New Approach in the Application of Conjugated Polymers: The Light-Activated Source of Versatile Singlet Oxygen Molecule
Abstract
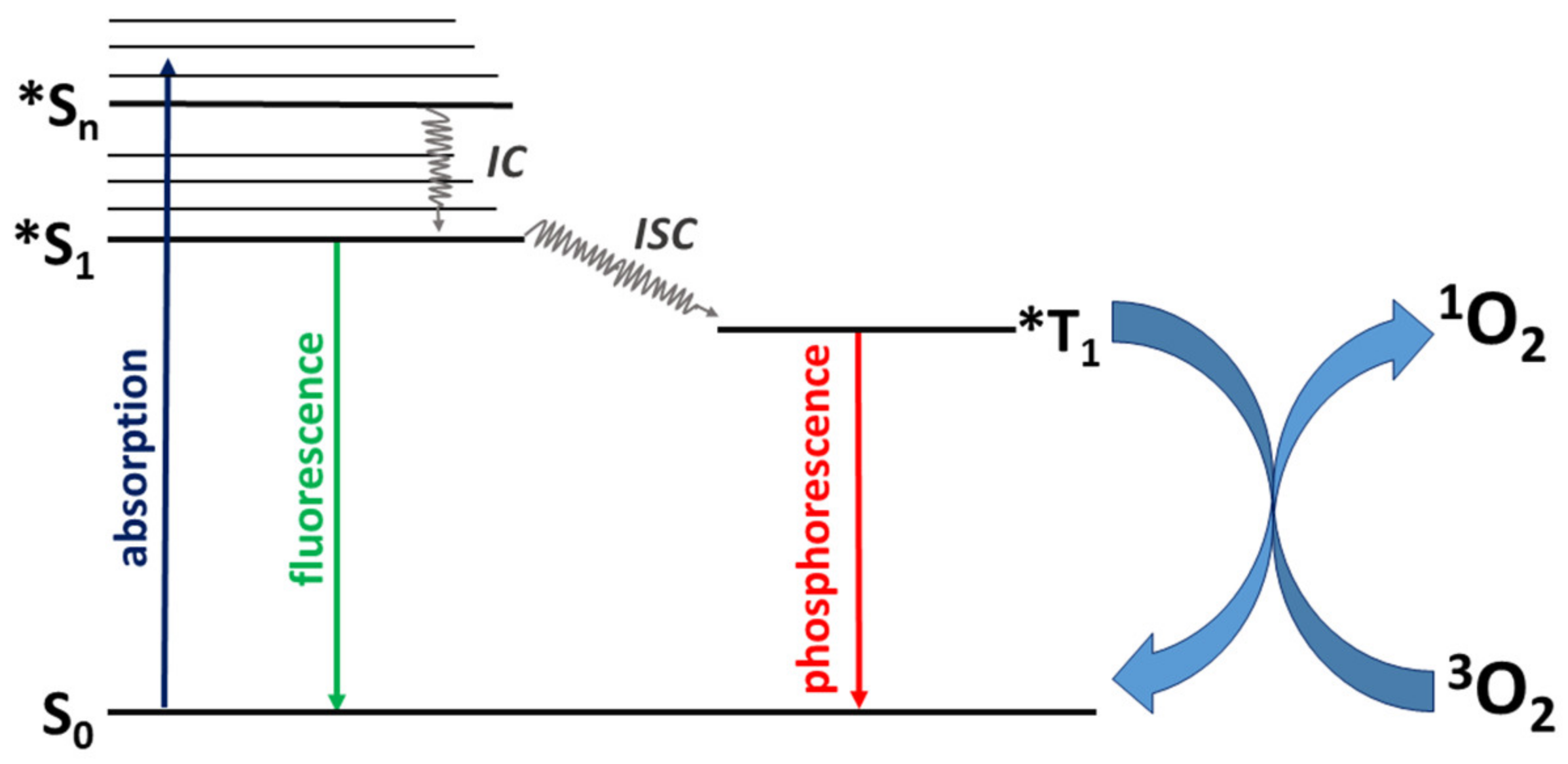
1. Introduction
2. Conjugated Polymers as 1O2 Source in Medical Applications
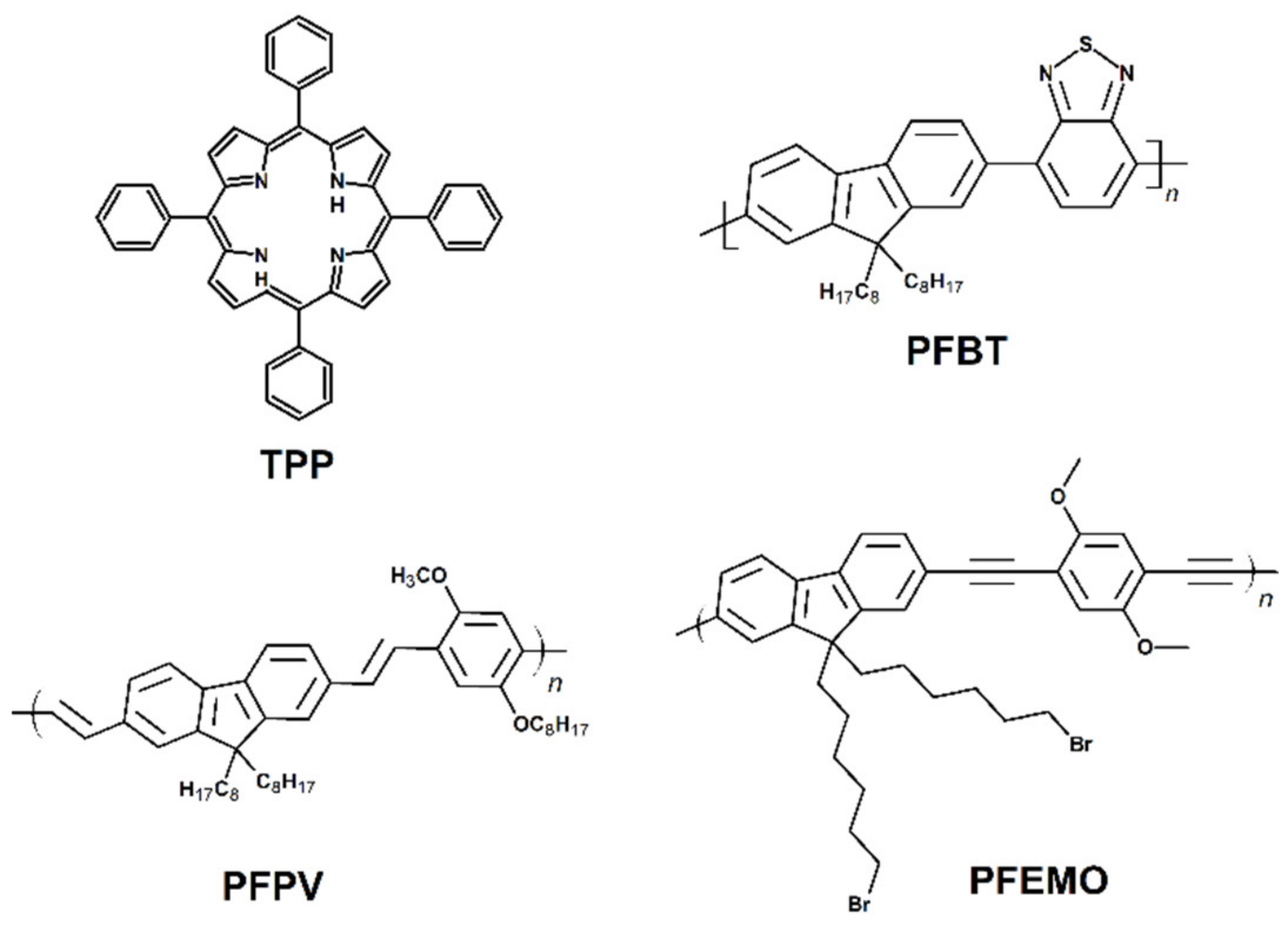
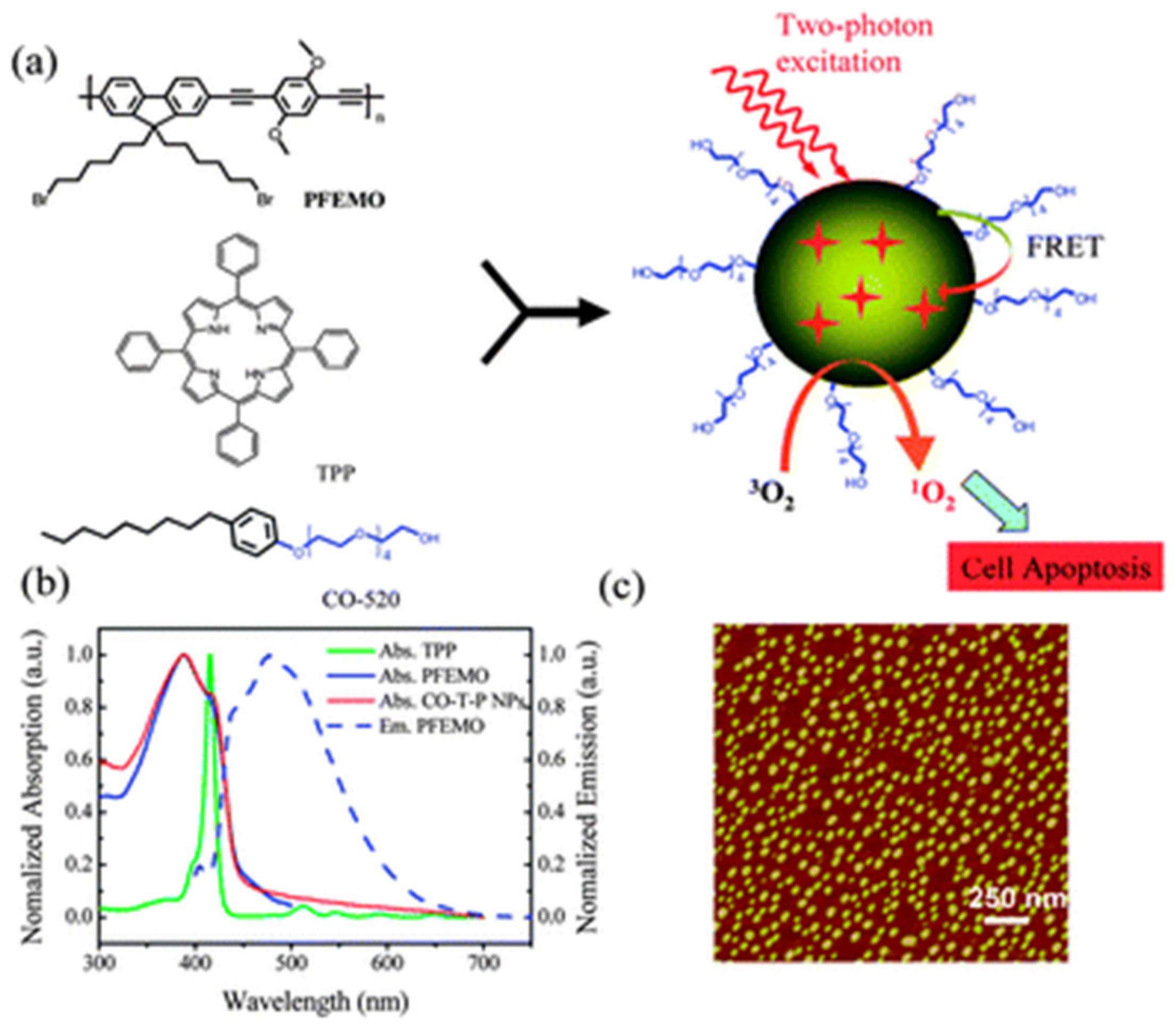
2.1. Photodynamic Therapy (PDT)
2.2. Photodynamic Antimicrobial Chemotherapy (PACT)
3. Conjugated Polymers as 1O2 Sources in Photo-Oxidation Processes
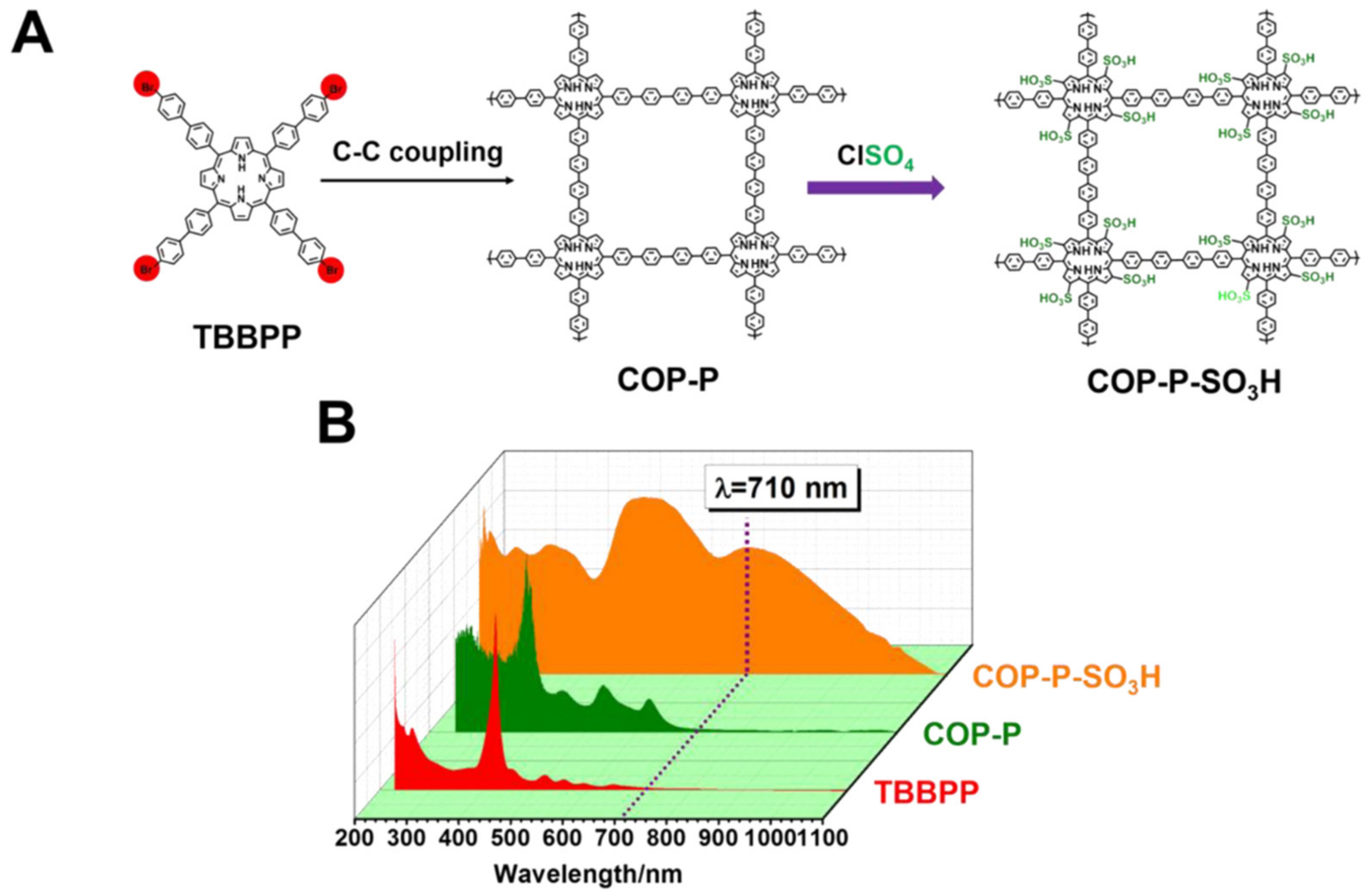
3.1. Synthesis of Fine Chemicals
3.2. Wastewater Treatment
4. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Ogilby, P.R. Singlet oxygen: There is indeed something new under the sun. Chem. Soc. Rev. 2010, 39, 3181–3209. [Google Scholar] [CrossRef] [PubMed]
- Fudickar, W.; Linker, T. Release of Singlet Oxygen from Aromatic Endoperoxides by Chemical Triggers. Angew. Chem. Int. Ed. 2018, 57, 12971–12975. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, C.; Wang, P.; Zhao, Y.; Yang, Y.; Wang, Y.; Yuan, H.; Qu, S.; Zhang, X.; Song, G.; et al. Light-free Generation of Singlet Oxygen through Manganese-Thiophene Nanosystems for pH-Responsive Chemiluminescence Imaging and Tumor Therapy. Chem 2020, 6, 2314–2334. [Google Scholar] [CrossRef]
- Kruk, I. Environmental Toxicology and Chemistry of Oxygen Species; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-662-14779-5. [Google Scholar]
- Schmidt, R. Photosensitized Generation of Singlet Oxygen. Photochem. Photobiol. 2007, 82, 1161–1177. [Google Scholar] [CrossRef]
- Wahlen, J.; de Vos, D.E.; Jacobs, P.A.; Alsters, P.L. Solid Materials as Sources for Synthetically Useful Singlet Oxygen. Adv. Synth. Catal. 2004, 346, 152–164. [Google Scholar] [CrossRef]
- Ghogare, A.A.; Greer, A. Using Singlet Oxygen to Synthesize Natural Products and Drugs. Chem. Rev. 2016, 116, 9994–10034. [Google Scholar] [CrossRef] [PubMed]
- Pibiri, I.; Buscemi, S.; Palumbo Piccionello, A.; Pace, A. Photochemically Produced Singlet Oxygen: Applications and Perspectives. ChemPhotoChem 2018, 2, 535–547. [Google Scholar] [CrossRef]
- Callaghan, S.; Senge, M.O. The good, the bad, and the ugly-controlling singlet oxygen through design of photosensitizers and delivery systems for photodynamic therapy. Photochem. Photobiol. Sci. 2018, 17, 1490–1514. [Google Scholar] [CrossRef] [PubMed]
- Noimark, S.; Dunnill, C.W.; Parkin, I.P. Shining light on materials—A self-sterilising revolution. Adv. Drug Deliv. Rev. 2013, 65, 570–580. [Google Scholar] [CrossRef]
- Ishii, K. Functional singlet oxygen generators based on phthalocyanines. Coord. Chem. Rev. 2012, 256, 1556–1568. [Google Scholar] [CrossRef]
- Imato, K.; Ohira, K.; Yamaguchi, M.; Enoki, T.; Ooyama, Y. Phenazine-based photosensitizers for singlet oxygen generation. Mater. Chem. Front. 2020, 4, 589–596. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet photosensitizers: From molecular design to applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, C.; Xu, K.; Zhao, J. Application of singlet energy transfer in triplet state formation: Broadband visible light-absorbing triplet photosensitizers, molecular structure design, related photophysics and applications. J. Mater. Chem. C 2015, 3, 8735–8759. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, D. New Photosensitizer Design Concept: Polymerization-Enhanced Photosensitization. Chem 2018, 4, 2013–2015. [Google Scholar] [CrossRef]
- Stasheuski, A.S.; Galievsky, V.A.; Stupak, A.P.; Dzhagarov, B.M.; Choi, M.J.; Chung, B.H.; Jeong, J.Y. Photophysical Properties and Singlet Oxygen Generation Efficiencies of Water-Soluble Fullerene Nanoparticles. Photochem. Photobiol. 2014, 90, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Hamano, T.; Okuda, K.; Mashino, T.; Hirobe, M.; Arakane, K.; Ryu, A.; Mashiko, S.; Nagano, T. Singlet oxygen production from fullerene derivatives: Effect of sequential functionalization of the fullerene core. Chem. Commun. 1997, 21–22. [Google Scholar] [CrossRef]
- Gandra, N.; Chiu, P.L.; Li, W.; Anderson, Y.R.; Mitra, S.; He, H.; Gao, R. Photosensitized singlet oxygen production upon two-photon excitation of single-walled carbon nanotubes and their functionalized analogues. J. Phys. Chem. C 2009, 113, 5182–5185. [Google Scholar] [CrossRef]
- Kholikov, K.; Ilhom, S.; Sajjad, M.; Smith, M.E.; Monroe, J.D.; San, O.; Er, A.O. Improved singlet oxygen generation and antimicrobial activity of sulphur-doped graphene quantum dots coupled with methylene blue for photodynamic therapy applications. Photodiagnosis Photodyn. Ther. 2018, 24, 7–14. [Google Scholar] [CrossRef]
