Thermal Decomposition Behavior of Prussian Blue in Various Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Methods
2.2. Characterization Methods
2.3. Adsorption Test
2.4. Thermal Oxidation of PB and PB-b
2.5. Pilot Plant Test
3. Results and Discussion
3.1. Thermogravimetric Properties of PB
3.2. Influence of the Presence of Alkali Metal Ions on the Thermogravimetric Properties of PB
3.3. Thermal Decomposition of PB-Cs: Studying the Possibility of Partial Decomposition
3.4. Scale-Up Study
3.5. Complete Oxidation of PB for Ruling out the Temperature rise due to the Presence of HCF
3.6. Pilot-Scale Test
3.7. Process Recommendation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCF | Hexacyanoferrate |
| PB | Prussian blue |
| PBA | Prussian blue analogs |
| PB-Cs | Cesium adsorbed Prussian blue |
| PB-b | Prussian blue microbead |
| PB-b-Cs | Cesium adsorbed Prussian blue microbead |
| r-Cs | Cesium radioisotopes |
| TG-DTA | Thermogravimetric – Differential Thermal Analysis |
References
- Valentini, M.G.; Meloni, S.; Maxia, V. Adsorption of monovalent ions on zinc ferrocyanide. J. Inorg. Nucl. Chem. 1972, 34, 1427–1436. [Google Scholar] [CrossRef]
- Ware, M. Prussian Blue: Artists’ Pigment and Chemists’ Sponge. J. Chem. Educ. 2008, 85, 612. [Google Scholar] [CrossRef]
- Kraft, A. On the discovery and history of Prussian blue. Bull. Hist. Chem. 2008, 33, 61–67. Available online: http://www.scs.illinois.edu/~mainzv/HIST/bulletin_open_access/v33-2/v33-2 p61-67.pdf (accessed on 1 March 2021).
- Ishizaki, M.; Akiba, S.; Ohtani, A.; Hoshi, Y.; Ono, K.; Matsuba, M.; Togashi, T.; Kananizuka, K.; Sakamoto, M.; Takahashi, A.; et al. Proton-exchange mechanism of specific Cs+ adsorption via lattice defect sites of Prussian blue filled with coordination and crystallization water molecules. Dalton Trans. 2013, 42, 16049–16055. [Google Scholar] [CrossRef]
- Chen, S.-M.; Peng, K.-T. The electrochemical properties of dopamine, epinephrine, norepinephrine, and their electrocatalytic reactions on cobalt(II) hexacyanoferrate films. J. Electroanal. Chem. 2003, 547, 179–189. [Google Scholar] [CrossRef]
- Parajuli, D.; Suzuki, Y.; Sato, M.; Takahashi, A.; Tanaka, H.; Kawamoto, T. Assessment of the measures for the extraction or fixation of radiocesium in soil. Geoderma 2016, 267, 169–173. [Google Scholar] [CrossRef]
- Lehto, J.; Haukka, S.; Harjula, R.; Blomberg, M. Mechanism of caesium ion exchange on potassium cobalt hexacyanoferrates(II). J. Chem. Soc. Dalton Trans. 1990, 1007–1011. [Google Scholar] [CrossRef]
- Parajuli, D.; Takahashi, A.; Noguchi, H.; Kitajima, A.; Tanaka, H.; Takasaki, M.; Yoshino, K.; Kawamoto, T. Comparative study of the factors associated with the application of metal hexacyanoferrates for environmental Cs decontamination. Chem. Eng. J. 2016, 283, 1322–1328. [Google Scholar] [CrossRef]
- Lee, K.-M.; Kido, G.; Yoshino, K.; Kawamoto, T.; Minami, K.; Takahashi, A.; Parajuli, D.; Tanaka, H. Improved adsorption properties of granulated copper hexacyanoferrate with multi-scale porous networks. RSC Adv. 2016, 6, 16234–16238. [Google Scholar] [CrossRef] [Green Version]
- Mimura, H.; Lehto, J.; Harjula, R. Ion Exchange of Cesium on Potassium Nickel Hexacyanoferrate (II) s. J. Nucl. Sci. Technol. 1997, 34, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, A.; Kitajima, A.; Parajuli, D.; Hakuta, Y.; Tanaka, H.; Ohkoshi, S.-I.; Kawamoto, T. Radioactive cesium removal from ash-washing solution with high pH and high K+-concentration using potassium zinc hexacyanoferrate. Chem. Eng. Res. Des. 2016, 109, 513–518. [Google Scholar] [CrossRef] [Green Version]
- Grandjean, F.; Samain, L.; Long, G.J. Characterization and utilization of Prussian blue and its pigments. Dalton Trans. 2016, 45, 18018–18044. [Google Scholar] [CrossRef]
