Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview
Abstract
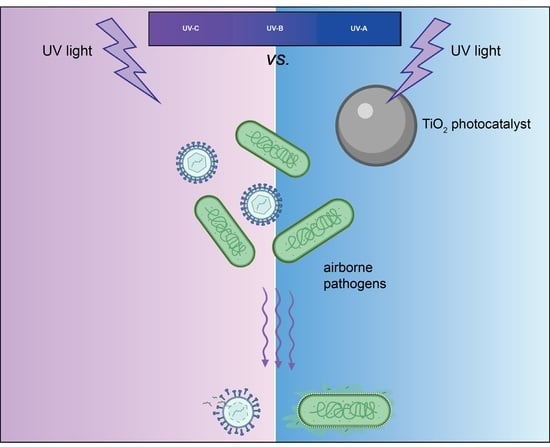
:1. Introduction
2. UV-Induced Disinfection
2.1. Mechanism of Action
2.1.1. Direct NAs Damage by UV Irradiation
2.1.2. Oxidative Damage to NAs and Proteins
2.2. Antimicrobial Effects of UV Light
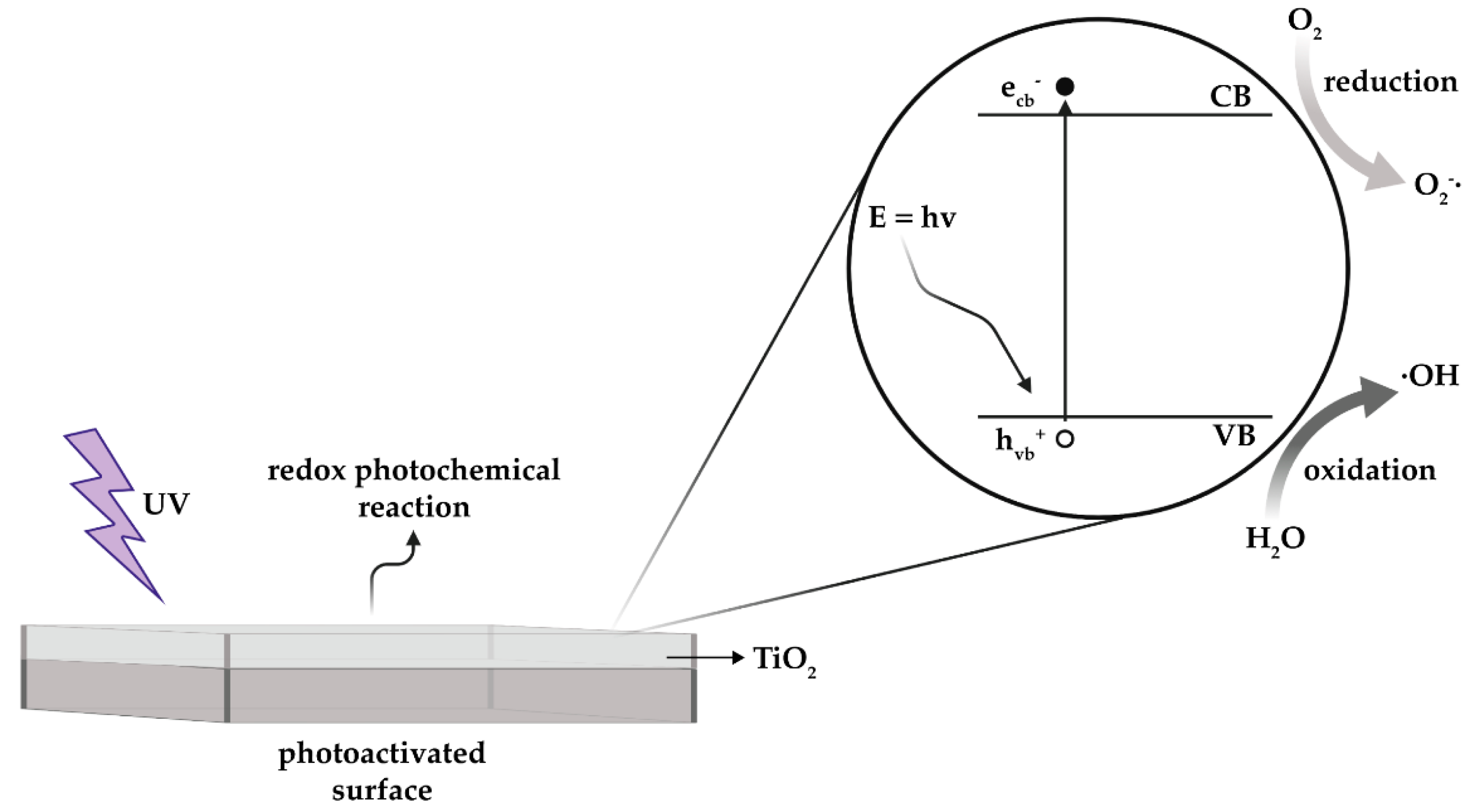
3. UV-Activated Photocatalysis
3.1. Operating Principles
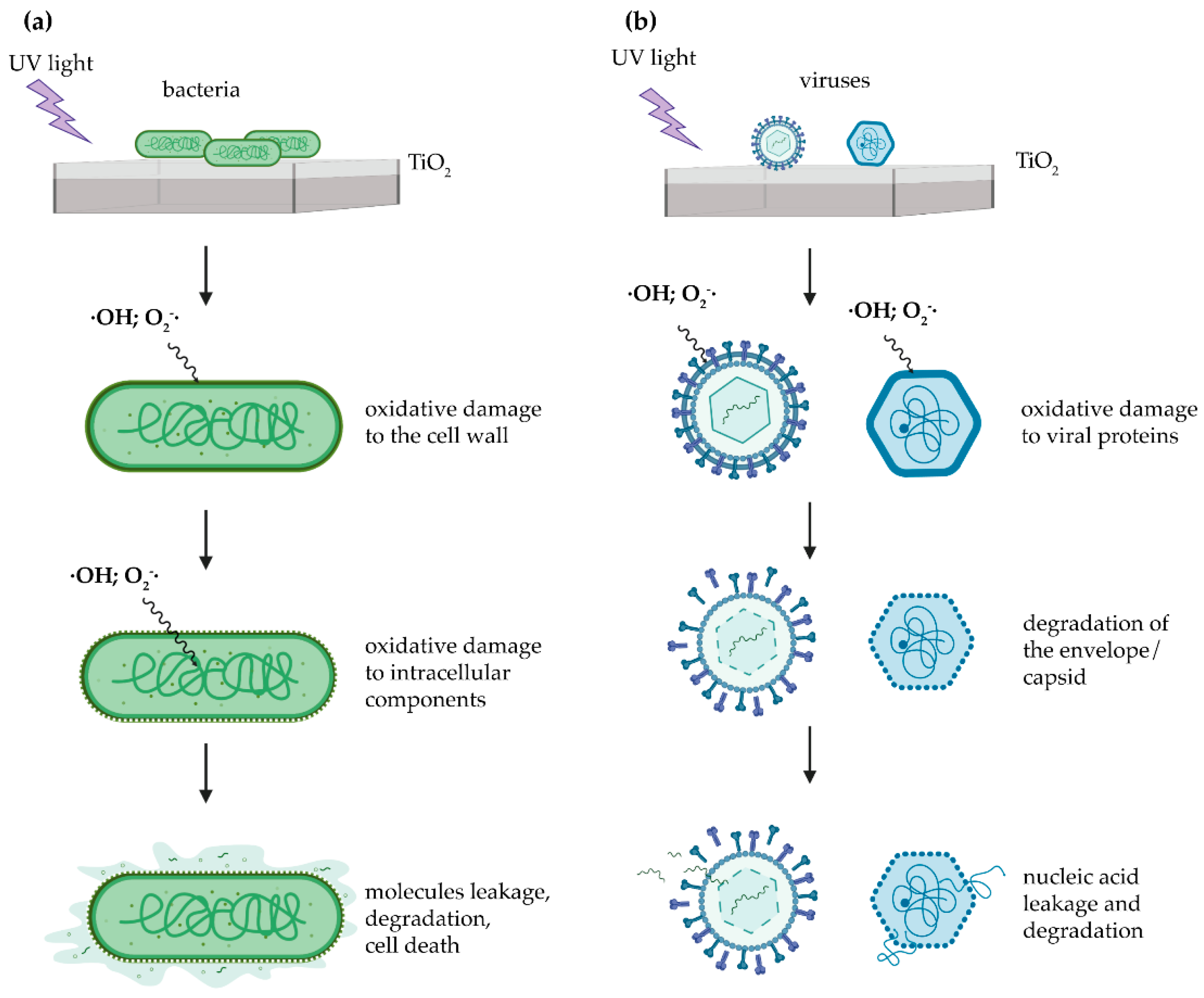
3.2. Photocatalysis-Induced Damage by ROS Formation
3.3. Antimicrobial Effects of UV Light-Induced TiO2 photocatalysis
4. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weiss, C.; Carriere, M.; Fusco, L.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Pasquali, M.; Pasquali, M.; Scott, J.A.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Kim, J.; Jang, J. Inactivation of airborne viruses using vacuum ultraviolet photocatalysis for a flow-through indoor air purifier with short irradiation time. Aerosol Sci. Technol. 2018, 52, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Wu, Y.; Shen, F.; Chen, Q.; Tan, M.; Yao, M. Bioaerosol science, technology, and engineering: Past, present, and future. Aerosol Sci. Technol. 2011, 45, 1337–1349. [Google Scholar] [CrossRef]
- Candiani, G.; Del Curto, B.; Cigada, A. Improving indoor air quality by using the new generation of corrugated cardboard-based filters. J. Appl. Biomater. Funct. Mater. 2012, 10, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Widmer, A.F.; Frei, R. Decontamination, disinfection, and sterilization. In Manual of Clinical Microbiology; American Society for Microbiology: Washington, DC, USA, 2011; pp. 143–173. [Google Scholar]
- Rutala, W.A.; Weber, D.J. Sterilization, High-Level Disinfection, and Environmental Cleaning. Infect. Dis. Clin. N. Am. 2011, 25, 45–76. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.F.; Hu, Z.B.; Liu, M.; Yang, H.L.; Kong, Q.X.; Liu, Y.H. Review of research on air-conditioning systems and indoor air quality control for human health. Int. J. Refrig. 2009, 32, 3–20. [Google Scholar] [CrossRef]
- Ryan, K.; McCabe, K.; Clements, N.; Hernandez, M.; Miller, S.L. Inactivation of airborne microorganisms using novel ultraviolet radiation sources in reflective flow-through control devices. Aerosol Sci. Technol. 2010, 44, 541–550. [Google Scholar]
- Liang, Y.; Wu, Y.; Sun, K.; Chen, Q.; Shen, F.; Zhang, J.; Yao, M.; Zhu, T.; Fang, J. Rapid inactivation of biological species in the air using atmospheric pressure nonthermal plasma. Environ. Sci. Technol. 2012, 46, 3360–3368. [Google Scholar] [CrossRef]
- Lee, B.U. Life comes from the air: A short review on bioaerosol control. Aerosol Air Qual. Res. 2011, 11, 921–927. [Google Scholar] [CrossRef] [Green Version]
- Hwang, G.B.; Jung, J.H.; Jeong, T.G.; Lee, B.U. Effect of hybrid UV-thermal energy stimuli on inactivation of S. epidermidis and B. subtilis bacterial bioaerosols. Sci. Total Environ. 2010, 408, 5903–5909. [Google Scholar] [CrossRef]
- Hollaender, A.; Du Buy, H.G.; Ingraham, H.S.; Wheeler, S.M. Control of air-borne microorganisms by ultraviolet floor irradiation. Science (80-) 1944, 99, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W. ISO 15714:2019 Method of Evaluating the UV Do; Springer Science and Business Media LLC: Berlin, Germany, 2009; ISBN 9783642019982. [Google Scholar]
- Darnell, M.E.R.; Subbarao, K.; Feinstone, S.M.; Taylor, D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods 2004, 121, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kujundzic, E.; Matalkah, F.; Howard, C.J.; Hernandez, M.; Miller, S.L. UV air cleaners and upper-room air ultraviolet germicidal irradiation for controlling airborne bacteria and fungal spores. J. Occup. Environ. Hyg. 2006, 3, 536–546. [Google Scholar] [CrossRef]