- Jovanović, S.P.; Syrgiannis, Z.; Budimir, M.D.; Milivojević, D.D.; Jovanovic, D.J.; Pavlović, V.B.; Papan, J.M.; Bartenwerfer, M.; Mojsin, M.M.; Stevanović, M.J.; et al. Graphene quantum dots as singlet oxygen producer or radical quencher—The matter of functionalization with urea/thiourea. Mater. Sci. Eng. C 2020, 109, 110539. [Google Scholar] [CrossRef] [PubMed]
- Peres, R.M.; Brêda, G.C.; Almeida, R.V.; Corrêa, R.J. Photochemistry of covalently bonded Graphene oxide—perylene diimide system for bacterial growth inhibition started by singlet oxygen. J. Photochem. Photobiol. A Chem. 2020, 407, 113058. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Reynolds, J.R.; Thompson, B.C.; Skotheim, T.A. Conjugated Polymers: Perspective, Theory, and New Materials, 4th ed.; Taylor & Francis: Abingdon, UK, 2019. [Google Scholar]
- Lo, M.; Ktari, N.; Gningue-Sall, D.; Madani, A.; Aaron, S.E.; Aaron, J.-J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole: A reactive and functional conductive polymer for the selective electrochemical detection of heavy metals in water. Emergent Mater. 2020, 3, 815–839. [Google Scholar] [CrossRef]
- Slodek, A.; Zych, D.; Golba, S.; Zimosz, S.; Gnida, P.; Schab-Balcerzak, E. Dyes based on the D/A-acetylene linker-phenothiazine system for developing efficient dye-sensitized solar cells. J. Mater. Chem. C 2019, 7, 5830–5840. [Google Scholar] [CrossRef]
- Fonseca, S.M.; Pina, J.; Arnaut, L.G.; de Melo, J.S.; Burrows, H.D.; Chattopadhyay, N.; Alcácer, L.; Charas, A.; Morgado, J.; Monkman, A.P.; et al. Triplet-state and singlet oxygen formation in fluorene-based alternating copolymers. J. Phys. Chem. B 2006, 110, 8278–8283. [Google Scholar] [CrossRef] [PubMed]
- Scurlock, R.D.; Wang, B.; Ogilby, P.R.; Sheats, J.R.; Clough, R.L. Singlet Oxygen as a Reactive Intermediate in the Photodegradation of an Electroluminescent Polymer. J. Am. Chem. Soc. 1995, 117, 10194–10202. [Google Scholar] [CrossRef]
- Cook, S.; Ohkita, H.; Durrant, J.R.; Kim, Y.; Benson-Smith, J.J.; Nelson, J.; Bradley, D.D.C. Singlet exciton transfer and fullerene triplet formation in polymer-fullerene blend films. Appl. Physic Lett. 2006, 89, 8–11. [Google Scholar] [CrossRef]
- Bregnhøj, M.; Prete, M.; Turkovic, V.; Petersen, A.U. Oxygen-dependent photophysics and photochemistry of prototypical compounds for organic photovoltai. Methods Appl. Fluoresc. 2020, 8, 14001. [Google Scholar] [CrossRef] [PubMed]
- Pacios, B.R.; Chatten, A.J.; Kawano, K.; Durrant, J.R.; Bradley, D.D.C.; Nelson, J. Effects of photo-oxidation on the Performance vinylene]:[6,6]-Phenyl C61-Butyric Acid Methyl Ester Solar Cells. Adv. Funct. Mater. 2006, 2117–2126. [Google Scholar] [CrossRef]
- Frausto, F.; Thomas, S.W. Ratiometric Singlet Oxygen Detection in Water Using Acene-Doped Conjugated Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 15768–15775. [Google Scholar] [CrossRef]
- Sun, P.; Wang, G.; Hou, H.; Yuan, P.; Deng, W.; Wang, C.; Lu, X.; Fan, Q.; Huang, W. A water-soluble phosphorescent conjugated polymer brush for tumor-targeted photodynamic therapy. Polym. Chem. 2017, 8, 5836–5844. [Google Scholar] [CrossRef]
- Meng, Z.; Hou, W.; Zhou, H.; Zhou, L.; Chen, H.; Wu, C. Therapeutic Considerations and Conjugated Polymer-Based Photosensitizers for Photodynamic Therapy. Macromol. Rapid Commun. 2018, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yuan, Y.; Cai, X.; Zhang, C.J.; Hu, F.; Liang, J.; Zhang, G.; Zhang, D.; Liu, B. Tuning the singlet-triplet energy gap: A unique approach to efficient photosensitizers with aggregation-induced emission (AIE) characteristics. Chem. Sci. 2015, 6, 5824–5830. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Mao, D.; Xu, S.; Hu, K.F.; Li, X.; Kong, D.; Liu, B. Polymerization-Enhanced Photosensitization. Chem 2018, 4, 1937–1951. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Li, X.; Ge, J.; Zhang, Q.; Jiang, J.; Zhang, G. Polymerization-enhanced intersystem crossing: New strategy to achieve long-lived excitons. Macromol. Rapid Commun. 2015, 36, 298–303. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.; Ashraf, S.M.; Riaz, U. Experimental and theoretical studies of benzoquinone modified poly(ortho-phenylenediamine): Singlet oxygen generating oligomers. Colloid Polym. Sci. 2020, 298, 1443–1453. [Google Scholar] [CrossRef]
