Role of Sintering Temperature in Production of Nepheline Ceramics-Based Geopolymer with Addition of Ultra-High Molecular Weight Polyethylene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.3. Physical and Mechanical Testing
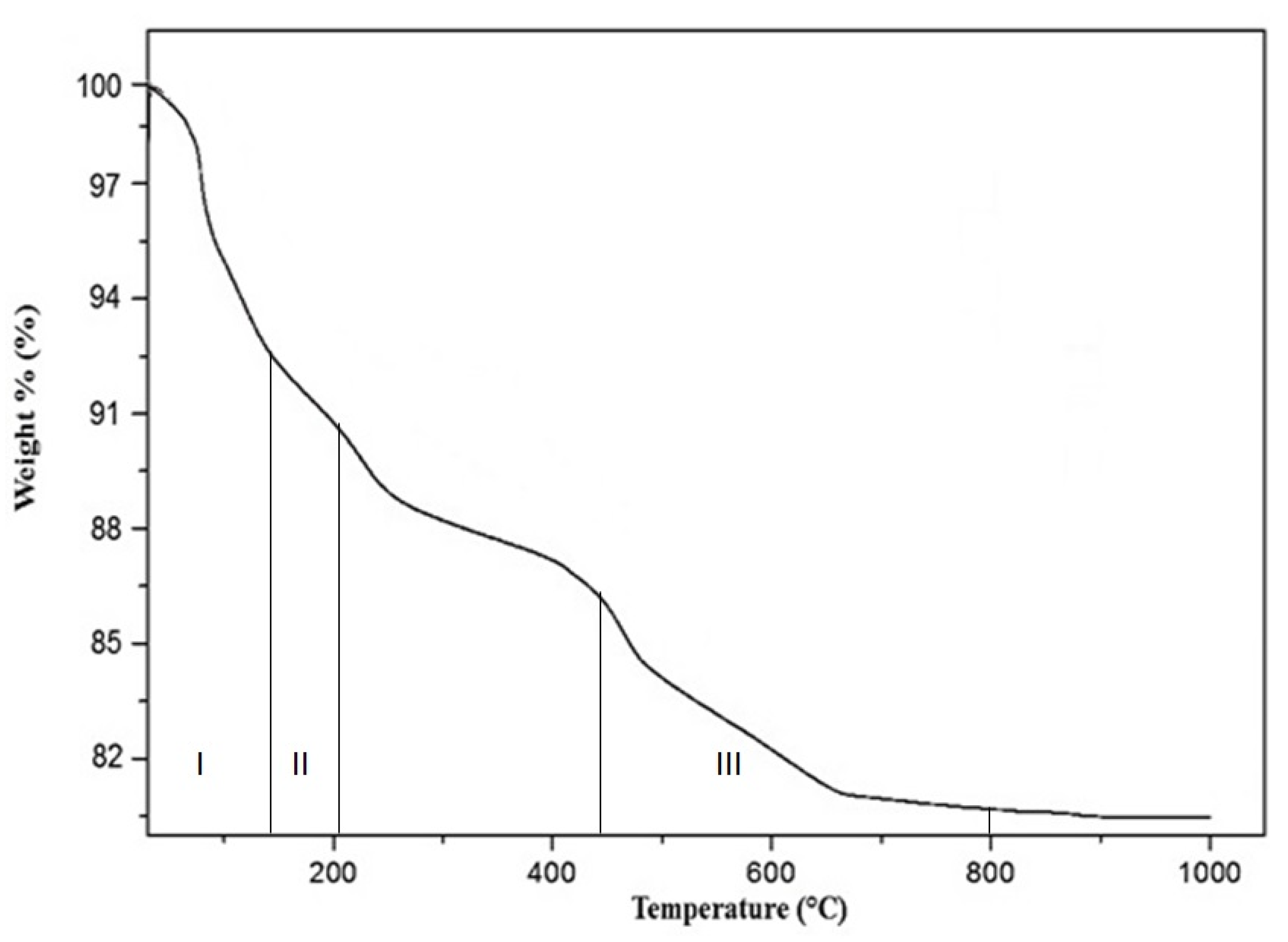
2.4. Thermal Analysis
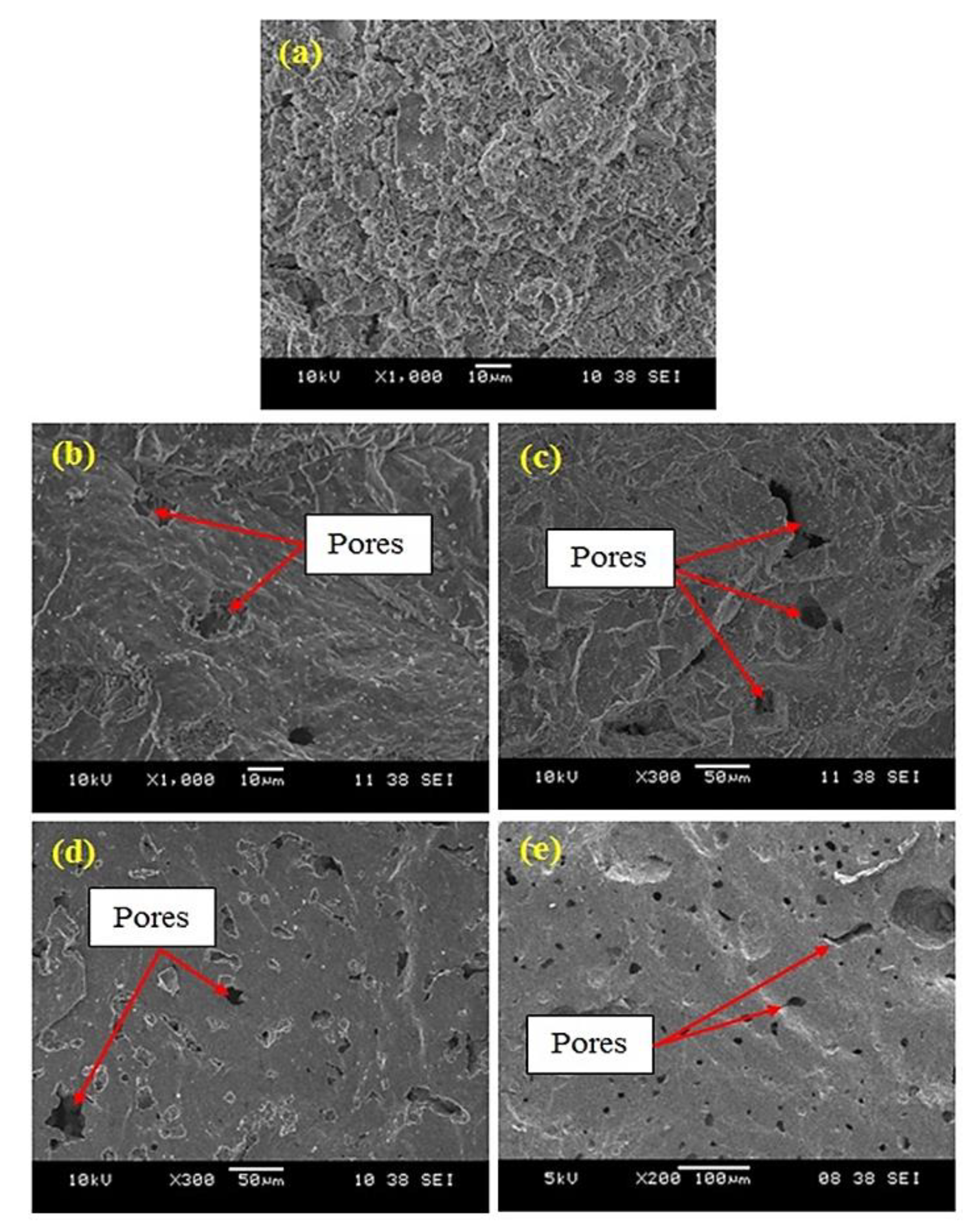
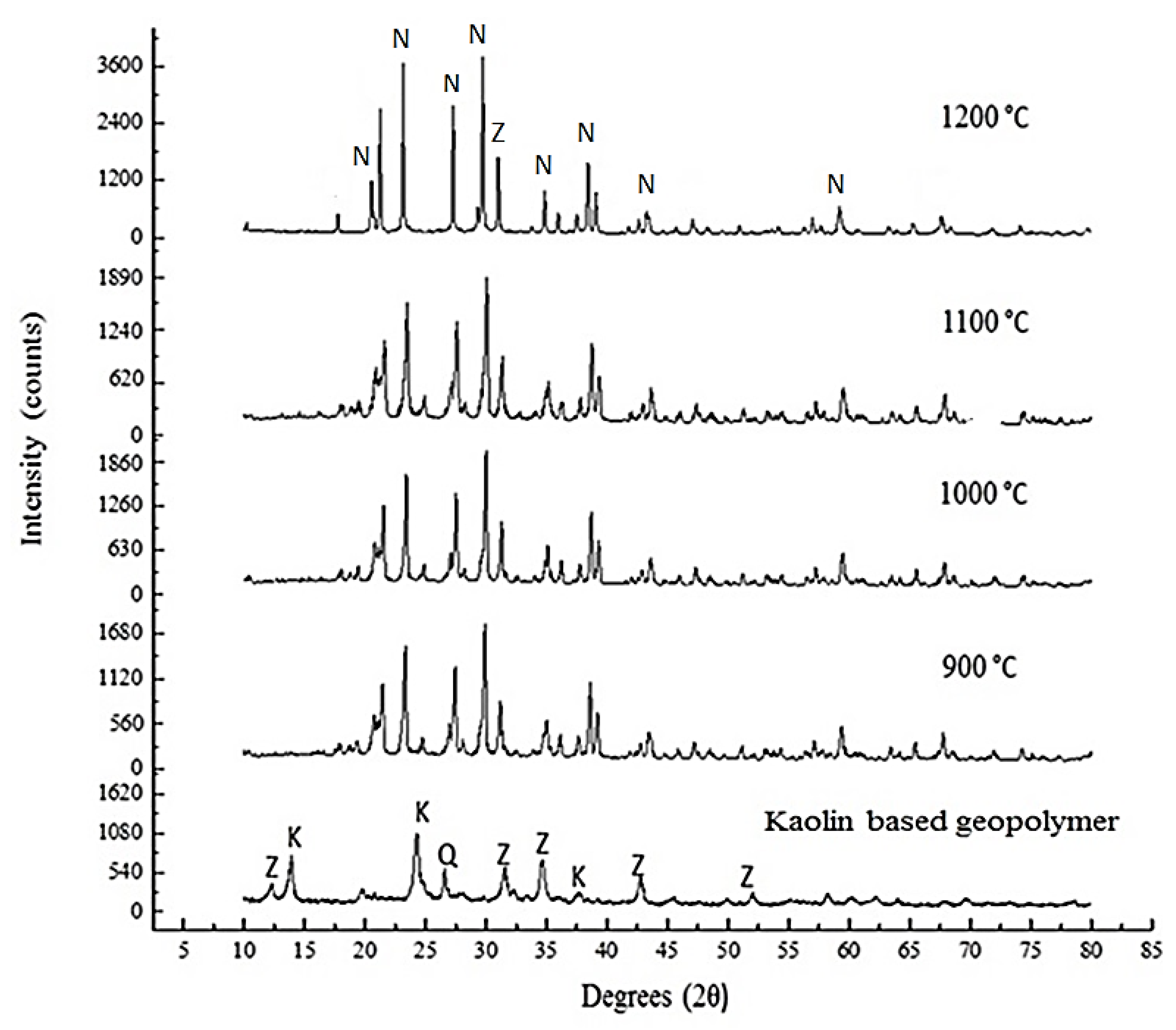
2.5. Microstructural Characterization Testing
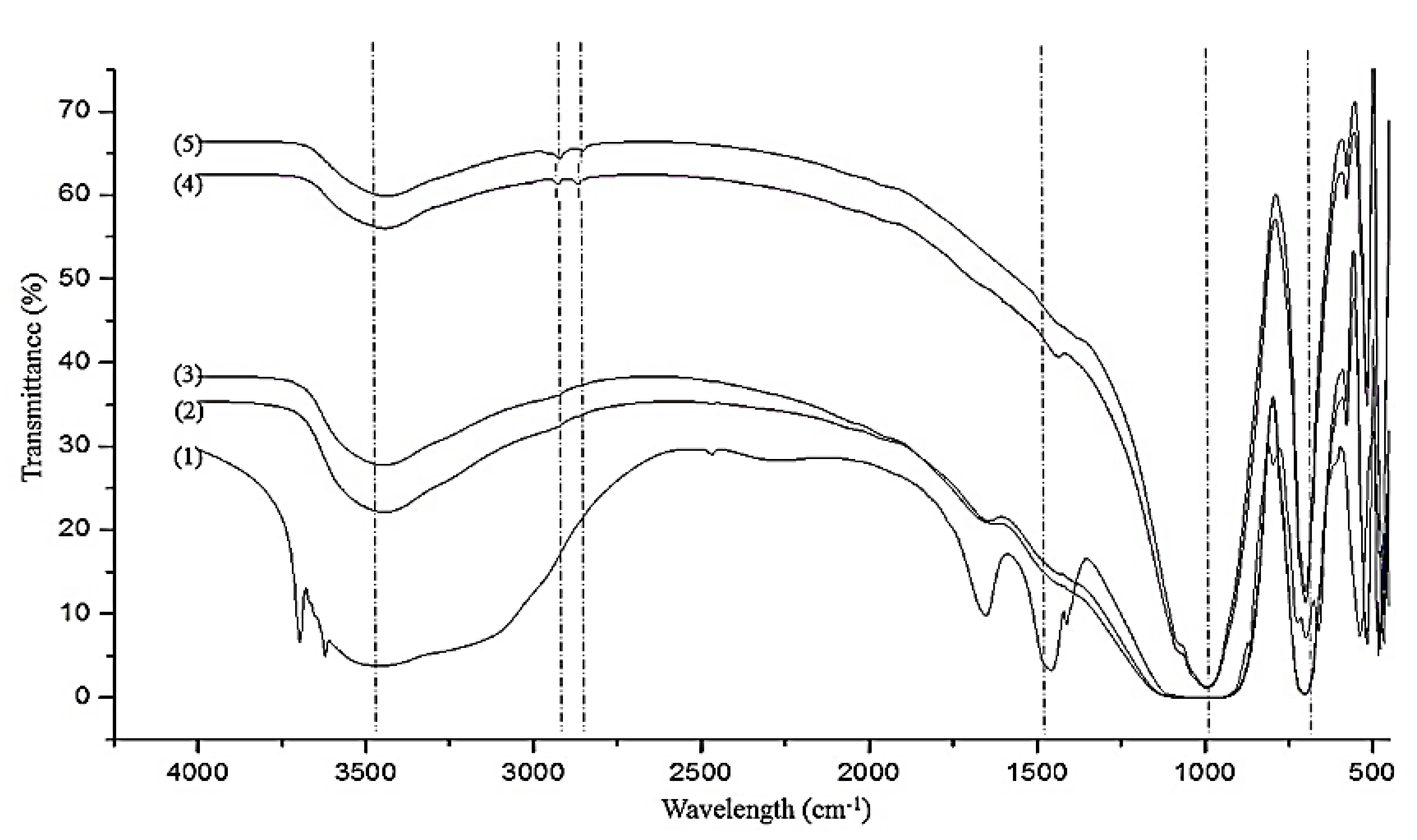
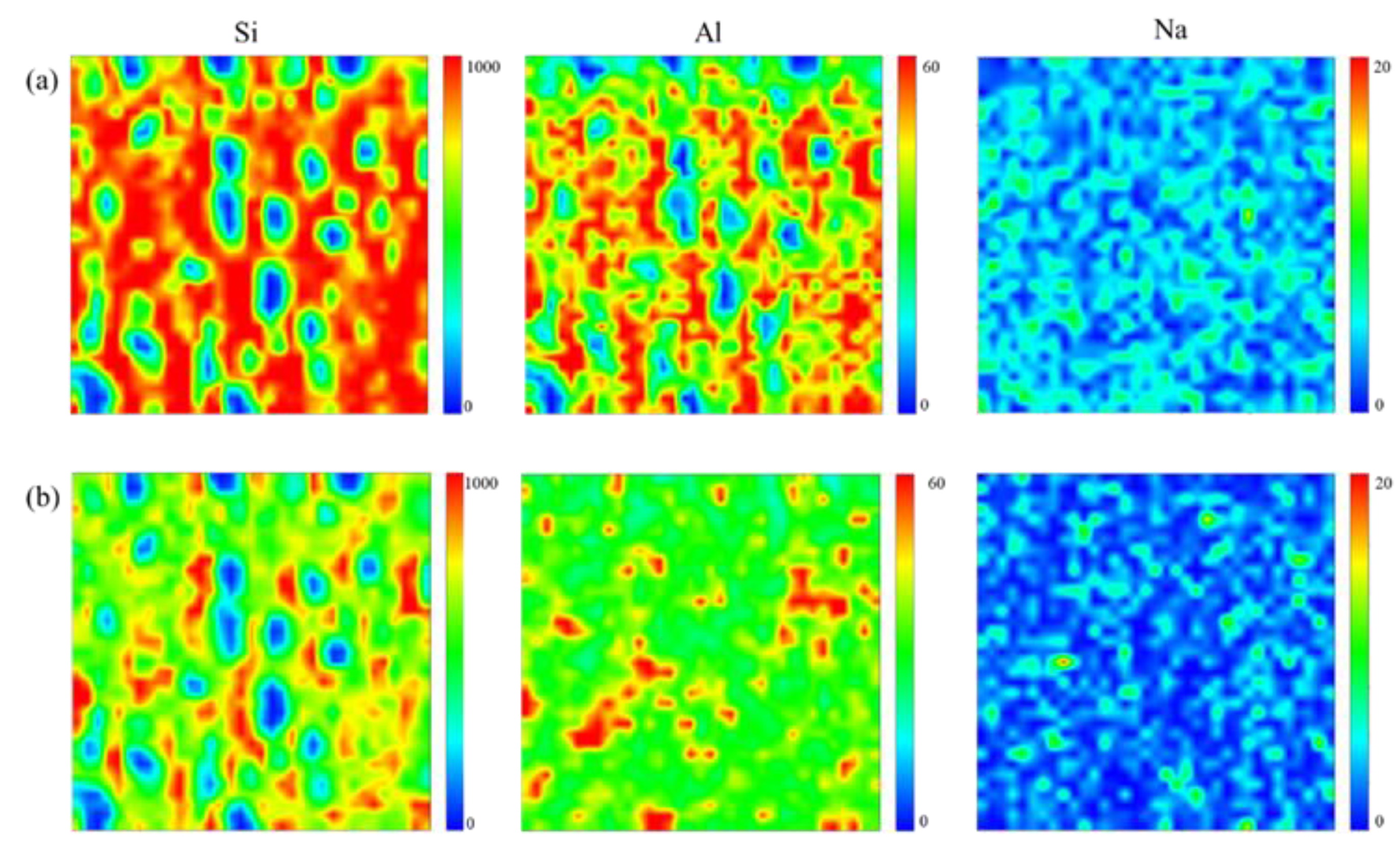
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, J.L.; Driemeyer, P.E.; Kriven, W.M. Formation of ceramics from metakaolin-based geopolymers. Part II: K-based geopolymer. J. Am. Ceram. Soc. 2009, 92, 607–615. [Google Scholar] [CrossRef]
- Raman, N.; Sudharsan, S.; Pothiraj, K. Synthesis and structural reactivity of inorganic–organic hybrid nanocomposites—A review. J. Saudi Chem. Soc. 2012, 16, 339–352. [Google Scholar] [CrossRef]