- Conner-Kerr, T.A.; Sullivan, P.K.; Gaillard, J.; Franklin, M.E.; Jones, R.M. The effects of ultraviolet radiation on antibiotic-resistant bacteria in vitro. Ostomy Wound. Manag. 1998, 44, 50–56. [Google Scholar] [PubMed]
- Budowsky, E.I.; Bresler, S.E.; Friedman, E.A.; Zheleznova, N.V. Principles of selective inactivation of viral genome—I. UV-induced inactivation of influenza virus. Arch. Virol. 1981, 68, 239–247. [Google Scholar] [CrossRef]
- Kowalski, W.J.; Bahnfleth, W.P.; Witham, D.L.; Severin, B.F.; Whittam, T.S. Mathematical modeling of ultraviolet germicidal irradiation for air disinfection. Quant. Microbiol. 2000, 2, 249–270. [Google Scholar] [CrossRef]
- Beck, S.E.; Rodriguez, R.A.; Hawkins, M.A.; Hargy, T.M.; Larason, T.C.; Linden, K.G. Comparison of UV-induced inactivation and RNA damage in MS2 phage across the germicidal UV spectrum. Appl. Environ. Microbiol. 2016, 82, 1468–1474. [Google Scholar] [CrossRef] [Green Version]
- Besaratinia, A.; Yoon, J.; Schroeder, C.; Bradforth, S.E.; Cockburn, M.; Pfeifer, G.P. Wavelength dependence of ultraviolet radiation-induced DNA damage as determined by laser irradiation suggests that cyclobutane pyrimidine dimers are the principal DNA lesions produced by terrestrial sunlight. FASEB J. 2011, 25, 3079–3091. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, D. Ultraviolet Light Fights New Virus. Engineering 2020, 6, 851–853. [Google Scholar] [CrossRef]
- Memarzadeh, F.; Olmsted, R.N.; Bartley, J.M. Applications of ultraviolet germicidal irradiation disinfection in health care facilities: Effective adjunct, but not stand-alone technology. Am. J. Infect. Control 2010, 38, S13–S24. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, M.; Wang, L.; Niu, F.; Yang, D.; Zhang, G. Evaluation survey of microbial disinfection methods in UV-LED water treatment systems. Sci. Total Environ. 2019, 659, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, L.A.; Noakes, C.J.; Beggs, C.B.; Sleigh, P.A.; Kerr, K.G. The ultraviolet susceptibility of aerosolised microorganisms and the role of photoreactivation. In Proceedings of the 2nd International Congress on Ultraviolet Technologies, Vienna, Austria, 9–11 July 2003; International Ozone Association: Scottsdale, AZ, USA, 2003; p. 10. [Google Scholar]
- Setlow, R.B.; Grist, E.; Thompson, K.; Woodhead, A.D. Wavelengths effective in induction of malignant melanoma. Proc. Natl. Acad. Sci. USA 1993, 90, 6666–6670. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.I. Ultraviolet Light in Human Health, Diseases and Environment; Springer: Berlin, Germany, 2017; Volume 996, ISBN 978-3-319-56017-5. [Google Scholar]
- Kebbi, Y.; Muhammad, A.I.; Sant’Ana, A.S.; do Prado-Silva, L.; Liu, D.; Ding, T. Recent advances on the application of UV-LED technology for microbial inactivation: Progress and mechanism. Compr. Rev. Food Sci. Food Saf. 2020, 1–27. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Asadzadeh-Khaneghah, S.; Feizpoor, S.; Rouhi, A. Review on heterogeneous photocatalytic disinfection of waterborne, airborne, and foodborne viruses: Can we win against pathogenic viruses? J. Colloid Interface Sci. 2020, 580, 503–514. [Google Scholar] [CrossRef]
- Cutler, T.D.; Zimmerman, J.J. Ultraviolet irradiation and the mechanisms underlying its inactivation of infectious agents. Anim. Health Res. Rev. 2011, 12, 15–23. [Google Scholar] [CrossRef]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherischia coli. Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B Environ. 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [Green Version]