- Johnson, K.R.; Vittardi, S.B.; Gracia-Nava, M.A.; Rack, J.J.; de Bettencourt-Dias, A. Wavelength-Dependent Singlet Oxygen Generation in Luminescent Lanthanide Complexes with a Pyridine-Bis(Carboxamide)-Terthiophene Sensitizer. Chem. Eur. J. 2020, 26, 7274–7280. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pu, K. Semiconducting Polymer Nanomaterials as Near-Infrared Photoactivatable Protherapeutics for Cancer. Acc. Chem. Res. 2020, 53, 752–762. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Wang, S. Conjugated Polymer Nanoparticles for Imaging, Cell Activity Regulation, and Therapy. Adv. Funct. Mater. 2019, 29, 1–20. [Google Scholar] [CrossRef]
- Liu, B.L. Conjugated Polymers for Biological and Biomedical Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018. [Google Scholar]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Xu, H. Recent Progress in the Biological Applications of Reactive Oxygen Species-Responsive Polymers. Polym. Rev. 2020, 60, 114–143. [Google Scholar] [CrossRef]
- Gesquiere, A. Photodynamic Therapy with Conjugated Polymer Nanoparticles: Recent Advances and Therapeutic Considerations. J. Cancer Treat. Diagn. 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Carmona, N.; Ouk, T.S.; Calvete, M.J.F.; Pereira, M.M.; Villandier, N.; Leroy-Lhez, S. Conjugating biomaterials with photosensitizers: Advances and perspectives for photodynamic antimicrobial chemotherapy. Photochem. Photobiol. Sci. 2020, 19, 445–461. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.J.; Dong, F.; Jiang, S.T.; Yin, G.Q.; Li, X.; Tian, Y.; Yang, H.B. Light-Controlled Generation of Singlet Oxygen within a Discrete Dual-Stage Metallacycle for Cancer Therapy. J. Am. Chem. Soc. 2019, 141, 8943–8950. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Qian, Y.; Xu, Z.; Lv, Z.; Tao, P.; Xie, M.; Liu, S.; Huang, W.; Zhao, Q. Enhancing singlet oxygen generation in semiconducting polymer nanoparticles through fluorescence resonance energy transfer for tumor treatment. Chem. Sci. 2019, 10, 5085–5094. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhu, L.; Qi, L.; Yan, L.; Xue, Y.; Wang, D.; Chen, J.F.; Dai, L. Two-Dimensional Fully Conjugated Polymeric Photosensitizers for Advanced Photodynamic Therapy. Chem. Mater. 2016, 28, 8651–8658. [Google Scholar] [CrossRef]
- Chang, K.; Tang, Y.; Fang, X.; Yin, S.; Xu, H.; Wu, C. Incorporation of Porphyrin to π-Conjugated Backbone for Polymer-Dot-Sensitized Photodynamic Therapy. Biomacromolecules 2016, 17, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, S.S.; Chen, Y.; Zhao, H.; Lv, F.; Liu, L.; Wang, S. In situ self-assembly of conjugated polyelectrolytes for cancer targeted imaging and photodynamic therapy. Biomater. Sci. 2020, 8, 2156–2163. [Google Scholar] [CrossRef]
- Li, S.; Chang, K.; Sun, K.; Tang, Y.; Cui, N.; Wang, Y.; Qin, W.; Xu, H.; Wu, C. Amplified Singlet Oxygen Generation in Semiconductor Polymer Dots for Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 3624–3634. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Liu, L.; Tang, H.; Feng, X.; Yang, Q.; Wang, S.; Bazan, G.C. Design guidelines for conjugated polymers with light-activated anticancer activity. Adv. Funct. Mater. 2011, 21, 4058–4067. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Q.H. Enhanced one- and two-photon excitation emission of a porphyrin photosensitizer by FRET from a conjugated polyelectrolyte. Macromol. Rapid Commun. 2009, 30, 504–508. [Google Scholar] [CrossRef]
- Doshi, M.; Krienke, M.; Khederzadeh, S.; Sanchez, H.; Copik, A.; Oyer, J.; Gesquiere, A.J. Conducting polymer nanoparticles for targeted cancer therapy. RSC Adv. 2015, 5, 37943–37956. [Google Scholar] [CrossRef]
- Wu, Y.; Zhen, Y.; Ma, Y.; Zheng, R.; Wang, Z.; Fu, H. Exceptional intersystem crossing in Di(perylene bisimide)s: A structural platform toward photosensitizers for singlet oxygen generation. J. Phys. Chem. Lett. 2010, 1, 2499–2502. [Google Scholar] [CrossRef]
- Kuehne, A.J.C. Conjugated Polymer Nanoparticles toward in Vivo Theranostics—Focus on Targeting, Imaging, Therapy, and the Importance of Clearance. Adv. Biosyst. 2017, 1, 1700100. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, W.; Manghnani, P.; Xu, S.; Wang, Y.; Goh, C.C.; Ng, L.G.; Liu, B. Polymerization-Enhanced Two-Photon Photosensitization for Precise Photodynamic Therapy. ACS Nano 2019, 13, 3095–3105. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, S.; Li, L.; Yao, S.Q.; Xu, Q.H. Highly efficient, conjugated-polymer-based nano-photosensitizers for selectively targeted two-photon photodynamic therapy and imaging of cancer cells. Chem. Eur. J. 2015, 21, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wang, B.; Lv, F.; Liu, L.; Wang, S. Conjugated-polymer-based energy-transfer systems for antimicrobial and anticancer applications. Adv. Mater. 2014, 26, 6978–6982. [Google Scholar] [CrossRef]