- Vincent, T.; Barre, Y.; Guari, Y.; Le Saout, G.; Guibal, E. Immobilization of metal hexacyanoferrates in chitin beads for cesium sorption: Synthesis and characterization. J. Mater. Chem. A 2014, 2, 10007–10021. [Google Scholar] [CrossRef]
- Barton, G.B.; Hepworth, J.L.; McClanahan, E.D.; Moore, R.L.; Van Tuyl, H.H. Chemical Processing Wastes. Recovering Fission Products. Ind. Eng. Chem. 1958, 50, 212–216. [Google Scholar] [CrossRef]
- Han, F.; Zhang, G.-H.; Gu, P. Adsorption kinetics and equilibrium modeling of cesium on copper ferrocyanide. J. Radioanal. Nucl. Chem. 2012, 295, 369–377. [Google Scholar] [CrossRef]
- Causse, J.; Tokarev, A.; Ravaux, J.; Moloney, M.; Barré, Y.; Grandjean, A. Facile one-pot synthesis of copper hexacyanoferrate nanoparticle functionalised silica monoliths for the selective entrapment of 137Cs. J. Mater. Chem. A 2014, 2, 9461–9464. [Google Scholar] [CrossRef]
- Tusa, E.; Power, F.; Oy, H. Successful Cesium Removal Campaign at the Loviisa NPP, Finland. Available online: http://www.wmsym.org/archives/2011/papers/11002.pdf (accessed on 26 March 2017).
- Parajuli, D.; Kitajima, A.; Takahashi, A.; Tanaka, H.; Ogawa, H.; Hakuta, Y.; Yoshino, K.; Funahashi, T.; Yamaguchi, M.; Osada, M.; et al. Application of Prussian blue nanoparticles for the radioactive Cs decontamination in Fukushima region. J. Environ. Radioact. 2016, 151, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Tanaka, H.; Hakuta, Y.; Takahashi, A.; Parajuli, D.; Minami, K.; Yasutaka, T.; Uchida, T. Radioactive cesium decontamination technology for ash. Synth. Engl. Ed. 2016, 9, 139–154. [Google Scholar] [CrossRef]
- Chen, R.; Tanaka, H.; Kawamoto, T.; Asai, M.; Fukushima, C.; Kurihara, M.; Ishizaki, M.; Watanabe, M.; Arisaka, M.; Nankawa, T. Thermodynamics and Mechanism Studies on Electrochemical Removal of Cesium Ions from Aqueous Solution Using a Nanoparticle Film of Copper Hexacyanoferrate. ACS Appl. Mater. Interfaces 2013, 5, 12984–12990. [Google Scholar] [CrossRef]
- Ding, D.; Lei, Z.; Yang, Y.; Feng, C.; Zhang, Z. Selective removal of cesium from aqueous solutions with nickel (II) hexacyanoferrate (III) functionalized agricultural residue–walnut shell. J. Hazard. Mater. 2014, 270, 187–195. [Google Scholar] [CrossRef]
- Bunting, R. Nuclear data sheets for A = 137. Nucl. Data Sheets 1975, 15, 335–369. [Google Scholar] [CrossRef]
- Hepworth, J.L.; McClanahan, E.D.J.; Moore, R.L. Cesium Packaging Studies Coonversion of Cesium Zinc Ferrocyanide tp a Cesium Chloride Product; Hanford Atomic Products Operation: Richland, WA, USA, 1957. [Google Scholar]
- Parajuli, D.; Tanaka, H.; Hakuta, Y.; Minami, K.; Fukuda, S.; Umeoka, K.; Kamimura, R.; Hayashi, Y.; Ouchi, M.; Kawamoto, T. Dealing with the Aftermath of Fukushima Daiichi Nuclear Accident: Decontamination of Radioactive Cesium Enriched Ash. Environ. Sci. Technol. 2013, 47, 3800–3806. [Google Scholar] [CrossRef]
- Parajuli, D.; Takahashi, A.; Tanaka, H.; Sato, M.; Fukuda, S.; Kamimura, R.; Kawamoto, T. Variation in available cesium concentration with parameters during temperature induced extraction of cesium from soil. J. Environ. Radioact. 2015, 140, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.F.; Bonnette, A.K., Jr. Thermal decomposition of Prussian blue: Isotopic labeling with Mössbauer-inactive Fe-56. J. Inorg. Nucl. Chem. 1974, 36, 1011–1016. [Google Scholar] [CrossRef]
- Krtil, J. Ion exchange of Cs and Rb on tungsten ferrocyanide. J. Inorg. Nucl. Chem. 1965, 27, 233–236. [Google Scholar] [CrossRef]
- Reguera, E.; Fernández-Bertrán, J.; Dago, A.; Diaz, C. Mössbauer spectroscopic study of Prussian Blue from different provenances. Hyperfine Interact. 1992, 73, 295–308. [Google Scholar] [CrossRef]