- Da Costa Filho, B.M.; Vilar, V.J.P. Strategies for the intensification of photocatalytic oxidation processes towards air streams decontamination: A review. Chem. Eng. J. 2020, 391, 123531. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Destaillats, H.; Sleiman, M.; Sullivan, D.P.; Jacquiod, C.; Sablayrolles, J.; Molins, L. Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions. Appl. Catal. B Environ. 2012, 128, 159–170. [Google Scholar] [CrossRef]
- Reed, N.G. The history of ultraviolet germicidal irradiation for air disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef]
- Pattison, D.I.; Davies, M.J. Actions of ultraviolet light on cellular structures. EXS 2006, 131–157. [Google Scholar]
- Davies, M.J. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003, 305, 761–770. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef]
- Kesavan, J.S.; Sagripanti, J.L. Disinfection of Airborne Organisms by Ultraviolet-C Radiation and Sunlight. Aerosol Sci. Technol. Appl. 2014, 9781119977, 417–439. [Google Scholar] [CrossRef]
- Pigeot-Rémy, S.; Simonet, F.; Atlan, D.; Lazzaroni, J.C.; Guillard, C. Bactericidal efficiency and mode of action: A comparative study of photochemistry and photocatalysis. Water Res. 2012, 46, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.P.; Douki, T.; Richard, M.J.; Cadet, J. DNA damage induced in cells by γ and UVA radiation as measured by HPLC/GC-MS and HPLC-EC and comet assay. Chem. Res. Toxicol. 2000, 13, 541–549. [Google Scholar] [CrossRef]
- Ariza-Mateos, A.; Prieto-Vega, S.; Díaz-Toledano, R.; Birk, A.; Szeto, H.; Mena, I.; Berzal-Herranz, A.; Gómez, J. RNA self-cleavage activated by ultraviolet light-induced oxidation. Nucleic Acids Res. 2012, 40, 1748–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravanat, J.L.; Douki, T.; Cadet, J. Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B Biol. 2001, 63, 88–102. [Google Scholar] [CrossRef]
- Wigginton, K.R.; Pecson, B.M.; Sigstam, T.; Bosshard, F.; Kohn, T. Virus inactivation mechanisms: Impact of disinfectants on virus function and structural integrity. Environ. Sci. Technol. 2012, 46, 12069–12078. [Google Scholar] [CrossRef]
- Mullenders, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britt, A.B. Repair of DNA damage induced by solar UV. Photosynth. Res. 2004, 81, 105–112. [Google Scholar] [CrossRef]
- Lytle, C.D.; Sagripanti, J.-L. Predicted Inactivation of Viruses of Relevance to Biodefense by Solar Radiation. J. Virol. 2005, 79, 14244–14252. [Google Scholar] [CrossRef] [Green Version]
- Courdavault, S.; Baudouin, C.; Charveron, M.; Canguilhem, B.; Favier, A.; Cadet, J.; Douki, T. Repair of the three main types of bipyrimidine DNA photoproducts in human keratinocytes exposed to UVB and UVA radiations. DNA Repair (Amst.) 2005, 4, 836–844. [Google Scholar] [CrossRef]
- Douki, T.; Reynaud-Angelin, A.; Cadet, J.; Sage, E. Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochemistry 2003, 42, 9221–9226. [Google Scholar] [CrossRef]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis, 2nd ed.; American Society for Microbiology: Washington, DC, USA, 2005; Volume 12, ISBN 1555813194. [Google Scholar]
- Tseng, C.C.; Li, C.S. Inactivation of virus-containing aerosols by ultraviolet germicidal irradiation. Aerosol Sci. Technol. 2005, 39, 1136–1142. [Google Scholar] [CrossRef]
- Wurtmann, E.J.; Wolin, S.L. RNA under attack: Cellular handling of RNA damage RNA under attack: Cellular handling of RNA damage E. J. Wurtmann et.al. Crit. Rev. Biochem. Mol. Biol. 2009, 44, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Mayer, B.K.; Yang, Y.; Gerrity, D.W.; Abbaszadegan, M. The Impact of Capsid Proteins on Virus Removal and Inactivation during Water Treatment Processes. Microbiol. Insights 2015, 8s2, MBI.S31441. [Google Scholar] [CrossRef]