- Grimland, J.L.; Wu, C.; Ramoutar, R.R.; Brumaghim, J.L.; McNeill, J. Photosensitizer-doped conjugated polymer nanoparticles with high cross-sections for one- and two-photon excitation. Nanoscale 2011, 3, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, L.; Wu, H.; Yao, S.Q.; Xu, Q.H. Photosensitizer-doped conjugated polymer nanoparticles for simultaneous two-photon imaging and two-photon photodynamic therapy in living cells. Nanoscale 2011, 3, 5140–5146. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; He, F.; Wu, J.; Xu, G.Q.; Yao, S.Q.; Xu, Q.H. Enhanced two-photon singlet oxygen generation by photosensitizer-doped conjugated polymer nanoparticles. Langmuir 2011, 27, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, X.F.; Xu, Q.H. Polyfluorene based conjugated polymer nanoparticles for two-photon live cell imaging. Sci. China Chem. 2018, 61, 88–96. [Google Scholar] [CrossRef]
- Shen, X.; Li, L.; Min Chan, A.C.; Gao, N.; Yao, S.Q.; Xu, Q.H. Water-Soluble Conjugated Polymers for Simultaneous Two-Photon Cell Imaging and Two-Photon Photodynamic Therapy. Adv. Opt. Mater. 2013, 1, 92–99. [Google Scholar] [CrossRef]
- Feng, Z.; Tao, P.; Zou, L.; Gao, P.; Liu, Y.; Liu, X.; Wang, H.; Liu, S.; Dong, Q.; Li, J.; et al. Hyperbranched Phosphorescent Conjugated Polymer Dots with Iridium(III) Complex as the Core for Hypoxia Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 28319–28330. [Google Scholar] [CrossRef]
- Duan, X.; Jiang, X.F.; Hu, D.; Liu, P.; Li, S.; Huang, F.; Ma, Y.; Xu, Q.H.; Cao, Y. Red emitting conjugated polymer based nanophotosensitizers for selectively targeted two-photon excitation imaging guided photodynamic therapy. Nanoscale 2019, 11, 185–192. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, W.; Ma, H.; Jiang, R.; Tang, Y.; Ji, Y.; Lu, X.; Hou, B.; Deng, W.; Huang, W.; et al. Photo-Induced Charge-Variable Conjugated Polyelectrolyte Brushes Encapsulating Upconversion Nanoparticles for Promoted siRNA Release and Collaborative Photodynamic Therapy under NIR Light Irradiation. Adv. Funct. Mater. 2017, 27, 1–14. [Google Scholar] [CrossRef]
- Feng, G.; Fang, Y.; Liu, J.; Geng, J.; Ding, D.; Liu, B. Multifunctional Conjugated Polymer Nanoparticles for Image-Guided Photodynamic and Photothermal Therapy. Small 2017, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, R.; Zhao, W.; Yu, B.; Zhou, J.; Liu, S.; Huang, W.; Zhao, Q. Photothermally Responsive Conjugated Polymeric Singlet Oxygen Carrier for Phase Change-Controlled and Sustainable Phototherapy for Hypoxic Tumor. Research 2020, 2020, 1–14. [Google Scholar] [CrossRef]
- Wainwright, M.; Crossley, K.B. Photosensitising agents—Circumventing resistance and breaking down biofilms: A review. Int. Biodeterior. Biodegrad. 2004, 53, 119–126. [Google Scholar] [CrossRef]
- Spagnul, C.; Turner, L.C.; Boyle, R.W. Immobilized photosensitizers for antimicrobial applications. J. Photochem. Photobiol. B Biol. 2015, 150, 11–30. [Google Scholar] [CrossRef]
- Sautrot-Ba, P.; Jockusch, S.; Nguyen, T.T.T.; Grande, D.; Chiapionne, A.; Abbad-Andaloussi, S.; Pan, M.; Méallet-Renault, R.; Versace, D.L. Photoinduced synthesis of antibacterial hydrogel from aqueous photoinitiating system. Eur. Polym. J. 2020, 138, 109936. [Google Scholar] [CrossRef]
- Ringot, C.; Sol, V.; Granet, R.; Krausz, P. Porphyrin-grafted cellulose fabric: New photobactericidal material obtained by “Click-Chemistry” reaction. Mater. Lett. 2009, 63, 1889–1891. [Google Scholar] [CrossRef]
- Xing, C.; Xu, Q.; Tang, H.; Liu, L.; Wang, S. Conjugated polymer/porphyrin complexes for efficient energy transfer and improving light-activated antibacterial activity. J. Am. Chem. Soc. 2009, 131, 13117–13124. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Goswami, S.; Corbitt, T.S.; Ji, E.; Dascier, D.; Whitten, D.G.; Schanze, K.S. Photophysics and light-activated biocidal activity of visible-light- absorbing conjugated oligomers. ACS Appl. Mater. Interfaces 2013, 5, 4516–4520. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Zhang, Z.; Zhao, Y.; Tang, Y. Efficient Antibacterial Performance and Effect of Structure on Property Based on Cationic Conjugated Polymers. Macromolecules 2018, 51, 7239–7247. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.; Chen, H.; Wang, X.; Liu, L.; Lv, F.; Wang, S. Biofilm Inhibition and Elimination Regulated by Cationic Conjugated Polymers. ACS Appl. Mater. Interfaces 2017, 9, 16933–16938. [Google Scholar] [CrossRef]