- Popoola, A.; Olorunniwo, O.; Ige, O. Corrosion resistance through the application of anti-corrosion coatings. In Developments in Corrosion Protection; InTech: Rijeka, Croatia, 2014. [Google Scholar]
- Rajeswari, K.; Chaitanya, S.; Biswas, P.; Suresh, M.B.; Rao, Y.S.; Johnson, R. Processing Research Binder burnout and sintering kinetic study of alumina ceramics shaped using methylcellulose. J. Ceram. Proc. Res. 2015, 16, 24–31. [Google Scholar]
- Taktak, R.; Baklouti, S.; Bouaziz, J. Effect of binders on microstructural and mechanical properties of sintered alumina. Mater. Char. 2011, 62, 912–916. [Google Scholar] [CrossRef]
- Mustafa Al Bakri, A.M.; Kamarudin, H.; Bnhussain, M.; Nizar, I.K.; Rafiza, A.R.; Izzat, A.M. Chemical Reactions in the Geopolymerisation Process Using Fly Ash-Based Geopolymer: A Review. J. Appl. Sci. Res. 2011, 7, 1199–1203. [Google Scholar]
- Mat Daud, Y.; Hussin, K.; Ruzaidi, C.M.; Osman, A.F.; Al Bakri Abdullah, M.M.; Binhussain, M. Characterization of Epoxy-Layered Silicates Filled with Fly Ash-Based Geopolymer. Appl. Mech. Mater. 2015, 754, 225–229. [Google Scholar] [CrossRef]
- Faris, M.A.; Abdullah, M.M.A.B.; Ismail, K.N.; Muniandy, R.; Ariffin, N. Performance of steel wool fiber reinforced geopolymer concrete. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2017; Volume 1885, p. 020219. [Google Scholar]
- Abdulkareem, O.A.; Abdullah, M.M.A.B.; Hussin, K.; Ismail, K.N.; Binhussain, M. Mechanical and Microstructural Evaluations of Lightweight Aggregate Geopolymer Concrete before and after Exposed to Elevated Temperatures. Materials 2013, 6, 4450–4461. [Google Scholar] [CrossRef]