- Rule Wigginton, K.; Menin, L.; Montoya, J.P.; Kohn, T. Oxidation of virus proteins during UV254 and singlet oxygen mediated inactivation. Environ. Sci. Technol. 2010, 44, 5437–5443. [Google Scholar] [CrossRef]
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012, 11, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Reactive species formed on proteins exposed to singlet oxygen. Photochem. Photobiol. Sci. 2004, 3, 17–25. [Google Scholar] [CrossRef]
- Sullivan, P.K.; Conner-Kerr, T.A. A comparative study of the effects of UVC irradiation on select procaryotic and eucaryotic wound pathogens. Ostomy. Wound. Manag. 2000, 46, 28–34. [Google Scholar]
- Mohr, H.; Steil, L.; Gravemann, U.; Thiele, T.; Hammer, E.; Greinacher, A.; Müller, T.H.; Völker, U. A novel approach to pathogen reduction in platelet concentrates using short-wave ultraviolet light. Transfusion 2009, 49, 2612–2624. [Google Scholar] [CrossRef]
- Buonanno, M.; Randers-Pehrson, G.; Bigelow, A.W.; Trivedi, S.; Lowy, F.D.; Spotnitz, H.M.; Hammer, S.M.; Brenner, D.J. 207-nm UV Light—A Promising Tool for Safe Low-Cost Reduction of Surgical Site Infections. I: In Vitro Studies. PLoS ONE 2013, 8, e76968. [Google Scholar] [CrossRef] [Green Version]
- Buonanno, M.; Stanislauskas, M.; Ponnaiya, B.; Bigelow, A.W.; Randers-Pehrson, G.; Xu, Y.; Shuryak, I.; Smilenov, L.; Owens, D.M.; Brenner, D.J. 207-nm UV light—A promising tool for safe low-cost reduction of surgical site infections. II: In-vivo safety studies. PLoS ONE 2016, 11, e013841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDevitt, J.J.; Rudnick, S.N.; Radonovich, L.J. Aerosol susceptibility of influenza virus to UV-C light. Appl. Environ. Microbiol. 2012, 78, 1666–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, D.; Buonanno, M.; Grilj, V.; Shuryak, I.; Crickmore, C.; Bigelow, A.W.; Randers-Pehrson, G.; Johnson, G.W.; Brenner, D.J. Far-UVC light: A new tool to control the spread of airborne-mediated microbial diseases. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Storm, N.; McKay, L.G.A.; Downs, S.N.; Johnson, R.I.; Birru, D.; de Samber, M.; Willaert, W.; Cennini, G.; Griffiths, A. Rapid and complete inactivation of SARS-CoV-2 by ultraviolet-C irradiation. Sci. Rep. 2020, 10, 1–5. [Google Scholar] [CrossRef]
- Bedell, K.; Buchaklian, A.H.; Perlman, S. Efficacy of an automated multiple emitter whole-room Ultraviolet-C disinfection system against coronaviruses MHV and MERS-CoV. Infect. Control Hosp. Epidemiol. 2016, 37, 598–599. [Google Scholar] [CrossRef] [Green Version]
- Ludwig-Begall, L.F.; Wielick, C.; Dams, L.; Nauwynck, H.; Demeuldre, P.-F.; Napp, A.; Laperre, J.; Haubruge, E.; Thiry, E. Decontamination of face masks and filtering facepiece respirators via ultraviolet germicidal irradiation, hydrogen peroxide vaporisation, and use of dry heat inactivates an infectious SARS-CoV-2 surrogate virus. medRxiv 2020, 3608. [Google Scholar] [CrossRef]
- Bianco, A.; Biasin, M.; Pareschi, G.; Cavalleri, A.; Cavatorta, C.; Fenizia, F.; Galli, P.; Lessio, L.; Lualdi, M.; Redaelli, E.; et al. UV-C Irradiation Is Highly Effective in Inactivating and Inhibiting SARS-CoV-2 Replication. SSRN Electron. J. 2020, 1–9. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Buonanno, M.; Welch, D.; Shuryak, I.; Brenner, D.J. Far-UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Daikoku, T.; Takemoto, M.; Yoshida, Y.; Okuda, T.; Takahashi, Y.; Ota, K.; Tokuoka, F.; Kawaguchi, A.T.; Shiraki, K. Decomposition of organic chemicals in the air and inactivation of aerosol-associated influenza infectivity by photocatalysis. Aerosol Air Qual. Res. 2015, 15, 1469–1484. [Google Scholar] [CrossRef] [Green Version]