- Zehra, N.; Dutta, D.; Malik, A.H.; Ghosh, S.S.; Iyer, P.K. Fluorescence Resonance Energy Transfer-Based Wash-Free Bacterial Imaging and Antibacterial Application Using a Cationic Conjugated Polyelectrolyte. ACS Appl. Mater. Interfaces 2018, 10, 27603–27611. [Google Scholar] [CrossRef]
- Xu, Q.; He, P.; Wang, J.; Chen, H.; Lv, F.; Liu, L.; Wang, S.; Yoon, J. Antimicrobial activity of a conjugated polymer with cationic backbone. Dye. Pigment. 2019, 160, 519–523. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, H.; Hu, R.; Chen, H.; Lv, F.; Liu, L.; Wang, S. Supramolecular conjugated polymer systems with controlled antibacterial activity. Langmuir 2017, 33, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, J.; Zhang, X.; Li, Z.; Tang, Y. Cationic Oligo(thiophene ethynylene) with Broad-Spectrum and High Antibacterial Efficiency under White Light and Specific Biocidal Activity against S. aureus in Dark. ACS Appl. Mater. Interfaces 2016, 8, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jett, S.D.; Crum, J.; Schanze, K.S.; Chi, E.Y.; Whitten, D.G. Understanding the dark and light-enhanced bactericidal action of cationic conjugated polyelectrolytes and oligomers. Langmuir 2013, 29, 781–792. [Google Scholar] [CrossRef]
- Wang, Y.; Corbitt, T.S.; Jett, S.D.; Tang, Y.; Schanze, K.S.; Chi, E.Y.; Whitten, D.G. Direct visualization of bactericidal action of cationic conjugated polyelectrolytes and oligomers. Langmuir 2012, 28, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.C.; Ghasimi, S.; Landfester, K.; Zhang, K.A.I. Enhanced visible light promoted antibacterial efficiency of conjugated microporous polymer nanoparticles via molecular doping. J. Mater. Chem. B 2016, 4, 5112–5118. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, R.; Wang, L.; Qiu, Y.; Zhang, G.; Deng, Q.; Zhang, H.; Yin, P.; Situ, B.; Zhan, C.; et al. An AIE-Active Conjugated Polymer with High ROS-Generation Ability and Biocompatibility for Efficient Photodynamic Therapy of Bacterial Infections. Angew. Chem. Int. Ed. 2020, 59, 9952–9956. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, L.; Wang, Y.; Feng, L. Bactericidal activity-tunable conjugated polymers as a human-friendly bactericide for the treatment of wound infections. Biomater. Sci. 2019, 7, 3788–3794. [Google Scholar] [CrossRef]
- Xing, C.; Yang, G.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Conjugated polymers for light-activated antifungal activity. Small 2012, 8, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Jagadesan, P.; Yu, Z.; Barboza-Ramos, I.; Lara, H.H.; Vazquez-Munoz, R.; López-Ribot, J.L.; Schanze, K.S. Light-Activated Antifungal Properties of Imidazolium-Functionalized Cationic Conjugated Polymers. Chem. Mater. 2020, 32, 6186–6196. [Google Scholar] [CrossRef]
- Schanze, K.S.; Whitten, D.G.; Kell, A.M.; Chi, E.Y.; Ista, L.K.; Monge, F.A.; Jagadesan, P.; Bondu, V.; Donabedian, P.L. Highly Effective Inactivation of SARS-CoV-2 by Conjugated Polymers and Oligomers. ACS Appl. Mater. Interfaces 2020, 12, 55688–55695. [Google Scholar] [CrossRef]
- Montagnon, T.; Kalaitzakis, D.; Triantafyllakis, M.; Stratakis, M.; Vassilikogiannakis, G. Furans and singlet oxygen—Why there is more to come from this powerful partnership. Chem. Commun. 2014, 50, 15480–15498. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z. Reactivities of singlet oxygen: Open-shell or closed-shell? Phys. Chem. Chem. Phys. 2020, 22, 13373–13377. [Google Scholar] [CrossRef]
- Chakraborty, J.; Nath, I.; Song, S.; Mohamed, S.; Khan, A.; Heynderickx, P.M.; Verpoort, F. Porous organic polymer composites as surging catalysts for visible-light-driven chemical transformations and pollutant degradation. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100319. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, J.; Ou, H.; Huang, C.; Anpo, M.; Wang, X. Optimizing the crystallization process of conjugated polymer photocatalysts to promote electron transfer and molecular oxygen activation. J. Catal. 2020, 389, 636–645. [Google Scholar] [CrossRef]
- Li, R.; Byun, J.; Huang, W.; Ayed, C.; Wang, L.; Zhang, K.A.I. Poly(benzothiadiazoles) and Their Derivatives as Heterogeneous Photocatalysts for Visible-Light-Driven Chemical Transformations. ACS Catal. 2018, 8, 4735–4750. [Google Scholar] [CrossRef]
- Ronzani, F.; Costarramone, N.; Blanc, S.; Benabbou, A.K.; Le Bechec, M.; Pigot, T.; Oelgemöller, M.; Lacombe, S. Visible-light photosensitized oxidation of α-terpinene using novel silica-supported sensitizers: Photooxygenation vs. photodehydrogenation. J. Catal. 2013, 303, 164–174. [Google Scholar] [CrossRef]