- Xiao, R.; Jiang, X.; Zhang, M.; Polaczyk, P.; Huang, B. Analytical investigation of phase assemblages of alkali-activated materials in CaO-SiO2-Al2O3 systems: The management of reaction products and designing of precursors. Mater. Des. 2020, 194, 108975. [Google Scholar] [CrossRef]
- Biondi, L.; Perry, M.; Vlachakis, C.; Wu, Z.; Hamilton, A.; McAlorum, J. Ambient cured fly ash geopolymer coatings for concrete. Materials 2019, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Bellum, R.R.; Nerella, R.; Madduru, S.R.C.; Indukuri, C.S.R. Mix Design and Mechanical Properties of Fly Ash and GGBFS-Synthesized Alkali-Activated Concrete (AAC). Infrastructures 2019, 4, 20. [Google Scholar] [CrossRef]
- Detphan, S.; Chindaprasirt, P. Preparation of fly ash and rice husk ash geopolymer. Int. J. Miner. Metall. Mater. 2009, 16, 720–726. [Google Scholar]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Thermal evolution of metakaolin geopolymers: Part 1—Physical evolution. J. Non. Cryst. Solids 2006, 352, 5541–5555. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G. Damage behavior of geopolymer composites exposed to elevated temperatures. Cement. Conc. Comp. 2008, 30, 986–991. [Google Scholar] [CrossRef]
- Kuenzel, C.; Grover, L.M.; Vandeperre, L.; Boccaccini, A.R.; Cheeseman, C.R. Production of nepheline/quartz ceramics from geopolymer mortars. J. Eur. Ceram. Soc. 2013, 33, 251–258. [Google Scholar] [CrossRef]
- Kriven, W.M.; Halbig, M.; Mathur, S. Developments in strategic materials and computational design III. Presented at the 36th International Conference on Advanced Ceramics and Composites, Daytona Beach, FL, USA, 22–27 January 2012; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Barbosa, V.F.F.; MacKenzie, K.J.D. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Peigang, H.; Dechang, J.; Meirong, W.; Yu, Z. Thermal evolution and crystallization kinetics of potassium-based geopolymer. Ceram. Int. 2010, 37, 59–63. [Google Scholar]
- Iwahiro, T.; Nakamura, Y.; Komatsu, R.; Ikeda, K. Crystallization behavior and characteristics of mullites formed from alumina–silica gels prepared by the geopolymer technique in acidic conditions. J. Eur. Ceram. Soc. 2001, 21, 2515–2519. [Google Scholar] [CrossRef]
- Lóh, N.J.; Simão, L.; Faller, C.A.; De Noni, A.; Montedo, O.R.K. A review of two-step sintering for ceramics. Ceram. Int. 2016, 42, 12556–12572. [Google Scholar] [CrossRef]
- ASTM C373-88(2006), Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products; ASTM International: West Conshohocken, PA, USA, 2006.