- Josset, S.; Taranto, J.; Keller, N.; Keller, V.; Lett, M.C. Photocatalytic Treatment of Bioaerosols: Impact of the Reactor Design. Environ. Sci. Technol. 2010, 44, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Pigeot-Remy, S.; Lazzaroni, J.C.; Simonet, F.; Petinga, P.; Vallet, C.; Petit, P.; Vialle, P.J.; Guillard, C. Survival of bioaerosols in HVAC system photocatalytic filters. Appl. Catal. B Environ. 2014, 144, 654–664. [Google Scholar] [CrossRef]
- Rodrigues-Silva, C.; Miranda, S.M.; Lopes, F.V.S.; Silva, M.; Dezotti, M.; Silva, A.M.T.; Faria, J.L.; Boaventura, R.A.R.; Vilar, V.J.P.; Pinto, E. Bacteria and fungi inactivation by photocatalysis under UVA irradiation: Liquid and gas phase. Environ. Sci. Pollut. Res. 2017, 24, 6372–6381. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Chen, C.N.; Tseng, T.T.; Wei, M.H.; Hsieh, J.H.; Tseng, W.J. Micellar layer-by-layer synthesis of TiO2/Ag hybrid particles for bactericidal and photocatalytic activities. J. Eur. Ceram. Soc. 2010, 30, 2849–2857. [Google Scholar] [CrossRef]
- Pal, A.; Pehkonen, S.O.; Yu, L.E.; Ray, M.B. Photocatalytic inactivation of airborne bacteria in a continuous-flow reactor. Ind. Eng. Chem. Res. 2008, 47, 7580–7585. [Google Scholar] [CrossRef]
- Keller, N.; Rebmann, G.; Barraud, E.; Zahraa, O.; Keller, V. Macroscopic carbon nanofibers for use as photocatalyst support. Catal. Today 2005, 101, 323–329. [Google Scholar]
- Sirimahachai, U.; Phongpaichit, S.; Wongnawa, S. Evaluation of bactericidal activity of TiO2 photocatalysts: A comparative study of laboratory-made and commercial TiO2 samples. Songklanakarin J. Sci. Technol. 2009, 31, 517–525. [Google Scholar]
- Sclafani, A.; Herrmann, J.M. Comparison of the photoelectronic and photocatalytic activities of various anatase and rutile forms of titania in pure liquid organic phases and in aqueous solutions. J. Phys. Chem. 1996, 100, 13655–13661. [Google Scholar] [CrossRef]
- Miyagi, T.; Kamei, M.; Mitsuhashi, T.; Ishigaki, T.; Yamazaki, A. Charge separation at the rutile/anatase interface: A dominant factor of photocatalytic activity. Chem. Phys. Lett. 2004, 390, 399–402. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Sunada, K.; Iyoda, T.; Hashimoto, K.; Fujishima, A. Photocatalytic bactericidal effect of TiO2 thin films: Dynamic view of the active oxygen species responsible for the effect. J. Photochem. Photobiol. A Chem. 1997, 106, 51–56. [Google Scholar] [CrossRef]
- Sunada, K.; Kikuchi, Y.; Hashimoto, K.; Fujishima, A. Bactericidal and detoxification effects of TiO2 thin film photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Kühn, K.P.; Chaberny, I.F.; Massholder, K.; Stickler, M.; Benz, V.W.; Sonntag, H.G.; Erdinger, L. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere 2003, 53, 71–77. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.; Lin, J.; Yip, H.; Wong, P.K. Photocatalytic activity, antibacterial effect, and photoinduced hydrophilicity of TiO2 films coated on a stainless steel substrate. Environ. Sci. Technol. 2003, 37, 2296–2301. [Google Scholar] [CrossRef]
- Brook, L.A.; Evans, P.; Foster, H.A.; Pemble, M.E.; Steele, A.; Sheel, D.W.; Yates, H.M. Highly bioactive silver and silver/titania composite films grown by chemical vapour deposition. J. Photochem. Photobiol. A Chem. 2007, 187, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Ditta, I.B.; Steele, A.; Liptrot, C.; Tobin, J.; Tyler, H.; Yates, H.M.; Sheel, D.W.; Foster, H.A. Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008, 79, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedmann, D.; Mendive, C.; Bahnemann, D. TiO2 for water treatment: Parameters affecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B Environ. 