- Tobin, J.M.; McCabe, T.J.D.; Prentice, A.W.; Holzer, S.; Lloyd, G.O.; Paterson, M.J.; Arrighi, V.; Cormack, P.A.G.; Vilela, F. Polymer-Supported Photosensitizers for Oxidative Organic Transformations in Flow and under Visible Light Irradiation. ACS Catal. 2017, 7, 4602–4612. [Google Scholar] [CrossRef]
- Ma, B.C.; Ghasimi, S.; Landfester, K.; Vilela, F.; Zhang, K.A.I. Conjugated microporous polymer nanoparticles with enhanced dispersibility and water compatibility for photocatalytic applications. J. Mater. Chem. A 2015, 3, 16064–16071. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, X.; Zhang, X.; Hou, H.; Dai, K.; Huang, Q.; Chen, H. Enhanced visible light photocatalytic activity of TiO2 assisted by organic semiconductors: A structure optimization strategy of conjugated polymers. J. Mater. Chem. A 2017, 6, 153–159. [Google Scholar] [CrossRef]
- Zhi, Y.; Ma, S.; Xia, H.; Zhang, Y.; Shi, Z.; Mu, Y.; Liu, X. Construction of donor-acceptor type conjugated microporous polymers: A fascinating strategy for the development of efficient heterogeneous photocatalysts in organic synthesis. Appl. Catal. B Environ. 2019, 244, 36–44. [Google Scholar] [CrossRef]
- Li, L.; Cai, Z. Structure control and photocatalytic performance of porous conjugated polymers based on perylene diimide. Polym. Chem. 2016, 7, 4937–4943. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, R.; Sun, S.; Ren, F.; Chen, X.; Wu, L.; Xing, R. Metal-Free Organic Optoelectronic Molecule as a Highly Efficient Photocatalyst for the Degradation of Organic Pollutants. ACS Omega 2019, 4, 6068–6076. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef]
- Rose, M. Nanoporous polymers: Bridging the gap between molecular and solid catalysts? ChemCatChem 2014, 6, 1166–1182. [Google Scholar] [CrossRef]
- Liras, M.; Iglesias, M.; Sánchez, F. Conjugated Microporous Polymers Incorporating BODIPY Moieties as Light-Emitting Materials and Recyclable Visible-Light Photocatalysts. Macromolecules 2016, 49, 1666–1673. [Google Scholar] [CrossRef]
- Zhi, Y.; Yao, Z.; Jiang, W.; Xia, H.; Shi, Z.; Mu, Y.; Liu, X. Conjugated Microporous Polymers as Heterogeneous Photocatalysts for Efficient Degradation of a Mustard-Gas Simulant. ACS Appl. Mater. Interfaces 2019, 11, 37578–37585. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.X.; Liang, H.P.; Anito, D.A.; Ding, X.; Han, B.H. Emerging applications of porous organic polymers in visible-light photocatalysis. J. Mater. Chem. A 2020, 8, 7003–7034. [Google Scholar] [CrossRef]
- Li, A.; Tan, C.; Yuan, T.; Liang, J.; Gao, D.; Tan, Y.; Jiang, Y. Efficient photocatalytic oxidation sensitized by conjugated polymers in a batch reaction and microreactors under visible light. J. Mater. Chem. A 2018, 6, 15927–15932. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Zhan, Z.P. Conjugated Microporous Polymers for Heterogeneous Catalysis. Chem. Asian J. 2018, 13, 9–19. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, Z.; Zeng, G.; Liu, Z.; Xiao, R.; Xu, P.; Wang, H.; Huang, D.; Liu, Y.; Shao, B.; et al. Recent advances in conjugated microporous polymers for photocatalysis: Designs, applications, and prospects. J. Mater. Chem. A 2020, 8, 6434–6470. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, X.; Pan, L.; Shi, C.; Zhang, X.; Zou, J.J. Heterogeneous Photocatalytic Organic Transformation Reactions Using Conjugated Polymers-Based Materials. ACS Catal. 2020, 10, 12256–12283. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ghasimi, S.; Landfester, K.; Zhang, K.A.I. Highly porous conjugated polymers for selective oxidation of organic sulfides under visible light. Chem. Commun. 2014, 50, 8177–8180. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Han, B.H. Metallophthalocyanine-based conjugated microporous polymers as highly efficient photosensitizers for singlet oxygen generation. Angew. Chem. Int. Ed. 2015, 54, 6536–6539. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Han, B.H. Copper phthalocyanine-based CMPs with various internal structures and functionalities. Chem. Commun. 2015, 51, 12783–12786. [Google Scholar] [CrossRef] [PubMed]
- Hynek, J.; Rathouský, J.; Demel, J.; Lang, K. Design of porphyrin-based conjugated microporous polymers with enhanced singlet oxygen productivity. RSC Adv. 2016, 6, 44279–44287. [Google Scholar] [CrossRef]