- Bernard-Granger, G.; Monchalin, N.; Guizard, C. Sintering of ceramic powders: Determination of the densification and grain growth mechanisms from the ‘grain size/relative density’ trajectory. Scr. Mater. 2007, 57, 137–140. [Google Scholar] [CrossRef]
- Olevsky, E.; Molinari, A. Instability of sintering of porous bodies. Int. J. Plast. 2000, 16, 1–37. [Google Scholar] [CrossRef]
- Liew, Y.M.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Luqman, M.; Khairul Nizar, I.; Ruzaidi, C.M.; Heah, C.Y. Processing and Characterization of Calcined Kaolin Cement Powder. Const. Buil. Mater. 2012, 30, 794–802. [Google Scholar] [CrossRef]
- Chinelatto, A.S.A.; Pallone, E.M.J.A.; de Souza, A.M.; Manosso, M.K.; Chinelatto, A.L.; Tomasi, R. Mechanisms of microstructure control in conventional sintering. In Sintering of Ceramics―New Emerging Techniques; Brazil, 2012; pp. 401–422. [Google Scholar]
- Zhao, D.; Su, H.; Liu, H.; Liu, Y.; Shen, Z.; Yao, B.; Guo, M.; Guo, Y.; Zhang, J.; Liu, L.; et al. Densification and microstructural evolution of bulk Al2O3–Y3Al5O12 (YAG) eutectic ceramic fabricated by spark plasma sintering. Ceram. Int. 2019, 45, 12337–12343. [Google Scholar] [CrossRef]
- Baccour, H.; Medhiou, M.; Jamoussi, F.; Mhiri, T. Influence of firing temperature on the ceramic properties of Triassic clays from Tunisia. J. Mater. Process. Technol. 2009, 209, 2812–2817. [Google Scholar] [CrossRef]
- Rashad, A.M.; Hassan, A.A.; Zeedan, S.R. An investigation on alkali-activated Egyptian metakaolin pastes blended with quartz powder subjected to elevated temperatures Alaa. Appl. Clay Sci. 2016, 132–133, 366–376. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, W.; Shi, D. Effect of elevated temperature on the properties of geopolymer synthesized from calcined ore-dressing tailing of bauxite and ground-granulated blast furnace slag. Constr. Buil. Mater. 2014, 69, 41–48. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]

| Composition | SiO2 | Al2O3 | Fe2O3 | K2O | TiO2 | MnO2 | ZrO2 | LOI |
|---|---|---|---|---|---|---|---|---|
| Percentage (%) | 54.0 | 31.7 | 4.89 | 6.05 | 1.41 | 0.11 | 0.10 | 1.74 |