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Visai, L.; de Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium oxide antibacterial surfaces in biomedical devices. Int. J. Artif. Organs 2011, 34, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Xu, C.; Ren, Z.; Yang, W.; Ma, Z.; Dai, D.; Fan, H.; Minton, T.K.; Yang, X. Stepwise photocatalytic dissociation of methanol and water on TiO2(110). J. Am. Chem. Soc. 2012, 134, 13366–13373. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Bogdan, J.; Zarzyńska, J.; Pławińska-Czarnak, J. Comparison of Infectious Agents Susceptibility to Photocatalytic Effects of Nanosized Titanium and Zinc Oxides: A Practical Approach. Nanoscale Res. Lett. 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol. 2005, 71, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.Y.; Lee, C.; Cho, M.; Yoon, J. Enhanced inactivation of E. coli and MS-2 phage by silver ions combined with UV-A and visible light irradiation. Water Res. 2008, 42, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Liu, X.; Zhang, P.; Ma, H.; Liu, G.; Xia, Z. The photodestruction of virus in Nano-TiO2 suspension. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2007, 22, 422–425. [Google Scholar] [CrossRef]
- Sjogren, J.C.; Sierka, R.A. Inactivation of phage MS2 by iron-aided titanium dioxide photocatalysis. Appl. Environ. Microbiol. 1994, 60, 344–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- ISO 15714:2019 Method of Evaluating the UV Dose to Airborne Microorganisms Transiting In-Duct Ultraviolet Germicidal Irradiation Devices 17; International Organization for Standardization: London, UK, 2019.
- ISO 27447:2009—Fine Ceramics (Advanced Ceramics and Advanced Technical Ceramics)—Test Method for Antibacterial Activity of Semiconducting Photocatalytic Materials; International Organization for Standardization: London, UK, 2009.
- Ishiguro, H.; Nakano, R.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of bacteriophages by TiO2-coated glass plates under low-intensity, long-wavelength UV irradiation. Photochem. Photobiol. Sci. 2011, 10, 1825–1829. [Google Scholar] [CrossRef]
- Doss, N.; Carré, G.; Keller, V.; André, P.; Keller, N. Photocatalytic Decontamination of Airborne T2 Bacteriophage Viruses in a Small-Size TiO2/Β-SiC Alveolar Foam LED Reactor. Water. Air. Soil Pollut. 2018, 229. [Google Scholar] [CrossRef]
- Khaiboullina, S.; Uppal, T.; Dhabarde, N.; Subramanian, V.R.; Verma, S.C. In Vitro Inactivation of Human Coronavirus by Titania Nanoparticle Coatings and UVC Radiation: Throwing Light on SARS-CoV-2. bioRxiv 2020, 6743. [Google Scholar] [CrossRef]
- Kim, B.; Kim, D.; Cho, D.; Cho, S. Bactericidal effect of TiO2 photocatalyst on selected food-borne pathogenic bacteria. Chemosphere 2003, 52, 277–281. [Google Scholar] [CrossRef]
- Rincón, A.G.; Pulgarin, C. Photocatalytical inactivation of E. coli: Effect of (continuous-intermittent) light intensity and of (suspended-fixed) TiO2 concentration. Appl. Catal. B Environ. 2003, 44, 263–284. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Robertson, P.K.J.; Officer, S.; Pollard, P.M.; McCullagh, C.; Robertson, J.M.C. Variables to be considered when assessing the photocatalytic destruction of bacterial pathogens. Chemosphere 2009, 74, 1374–1378. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. Antibacterial activity of TiO2 photocatalyst alone or in coatings on E. coli: The influence of methodological aspects. Coatings 2014, 4, 670–686. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Safatov, A.S.; Kiselev, S.A.; Marchenko, V.Y.; Sergeev, A.A.; Skarnovich, M.O.; Emelyanova, E.K.; Smetannikova, M.A.; Buryak, G.A.; Vorontsov, A.V. Inactivation and mineralization of aerosol deposited model pathogenic microorganisms over TiO2 and Pt/TiO2. Environ. Sci. Technol. 2010, 44, 5121–5126. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kwak, S.Y. Photocatalytic inactivation of E. coli with a mesoporous TiO2 coated film using the film adhesion method. Environ. Sci. Technol. 2009, 43, 148–151. [Google Scholar] [CrossRef]