- Park, K.C.; Cho, J.; Lee, C.Y. Porphyrin and pyrene-based conjugated microporous polymer for efficient sequestration of CO2 and iodine and photosensitization for singlet oxygen generation. RSC Adv. 2016, 6, 75478–75481. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.; Chen, L.; Wu, Y.; Liu, L.; Addicoat, M.; Irle, S.; Dong, Y.; Jiang, D. Two-dimensional artificial light-harvesting antennae with predesigned high-order structure and robust photosensitising activity. Sci. Rep. 2016, 6, 32944. [Google Scholar] [CrossRef]
- Xu, Q.; Gao, Y.; Wu, X.; Hang, H.; Li, H.; Chen, Y.; Wang, W.; Tong, H. Subphthalocyanine-based conjugated porous polymers for efficient singlet oxygen generation. New J. Chem. 2019, 43, 16385–16390. [Google Scholar] [CrossRef]
- Wu, W.; Xu, S.; Qi, G.; Zhu, H.; Hu, F.; Liu, Z.; Zhang, D.; Liu, B. A Cross-linked Conjugated Polymer Photosensitizer Enables Efficient Sunlight-Induced Photooxidation. Angew. Chem. Int. Ed. 2019, 58, 3062–3066. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Lu, J.; Zhang, J. Carbazole-triazine based donor-acceptor porous organic frameworks for efficient visible-light photocatalytic aerobic oxidation reactions. J. Mater. Chem. A 2018, 6, 15154–15161. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ghasimi, S.; Landfester, K.; Zhang, K.A.I. Molecular Structural Design of Conjugated Microporous Poly(Benzooxadiazole) Networks for Enhanced Photocatalytic Activity with Visible Light. Adv. Mater. 2015, 27, 6265–6270. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, W.; Li, R.; Gehrig, D.; Blom, P.W.M.; Landfester, K.; Zhang, K.A.I. Structural Design Principle of Small-Molecule Organic Semiconductors for Metal-Free, Visible-Light-Promoted Photocatalysis. Angew. Chem. Int. Ed. 2016, 55, 9783–9787. [Google Scholar] [CrossRef]
- Zhang, K.; Vobecka, Z.; Tauer, K.; Antonietti, M.; Vilela, F. π-Conjugated polyHIPEs as highly efficient and reusable heterogeneous photosensitizers. Chem. Commun. 2013, 49, 11158–11160. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ghasimi, S.; Landfester, K.; Zhang, K.A.I. A conjugated porous poly-benzobisthiadiazole network for a visible light-driven photoredox reaction. J. Mater. Chem. A 2014, 2, 18720–18724. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated polymers for visible-light-driven photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Ren, S.; Dawson, R.; Adams, D.J.; Cooper, A.I. Low band-gap benzothiadiazole conjugated microporous polymers. Polym. Chem. 2013, 4, 5585–5590. [Google Scholar] [CrossRef]
- Zhang, K.; Kopetzki, D.; Seeberger, P.H.; Antonietti, M.; Vilela, F. Surface area control and photocatalytic activity of conjugated microporous poly(benzothiadiazole) networks. Angew. Chem. Int. Ed. 2013, 52, 1432–1436. [Google Scholar] [CrossRef] [PubMed]
- Urakami, H.; Zhang, K.; Vilela, F. Modification of conjugated microporous poly-benzothiadiazole for photosensitized singlet oxygen generation in water. Chem. Commun. 2013, 49, 2353–2355. [Google Scholar] [CrossRef] [PubMed]
- Banfi, S.; Nasini, G.; Zaza, S.; Caruso, E. Synthesis and photo-physical properties of a series of BODIPY dyes. Tetrahedron 2013, 69, 4845–4856. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, X.; Zong, S.; Tang, C.; Cui, Y.; Zheng, Q. BODIPY-doped silica nanoparticles with reduced dye leakage and enhanced singlet oxygen generation. Sci. Rep. 2015, 5, 12602. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Geng, Y.; Fan, W.; Li, Z.; Zhan, L.; Wu, X.; Zheng, J.; Zhao, J.; Wu, M. BODIPY-based photosensitizers with intense visible light harvesting ability and high 1O2 quantum yield in aqueous solution. RSC Adv. 2014, 4, 51349–51352. [Google Scholar] [CrossRef]
- Jiang, X.D.; Xi, D.; Le Guennic, B.; Guan, J.; Jacquemin, D.; Guan, J.; Xiao, L.J. Synthesis of NIR naphthyl-containing aza-BODIPYs and measure of the singlet oxygen generation. Tetrahedron 2015, 71, 7676–7680. [Google Scholar] [CrossRef]
- Zhang, X.F.; Yang, X. Photosensitizer that selectively generates singlet oxygen in nonpolar environments: Photophysical mechanism and efficiency for a covalent BODIPY dimer. J. Phys. Chem. B 2013, 117, 9050–9055. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Kundu, S.; Giri, A.; Patra, A. A smart photosensitizer based on a red emitting solution processable porous polymer: Generation of reactive oxygen species. Chem. Commun. 2018, 54, 9123–9126. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.M.; Liu, J.; Hayes, H.; Demleitner, M.; Ellis, D.; Arrighi, V.; Xu, Z.; Vilela, F. BODIPY-based conjugated microporous polymers as reusable heterogeneous photosensitisers in a photochemical flow reactor. Polym. Chem. 2016, 7, 6662–6670. [Google Scholar] [CrossRef]