| Sintering Temperature | 900 °C | 1000 °C | 1100 °C | 1200 °C | ||||
|---|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | Average | SD | |
| Flexural Strength (MPa) | 42.35 | 1.81 | 50.12 | 1.71 | 65.65 | 1.82 | 92 | 1.51 |
| Theoretical density (g/cm3) | 2.53 | 0.02 | 2.21 | 0.03 | 2.05 | 0.02 | 1.88 | 0.02 |
| Total Porosity (%) | 25.35 | 0.5 | 26.93 | 0.42 | 28.23 | 0.3 | 34.01 | 0.61 |
| Volumetric Shrinkage (%) | 10.02 | 0.62 | 13.54 | 0.32 | 16.55 | 0.54 | 18.32 | 0.63 |
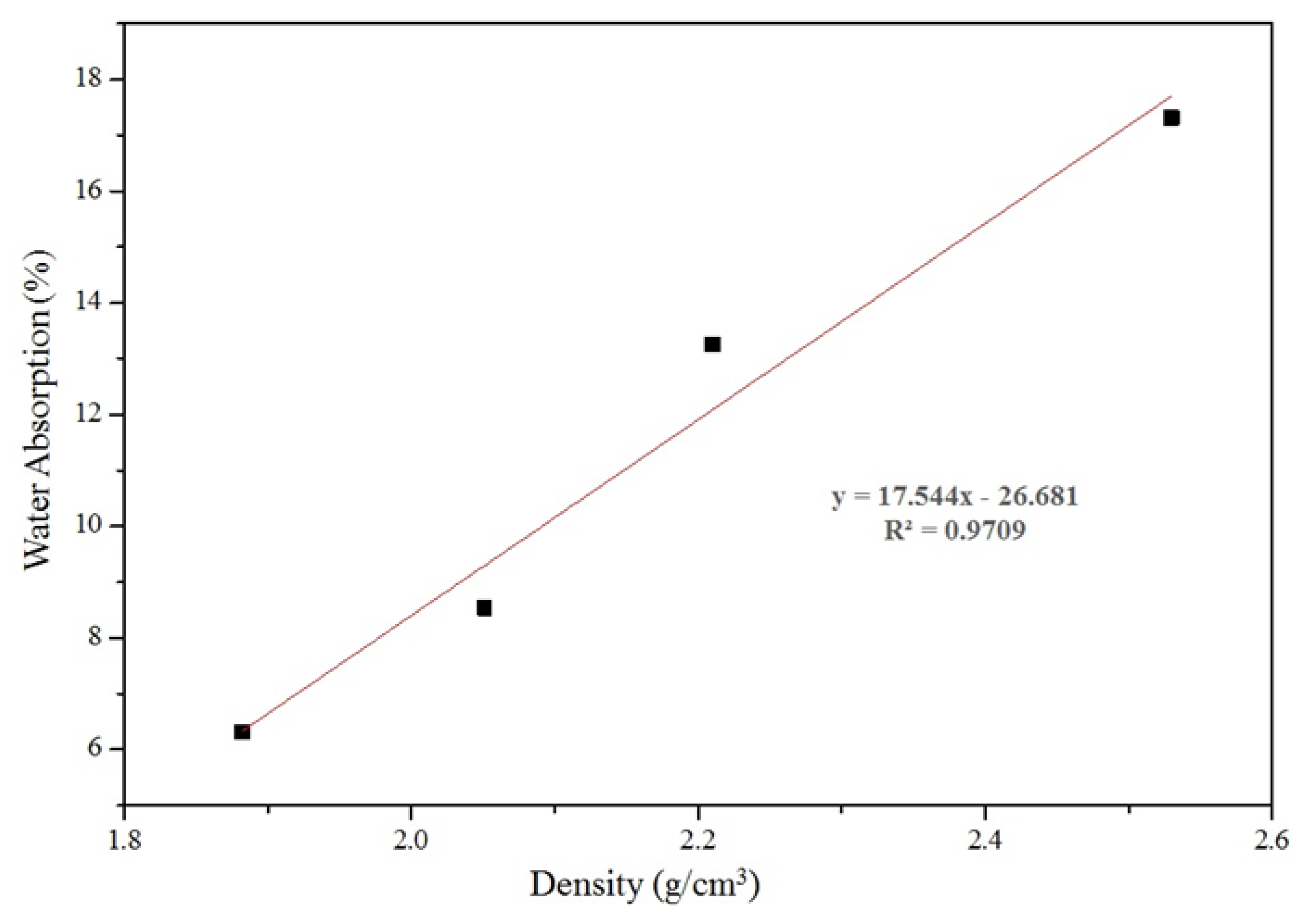
| Water Absorption (%) | 6.32 | 0.63 | 8.54 | 0.32 | 13.26 | 0.51 | 17.31 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.; Abdullah, M.M.A.B.; Ibrahim, W.M.W.; Hussin, K.; Ahmad Zaidi, F.H.; Chaiprapa, J.; Wysłocki, J.J.; Błoch, K.; Nabiałek, M. Role of Sintering Temperature in Production of Nepheline Ceramics-Based Geopolymer with Addition of Ultra-High Molecular Weight Polyethylene. Materials 2021, 14, 1077. https://doi.org/10.3390/ma14051077
Ahmad R, Abdullah MMAB, Ibrahim WMW, Hussin K, Ahmad Zaidi FH, Chaiprapa J, Wysłocki JJ, Błoch K, Nabiałek M. Role of Sintering Temperature in Production of Nepheline Ceramics-Based Geopolymer with Addition of Ultra-High Molecular Weight Polyethylene. Materials. 2021; 14(5):1077. https://doi.org/10.3390/ma14051077
Chicago/Turabian StyleAhmad, Romisuhani, Mohd Mustafa Al Bakri Abdullah, Wan Mastura Wan Ibrahim, Kamarudin Hussin, Fakhryna Hannanee Ahmad Zaidi, Jitrin Chaiprapa, Jerzy J. Wysłocki, Katarzyna Błoch, and Marcin Nabiałek. 2021. "Role of Sintering Temperature in Production of Nepheline Ceramics-Based Geopolymer with Addition of Ultra-High Molecular Weight Polyethylene" Materials 14, no. 5: 1077. https://doi.org/10.3390/ma14051077
APA StyleAhmad, R., Abdullah, M. M. A. B., Ibrahim, W. M. W., Hussin, K., Ahmad Zaidi, F. H., Chaiprapa, J., Wysłocki, J. J., Błoch, K., & Nabiałek, M. (2021). Role of Sintering Temperature in Production of Nepheline Ceramics-Based Geopolymer with Addition of Ultra-High Molecular Weight Polyethylene. Materials, 14(5), 1077. https://doi.org/10.3390/ma14051077