- Hajkova, P.; Spatenka, P.; Horsky, J.; Horska, I.; Kolouch, A. Photocatalytic effect of TiO2 films on viruses and bacteria. In Plasma Processes and Polymers; Wiley Online Library: Hoboken, NJ, USA, 2007; Volume 4, pp. 397–401. [Google Scholar]
- Nakano, R.; Ishiguro, H.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012, 11, 1293–1298. [Google Scholar] [CrossRef]
- Kim, S.H.; Shahbaz, H.M.; Park, D.; Chun, S.; Lee, W.; Oh, J.W.; Lee, D.U.; Park, J. A combined treatment of UV-assisted TiO2 photocatalysis and high hydrostatic pressure to inactivate internalized murine norovirus. Innov. Food Sci. Emerg. Technol. 2017, 39, 188–196. [Google Scholar] [CrossRef]
- Moongraksathum, B.; Chien, M.-Y.; Chen, Y.-W. Antiviral and Antibacterial Effects of Silver-Doped TiO2 Prepared by the Peroxo Sol-Gel Method. J. Nanosci. Nanotechnol. 2019, 19, 7356–7362. [Google Scholar] [CrossRef]
- Moon, E.W.; Lee, H.W.; Rok, J.H.; Ha, J.H. Photocatalytic inactivation of viral particles of human norovirus by Cu-doped TiO2 non-woven fabric under UVA-LED wavelengths. Sci. Total Environ. 2020, 749, 141574. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Jiang, J.; Gu, W.; Sun, C.; Wu, D.; Yang, T.; Yang, G. Photocatalytic inactivation efficiency of anatase nano-TiO2 sol on the H9N2 avian influenza virus. Photochem. Photobiol. 2010, 86, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Hallmich, C.; Gehr, R. Effect of pre- and post-UV disinfection conditions on photoreactivation of fecal coliforms in wastewater effluents. Water Res. 2010, 44, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Suri, R.P.S.; Thornton, H.M.; Muruganandham, M. Disinfection of water using Pt- and Ag-doped TiO2 photocatalysts. Environ. Technol. (U.K.) 2012, 33, 1651–1659. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; Yu, J.C.; Wong, P.K. Advances in photocatalytic disinfection of bacteria: Development of photocatalysts and mechanisms. J. Environ. Sci. (China) 2015, 34, 232–247. [Google Scholar] [CrossRef]

| Target | Photocatalyst | Light Parameters | Irradiation Time | Antibacterial Efficiency | Estimated Minimum UV Dose (According to Equation (1)) | Reference |
|---|---|---|---|---|---|---|
| S. choleraesuis, V. parahaemolyticus, L. monocytogenes | 10 mg mL−1 (Petri dish) | λ = 360 nm (UV-A); I = 0.4 mW cm−2 | 30 min 1 h 1.5 h 2 h | 100% at Tirr ≥ 2 h | 0.3 J cm−2 (or W s cm−2) | [109] |
| 0.25–1.25 mg mL−1 (batch reactor) | λ = 360 nm (UV-A); I = 0.1 mW cm−2 | 3 h 4 h | 100% at Tirr ≥ 3 h | 0.1 J cm−2 | ||
| E. coli | 0.025–1 mg mL−1 | λ = 400–800 nm (Vis–IR); I = 0.04 mW cm−2 I = 0.1 mW cm−2 | 2 h | 100% at Tirr ≥ 40 min 100% at Tirr ≥ 25 min | 96 J cm−2 150 J cm−2 | [110] |
| S. aureus, S. typhimurium, P. aeruginosa, E. coli | 1 mg mL−1 | λ = 368 nm (UV-A); I = n.d. | 30 min 1 h 1.5 h 2 h 2.5 h | 100% at Tirr ≥ 1 h | / | [111] |
| E. coli | 1 mg mL−1 | λ = 310–400 nm (UV-A); I = 0.5 mW cm−2 | 30 min 1 h 1.5 h 2 h 2.5 h 3 h 3.5 h 4 h | 100% at Tirr ≥ 4 h | 1.8 J cm−2 | [112] |
| E. coli | 0.25 mg mL−1 | λ = 355–375 nm (UV-A); I = 3.6 mW cm−2 λ = 254 nm (UV-C); I = 3.6 mW cm−2 | 30 min 1 h 1.5 h 2 h | 100% at Tirr ≥ 90 min (UV-A) 100% at Tirr ≥ 30 min (UV-C) | 19 J cm−2 (UV-A) 6.5 J cm−2 (UV-C) | [44] |
| Target | Photoreactor | Light Parameters | Irradiation Time | Antibacterial Efficiency | Estimated Minimum UV Dose (According to Equation (1)) | Reference |
|---|---|---|---|---|---|---|