- Blacha-Grzechnik, A.; Drewniak, A.; Walczak, K.Z.; Szindler, M.; Ledwon, P. Efficient generation of singlet oxygen by perylene diimide photosensitizers covalently bound to conjugate polymers. J. Photochem. Photobiol. A Chem. 2020, 388, 112161. [Google Scholar] [CrossRef]
- Piwowar, K.; Blacha-Grzechnik, A.; Turczyn, R.; Zak, J. Electropolymerized phenothiazines for the photochemical generation of singlet oxygen. Electrochim. Acta 2014, 141, 182–188. [Google Scholar] [CrossRef]
- Blacha-Grzechnik, A.; Piwowar, K.; Zdyb, T.; Krzywiecki, M. Formation of poly(Azure A)-C60 photoactive layer as a novel approach in the heterogeneous photogeneration of singlet oxygen. Appl. Surf. Sci. 2018, 457, 221–228. [Google Scholar] [CrossRef]
- Blacha-Grzechnik, A.; Krzywiecki, M.; Motyka, R.; Czichy, M. Electrochemically Polymerized Terthiopehene-C60 Dyads for the Photochemical Generation of Singlet Oxygen. J. Phys. Chem. C 2019, 123, 25915–25924. [Google Scholar] [CrossRef]
- Nyga, A.; Motyka, R.; Bussetti, G.; Calloni, A.; Sangarashettyhalli, M.; Fijak, S.; Pluczyk-Malek, S.; Data, P.; Blacha-Grzechnik, A. Electrochemically deposited poly(selenophene)-fullerene photoactive layer: Tuning of the spectroscopic properties towards visible light-driven generation of singlet oxygen. Appl. Surf. Sci. 2020, 525, 146594. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Cui, Z.; Hu, Y.; Hao, X.; Wang, Y.; Zou, Z. Ultrathin conjugated polymer nanosheets as highly efficient photocatalyst for visible light driven oxygen activation. Appl. Catal. B Environ. 2020, 277, 119228. [Google Scholar] [CrossRef]
- Cui, X.; Li, Y.; Dong, W.; Liu, D.; Duan, Q. Microwave-assisted synthesis of novel imine-linked copper porphyrin conjugated microporous polymers as heterogeneous photocatalysts. React. Funct. Polym. 2020, 154, 104633. [Google Scholar] [CrossRef]
- Chakraborty, J.; Nath, I.; Jabbour, C.; Aljammal, N.; Song, S.; Kao, C.M.; Heynderickx, P.M.; Verpoort, F. Novel rapid room temperature synthesis of conjugated microporous polymer for metal-free photocatalytic degradation of fluoroquinolones. J. Hazard. Mater. 2020, 398, 122928. [Google Scholar] [CrossRef] [PubMed]
- Piwowar, K.; Blacha-Grzechnik, A.; Bernas, P.; Zak, J. Phenol degradation in heterogeneous system generating singlet oxygen employing light activated electropolymerized phenothiazines. Appl. Surf. Sci. 2015, 359, 426–431. [Google Scholar] [CrossRef]
- Tanaka, S.; Enoki, T.; Imoto, H.; Ooyama, Y.; Ohshita, J.; Kato, T.; Naka, K. Highly Efficient Singlet Oxygen Generation and High Oxidation Resistance Enhanced by Arsole-Polymer-Based Photosensitizer: Application as a Recyclable Photooxidation Catalyst. Macromolecules 2020, 53, 2006–2013. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Li, Y.; Wang, H.; Zuo, Q.; Duan, Q. A 1D porphyrin-based rigid conjugated polymer as efficient and recyclable visible-light driven photocatalyst. React. Funct. Polym. 2019, 143, 104340. [Google Scholar] [CrossRef]
- Shen, J.; Steinbach, R.; Tobin, J.M.; Mouro Nakata, M.; Bower, M.; McCoustra, M.R.S.; Bridle, H.; Arrighi, V.; Vilela, F. Photoactive and metal-free polyamide-based polymers for water and wastewater treatment under visible light irradiation. Appl. Catal. B Environ. 2016, 193, 226–233. [Google Scholar] [CrossRef]
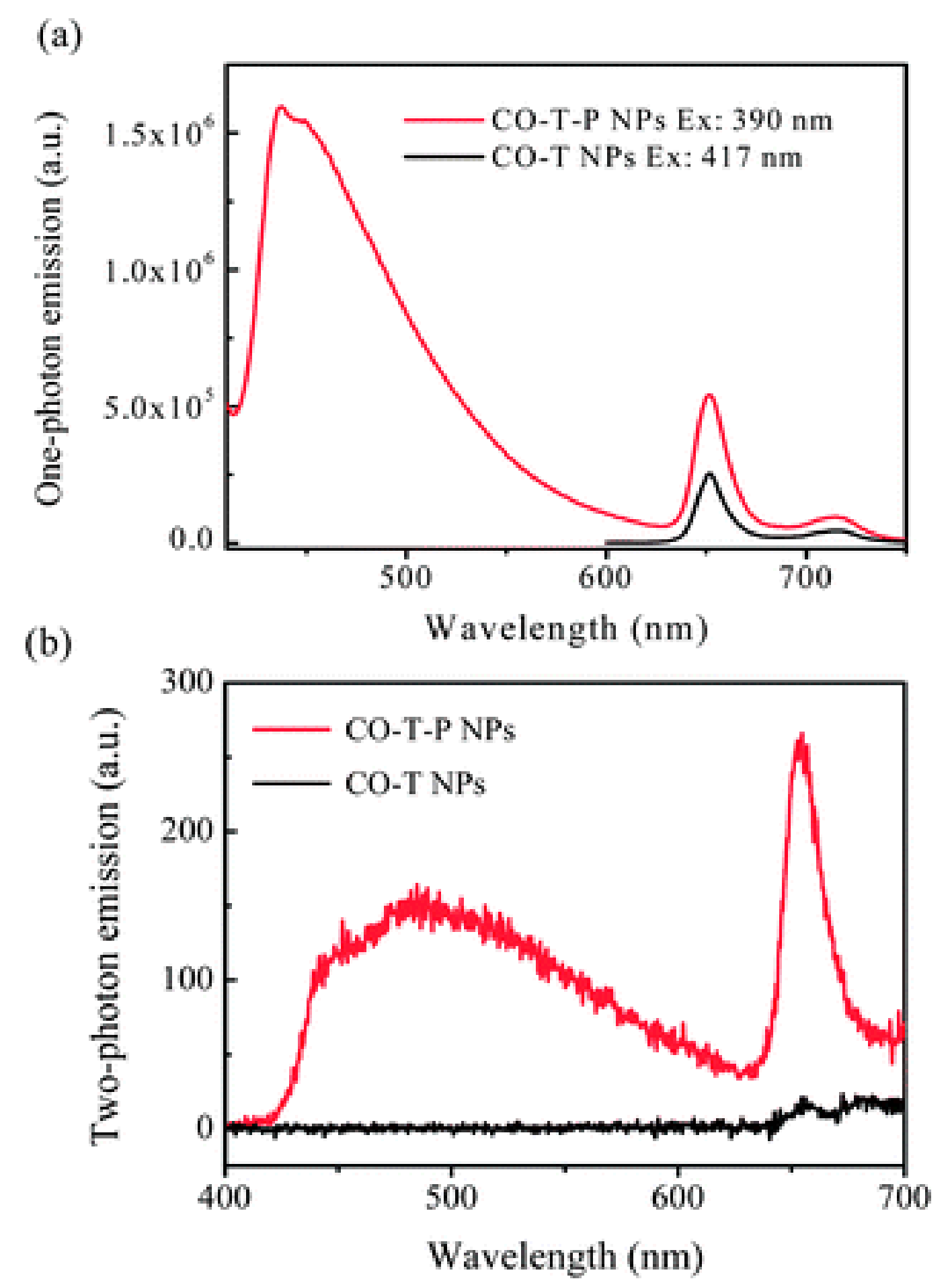
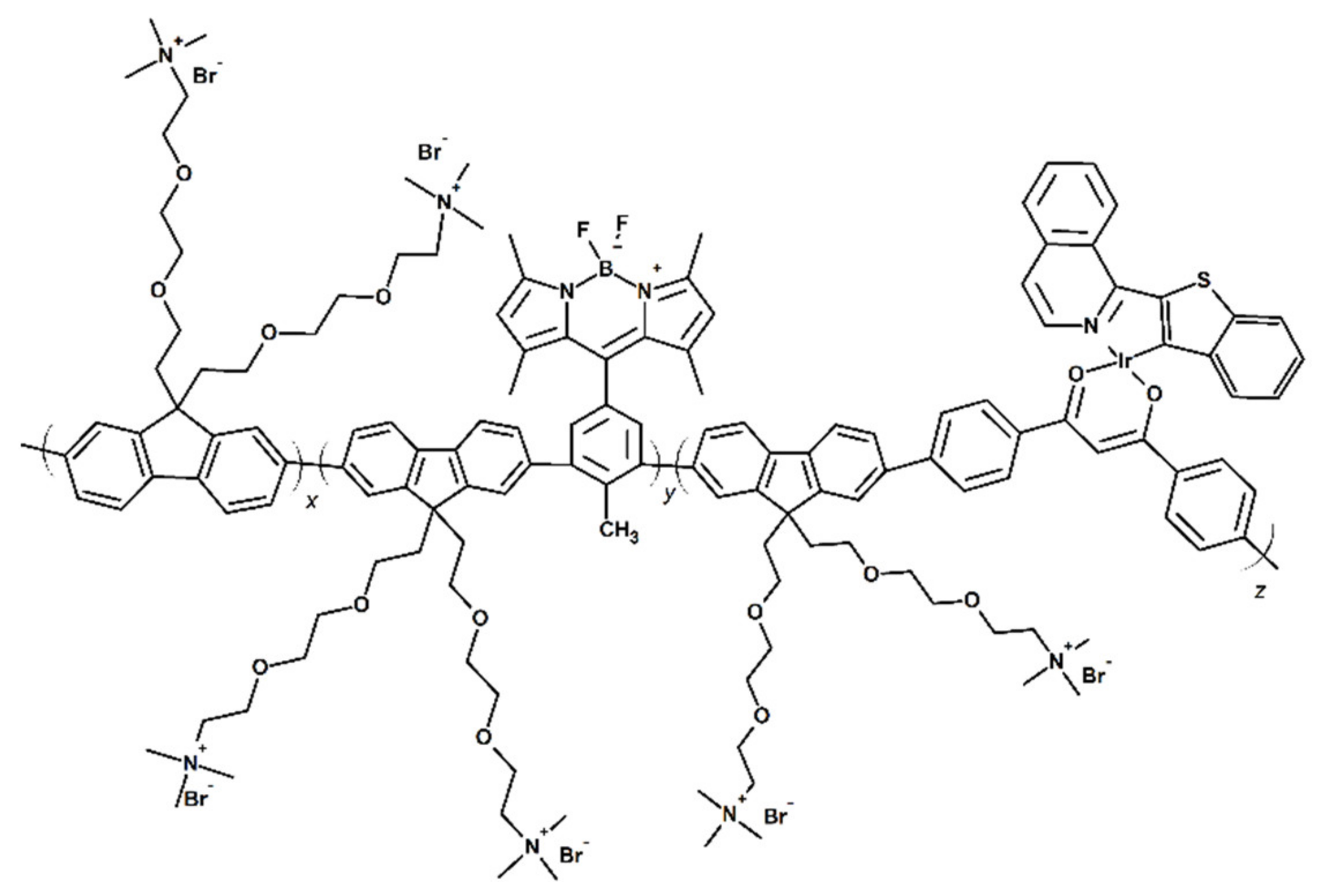
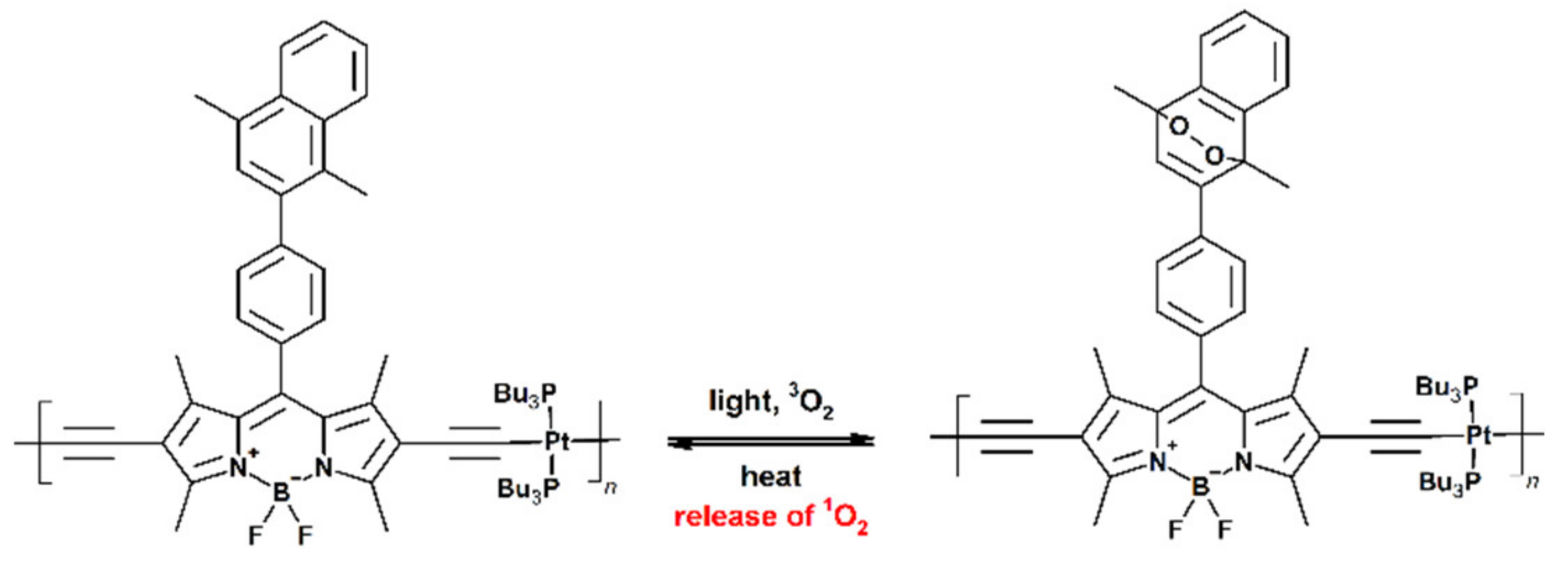
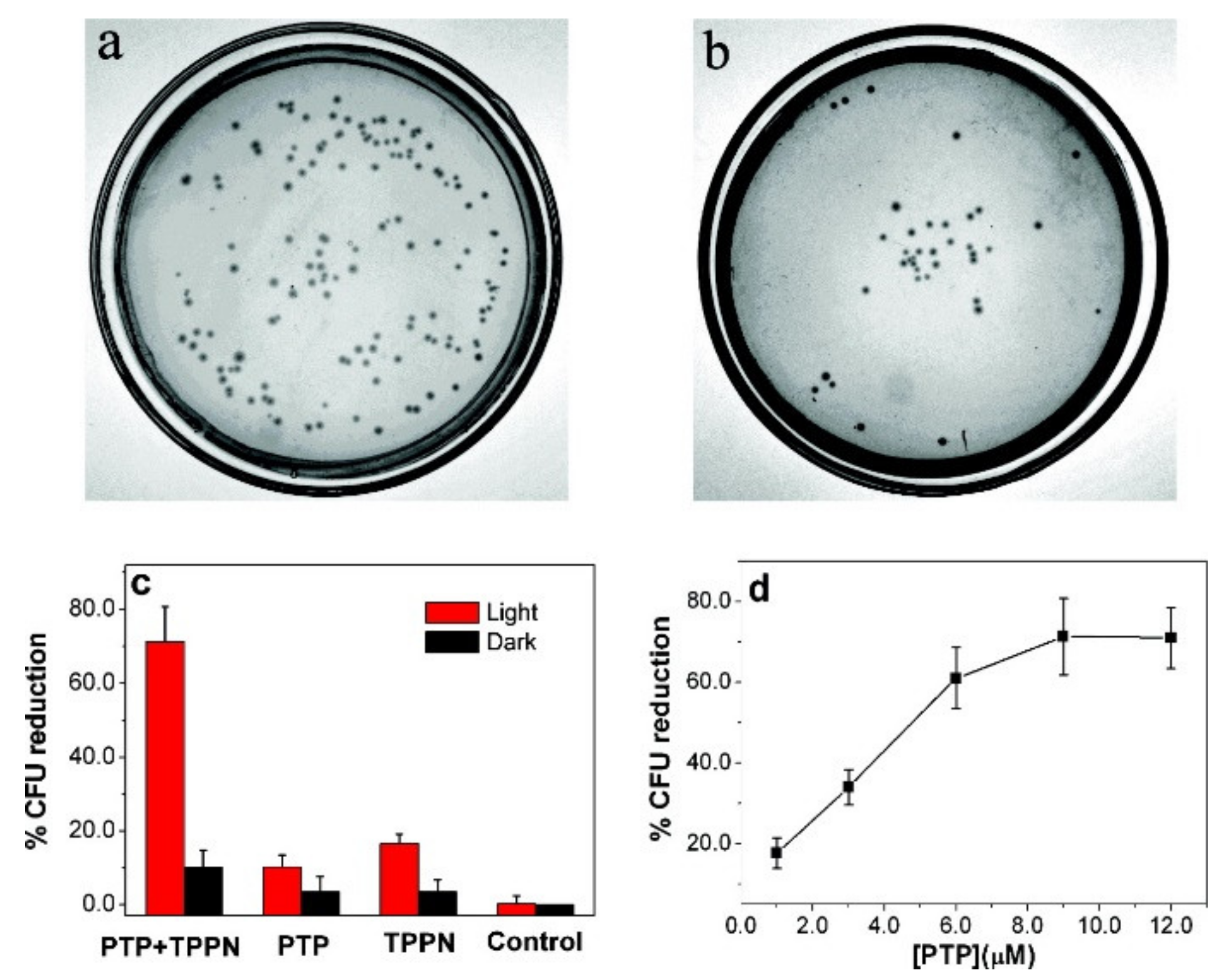
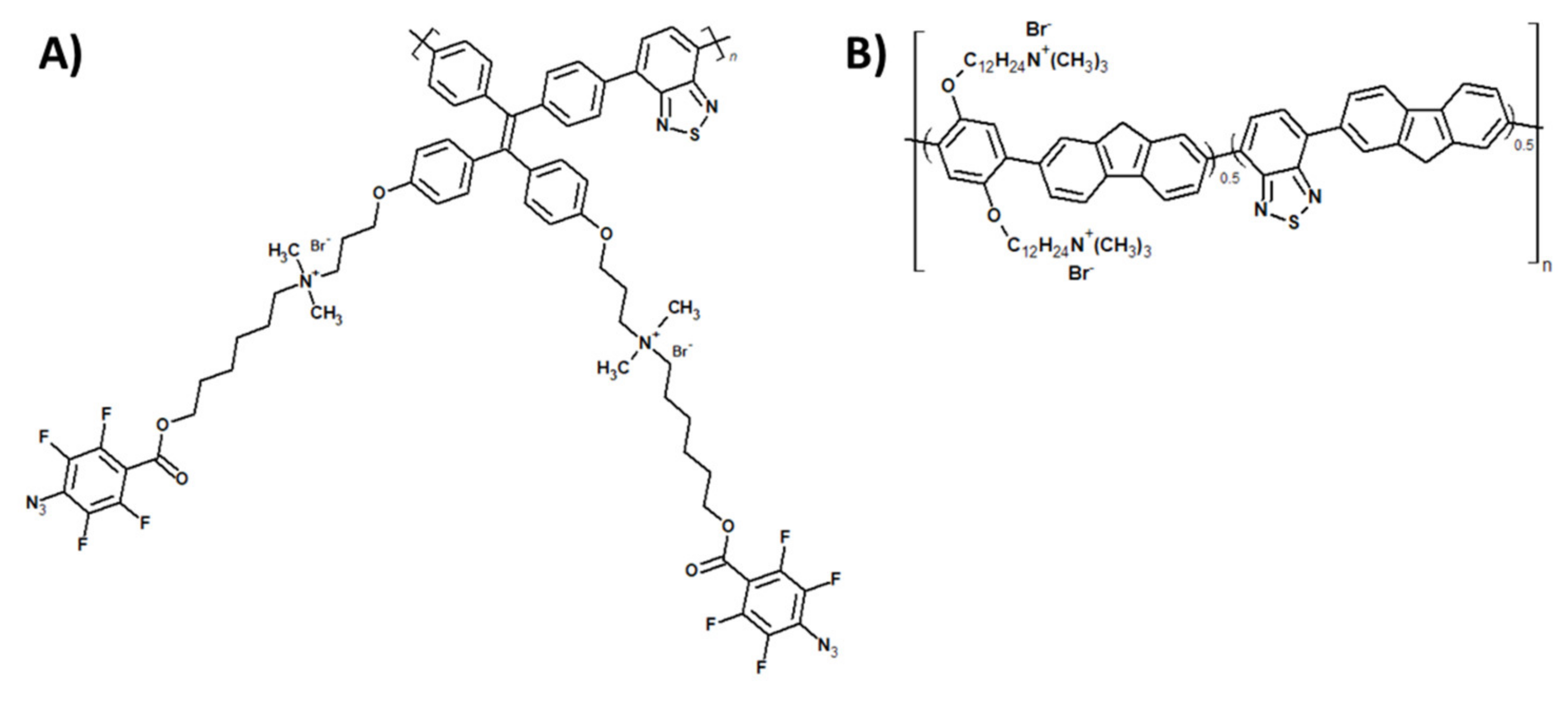

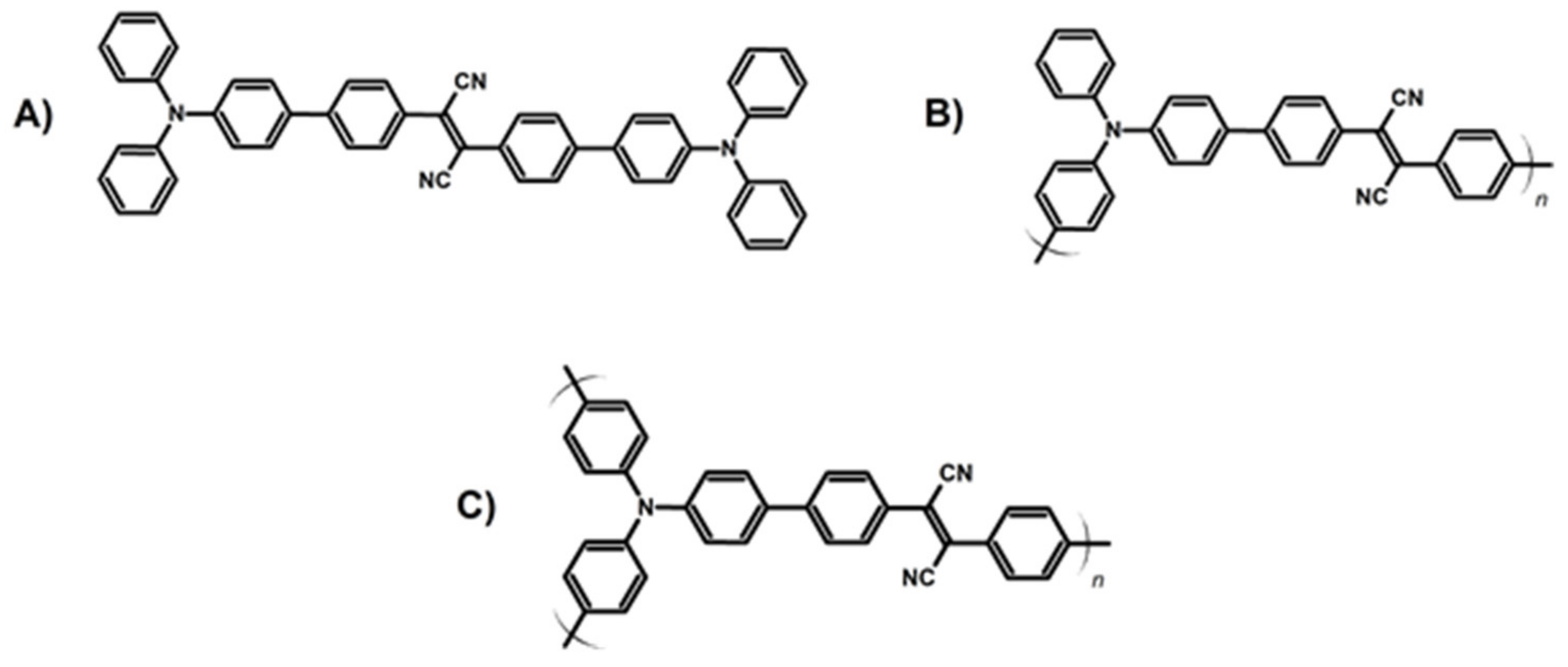
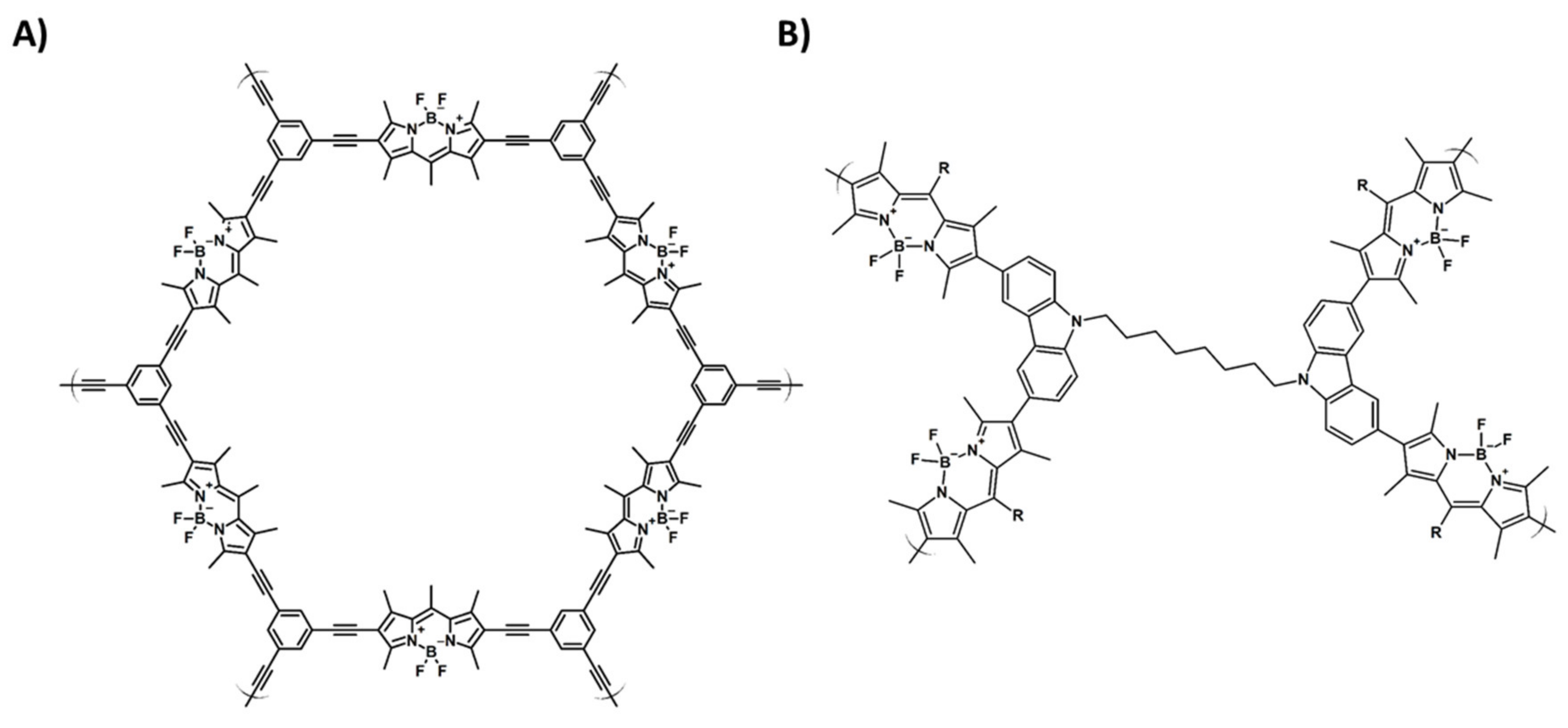
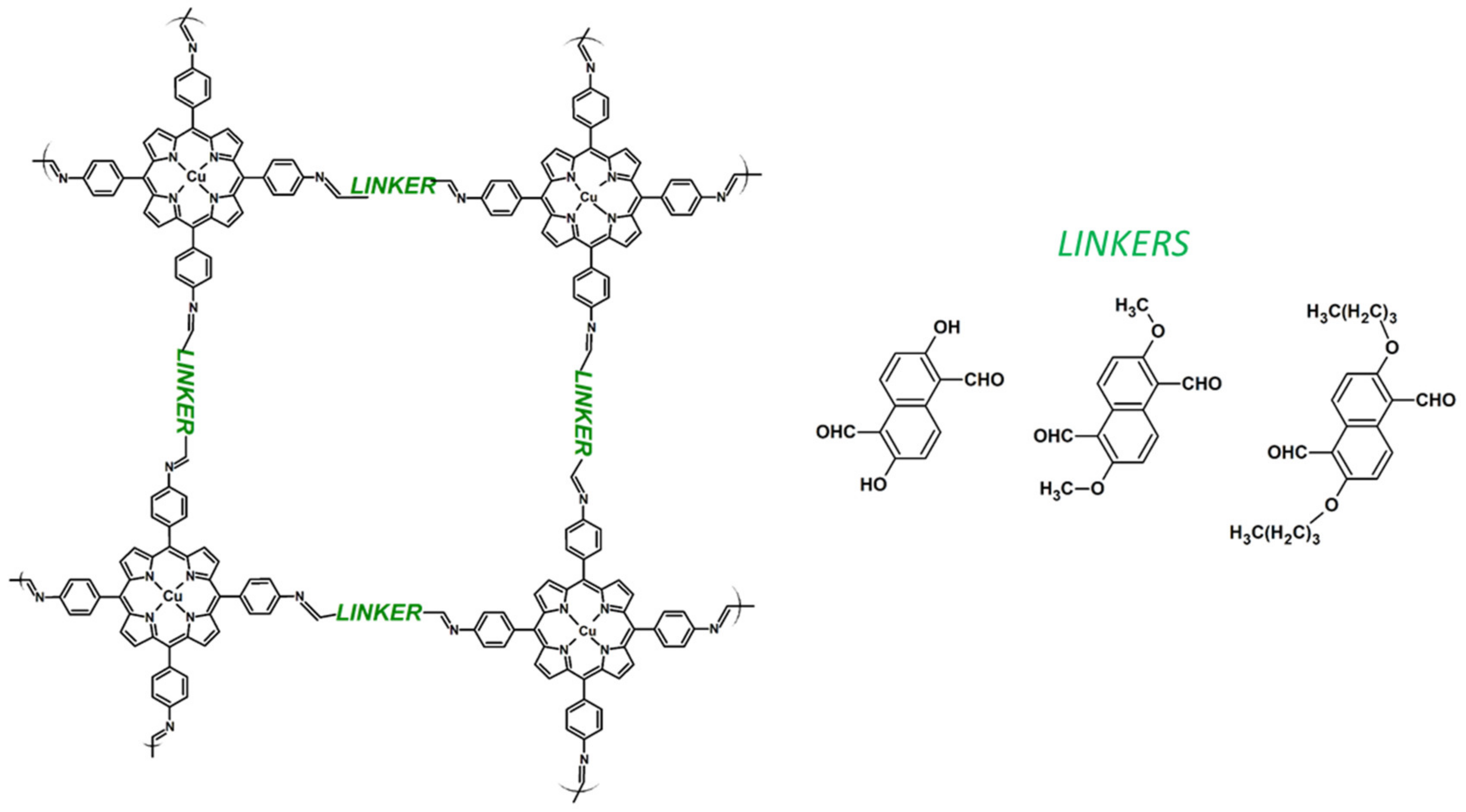
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blacha-Grzechnik, A. New Approach in the Application of Conjugated Polymers: The Light-Activated Source of Versatile Singlet Oxygen Molecule. Materials 2021, 14, 1098. https://doi.org/10.3390/ma14051098
Blacha-Grzechnik A. New Approach in the Application of Conjugated Polymers: The Light-Activated Source of Versatile Singlet Oxygen Molecule. Materials. 2021; 14(5):1098. https://doi.org/10.3390/ma14051098
Chicago/Turabian StyleBlacha-Grzechnik, Agata. 2021. "New Approach in the Application of Conjugated Polymers: The Light-Activated Source of Versatile Singlet Oxygen Molecule" Materials 14, no. 5: 1098. https://doi.org/10.3390/ma14051098
APA StyleBlacha-Grzechnik, A. (2021). New Approach in the Application of Conjugated Polymers: The Light-Activated Source of Versatile Singlet Oxygen Molecule. Materials, 14(5), 1098. https://doi.org/10.3390/ma14051098





