.84 ± 0.02 | 1.67 ± 0.03 | 41.2 ± 0.9 |
| LF-10 | 10 | 2.97 ± 0.02 | 1.69 ± 0.03 | 43.1 ± 1.0 |
| LF-15 | 15 | 3.09 ± 0.02 | 1.70 ± 0.03 | 44.9 ± 1.0 |
| LF-20 | 20 | 3.22 ± 0.02 | 1.71 ± 0.03 | 46.8 ± 1.1 |
| Sample | TH (°C) | TC (°C) | ΔT (K) | Φ (W/m2) | U (W/m2·K) | L (m) | λexp (W/m·K) | E.S. (%) |
|---|---|---|---|---|---|---|---|---|
| LF-0 | 61.2 | 30.5 | 30.7 | 166.2 | 5.42 | 0.123 | 0.67 ± 0.01 | 0.0 |
| LF-5 | 61.2 | 26.4 | 34.8 | 165.6 | 4.76 | 0.120 | 0.57 ± 0.01 | 14.3 ± 0.4 |
| LF-10 | 61.2 | 26.0 | 35.2 | 160.5 | 4.56 | 0.121 | 0.55 ± 0.01 | 17.2 ± 0.5 |
| LF-15 | 61.2 | 24.7 | 36.5 | 134.0 | 3.67 | 0.122 | 0.45 ± 0.01 | 32.8 ± 1.0 |
| LF-20 | 61.2 | 23.7 | 37.5 | 124.4 | 3.32 | 0.120 | 0.40 ± 0.01 | 40.1 ± 1.2 |
| Sample | Specific Heat Capacity, Ce (J/(kg·K)) | Decrement 1 (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Test 1 | Test 2 | Test 3 | Test 4 | Average | Standard Dev. | Rule of Mixture | ||
| LF-0 | 922 | 934 | 926 | 939 | 931 | 8 | 931 ± 3 | 0.0 |
| LF-5 | 933 | 920 | 929 | 925 | 927 | 6 | 913 ± 3 | 0.43 ± 0.01 |
| LF-10 | 898 | 912 | 900 | 917 | 907 | 9 | 895 ± 3 | 2.58 ± 0.03 |
| LF-15 | 887 | 896 | 883 | 902 | 892 | 9 | 877 ± 3 | 4.19 ± 0.05 |
| LF-20 | 866 | 852 | 875 | 847 | 860 | 13 | 859 ± 3 | 7.63 ± 0.13 |
| Sample | Density 1, d (kg/m3) | Specific Heat Capacity, Ce (kJ/(kg·K)) | d·Ce (kJ/m3·K) | Thermal Conductivity, λ (kW/(m·K)) | Diffusivity, a (m2/s) | Improvement 2 (%) |
|---|---|---|---|---|---|---|
| LF-0 | 1649 | 0.931 | 1535 | 666 | 0.434 ± 0.020 | 0.0 |
| LF-5 | 1667 | 0.927 | 1545 | 571 | 0.369 ± 0.016 | 14.9 ± 1.3 |
| LF-10 | 1688 | 0.907 | 1531 | 552 | 0.360 ± 0.017 | 17.0 ± 1.6 |
| LF-15 | 1705 | 0.892 | 1521 | 448 | 0.294 ± 0.014 | 32.2 ± 3.0 |
| LF-20 | 1714 | 0.860 | 1474 | 399 | 0.271 ± 0.014 | 37.6 ± 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masdeu, F.; Carmona, C.; Horrach, G.; Muñoz, J. Effect of Iron (III) Oxide Powder on Thermal Conductivity and Diffusivity of Lime Mortar. Materials 2021, 14, 998. https://doi.org/10.3390/ma14040998
Masdeu F, Carmona C, Horrach G, Muñoz J. Effect of Iron (III) Oxide Powder on Thermal Conductivity and Diffusivity of Lime Mortar. Materials. 2021; 14(4):998. https://doi.org/10.3390/ma14040998
Chicago/Turabian StyleMasdeu, Francesc, Cristian Carmona, Gabriel Horrach, and Joan Muñoz. 2021. "Effect of Iron (III) Oxide Powder on Thermal Conductivity and Diffusivity of Lime Mortar" Materials 14, no. 4: 998. https://doi.org/10.3390/ma14040998
APA StyleMasdeu, F., Carmona, C., Horrach, G., & Muñoz, J. (2021). Effect of Iron (III) Oxide Powder on Thermal Conductivity and Diffusivity of Lime Mortar. Materials, 14(4), 998. https://doi.org/10.3390/ma14040998