| E. coli | TiO2-coated Petri dish | λ = 310–400 nm (UV-A); I = 0.25 mW cm−2 | 2 h 4 h 6 h | 100% at Tirr ≥ 2 h | 7.2 J cm−2 | [112] |
| M. smegmatis, B. thuringiensis | TiO2 and Pt/TiO2-coated glass | λ = 350–400 nm (UV-A); I = 0.65 mW cm−2 | 10 min 20 min 30 min | 99.8% at Tirr ≥ 30 min | 1.1 J cm−2 | [113] |
| E. coli | TiO2-coated glass | λ = 315–400 nm (UV-A); I = 1 mW cm−2 | 15 min 30 min 1 h 1.5 h | 100% at Tirr ≥ 30 min | 1.8 J cm−2 | [114] |
| E. coli | TiO2-coated filter | λ = 355–375 nm (UV-A) λ = 280–320 nm (UV-B) λ = 254 nm (UV-C); I = 3.6 mW cm−2 | 2 h 4 h 6 h | 100% at Tirr ≥ 4 h | 518 J cm−2 | [76] |
| E. coli, P. aeruginosa, C. freundii, S. aureus, S. saprophyticus MRSA | TiO2-coated cellulose acetate monoliths | λ = 365 nm (UV-A); I = n.d. | 5 min 10 min 15 min 20 min | 100% at Tirr = 20 min | / | [77] |
| E. coli | TiO2 film | λ = 365 nm (UV-A); I = n.d. | 1 h 3 h 6 h 8 h | 100% at Tirr ≥ 6 h | / | [115] |
| E. coli | Continuous annular reactor with TiO2-coated filter | λ = 365 nm (UV-A); I = 0.5 mW cm−2 I = 3.4 mW cm−2 | 1.1 min | 100% | 0.03 J cm−2 0.204 J cm−2 | [79] |
| Target | Photoreactor | Light Parameters | Irradiation Time | Antiviral Efficacy | Estimated Minimum UV Dose (According to Equation (1)) | Reference |
|---|---|---|---|---|---|---|
| Influenza virus H1N1 | TiO2-coated porous ceramic substrate | λ = 365 nm (UV-A); I = 1 mW cm−2 | 4 min 10 min 15 min 30 min | 100% at Tirr ≥ 5 min | 0.3 J cm−2 | [74] |
| Vaccinia virus, influenza virus H3N2 | TiO2 and Pt/TiO2-coated glass | λ = 350–400 nm (UV-A); I = 0.65 mW cm−2 | 10 min 20 min 30 min | 99.8% at Tirr ≥ 30 min | 1.1 J cm−2 | [113] |
| Influenza virus H1N1 | TiO2-coated glass | λ = 352 nm (UV-A); I = 0.001 mW cm−2 I = 0.01 mW cm−2 I = 0.1 mW cm−2 I = 1 mW cm−2 | 2 h 4 h 6 h 8 h | 100% | 0.8–14.4 J cm−2 | [116] |
| HSV-1 virus | TiO2 film | λ = 365 nm (UV-A); I = n.d. | 6 h | 100% | / | [115] |
| Noravirus | TiO2 photocatalytic reactor | λ = 254 nm (UV-C) | 4 min 10 min 15 min 20 min | 100% at Tirr ≥ 10 min | 2.7 J cm−2 | [117] |
| Qβ and T4 bacteriophages | TiO2-coated glass | λ = 351 nm (UV-A) I = 0.001 mW cm−2 I = 0.01 mW cm−2 I = 0.1 mW cm−2 | 4 h 8 h 24 h | 99.99% at Tirr ≥ 8 h (with I = 0.1 mW cm−2) | 28.8 J cm−2 | [106] |
| Influenza virus H1N1, Enterovirus type 71 | 1% wt Ag/TiO2-coated glass | λ = 365 nm (UV-A) | 20 min | 99.99% at Tirr = 20 min | / | [118] |
| Human norovirus | Cu/TiO2 nonwoven fabric | λ = 365–405 nm (UV-A); I = 5000 mW cm−2 | 1–60 min | 99% at Tirr ≥ 48 min | / | [119] |
| T4 bacteriophage | TiO2-coated βSiC foam | λ = 392 nm (UV-A); I = 11.7 mW cm−2 | 15 min 30 min 45 min 60 min | 99.9% at Tirr ≥ 60 min | 42.12 J cm−2 | [107] |
| Avian influenza virus H9N2 | TiO2-coated Petri dish | λ = 365 nm (UV-A); I = 0.5 mW cm−2; I = 1 mW cm−2; I = 1.5 mW cm−2 | 30 min 1.5 h 2.5 h | 100% at Tirr = 2.5 h | 4.5 J cm−2 | [120] |
| Human coronavirus HCoV-NL63 | TiO2-coated glass | λ = 254 nm (UV-C); I = 2.9 mW cm−2; I = 4.3 mW cm−2; I = 13 mW cm−2 | 1 min 5 min 10 min | 100% at Tirr = 1 min (with I = 2.9 mW cm−2) | 0.17 J cm−2 | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials 2021, 14, 1075. https://doi.org/10.3390/ma14051075
Bono N, Ponti F, Punta C, Candiani G. Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials. 2021; 14(5):1075. https://doi.org/10.3390/ma14051075
Chicago/Turabian StyleBono, Nina, Federica Ponti, Carlo Punta, and Gabriele Candiani. 2021. "Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview" Materials 14, no. 5: 1075. https://doi.org/10.3390/ma14051075
APA StyleBono, N., Ponti, F., Punta, C., & Candiani, G. (2021). Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials, 14(5), 1075. https://doi.org/10.3390/ma14051075