- Inoue, H.; Nakazawa, T.; Mitsuhashi, T.; Shirai, T.; Fluck, E. Characterization of Prussian blue and its thermal decomposition products. Hyperfine Interact. 1989, 46, 723–731. [Google Scholar] [CrossRef]
- Aparicio, C.; Machala, L.; Marusak, Z. Thermal decomposition of Prussian blue under inert atmosphere. J. Therm. Anal. Calorim. 2011, 110, 661–669. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Borai, E.; Harjula, R.; Malinen, L.; Paajanen, A. Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J. Hazard. Mater. 2009, 172, 416–422. [Google Scholar] [CrossRef]
- Balencie, J.; Burger, D.; Rehspringer, J.-L.; Estournès, C.; Vilminot, S.; Richard-Plouet, M.; Boos, A. Perlite for permanent confinement of cesium. J. Nucl. Mater. 2006, 352, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Klika, Z.; Kraus, L.; Vopálka, D. Cesium Uptake from Aqueous Solutions by Bentonite: A Comparison of Multicomponent Sorption with Ion-Exchange Models. Langmuir 2007, 23, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, B.; Liao, J.; Feng, Y.; Zhang, N.; Zhao, J.; Wen, W.; Yang, Y.; Liu, N. Behavior and analysis of Cesium adsorption on montmorillonite mineral. J. Environ. Radioact. 2009, 100, 914–920. [Google Scholar] [CrossRef]
- Bostick, B.C.; Vairavamurthy, M.A.; Karthikeyan, K.G.; Chorover, J. Cesium Adsorption on Clay Minerals: An EXAFS Spectroscopic Investigation. Environ. Sci. Technol. 2002, 36, 2670–2676. [Google Scholar] [CrossRef]
- Kröcher, O.; Elsener, M. Hydrolysis and oxidation of gaseous HCN over heterogeneous catalysts. Appl. Catal. B: Environ. 2009, 92, 75–89. [Google Scholar] [CrossRef]
- Zbořil, R.; Machala, L.; Mashlan, M.; Sharma, V. Iron (III) Oxide Nanoparticles in the Thermally Induced Oxidative Decomposition of Prussian Blue, Fe4[Fe(CN)6]3. Cryst. Growth Des. 2004, 4, 1317–1325. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Hu, M.; Imura, M.; Salunkhe, R.R.; Umezawa, N.; Hamoudi, H.; Belik, A.A.; Yamauchi, Y. Single-Crystal-like Nanoporous Spinel Oxides: A Strategy for Synthesis of Nanoporous Metal Oxides Utilizing Metal-Cyanide Hybrid Coordination Polymers. Chem. A Eur. J. 2014, 20, 17375–17384. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Hu, M.; Hayashi, N.; Tsujimoto, Y.; Ishihara, S.; Imura, M.; Suzuki, N.; Huang, Y.-Y.; Sakka, Y.; Ariga, K.; et al. Thermal Conversion of Hollow Prussian Blue Nanoparticles into Nanoporous Iron Oxides with Crystallized Hematite Phase. Eur. J. Inorg. Chem. 2014, 2014, 1137–1141. [Google Scholar] [CrossRef]
- Zhang, L.; Bin Wu, H.; Madhavi, S.; Hng, H.H.; Lou, X.W. (David) Formation of Fe2O3 Microboxes with Hierarchical Shell Structures from Metal–Organic Frameworks and Their Lithium Storage Properties. J. Am. Chem. Soc. 2012, 134, 17388–17391. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Y.; Chen, Q. FexCo3−xO4 nanoporous particles stemmed from metal–organic frameworks Fe3[Co(CN)6]2: A highly efficient material for removal of organic dyes from water. J. Alloy. Compd. 2013, 559, 57–63. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Belik, A.A.; Liu, C.-H.; Hsieh, H.-Y.; Liao, Y.-T.; Malgras, V.; Yamauchi, Y.; Wu, K.C.-W. Prussian Blue Derived Nanoporous Iron Oxides as Anticancer Drug Carriers for Magnetic-Guided Chemotherapy. Chem. Asian J. 2015, 10, 1457–1462. [Google Scholar] [CrossRef]
- Hu, M.; Jiang, J.-S.; Zeng, Y. Prussian blue microcrystals prepared by selective etching and their conversion to mesoporous magnetic iron(iii) oxides. Chem. Commun. 2009, 46, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.B.; Chikyow, T. Recent advances in Prussian blue and Prussian blue analogues: Synthesis and thermal treatments, Coord. Chem. Rev. 2017, 352, 328–345. [Google Scholar] [CrossRef]
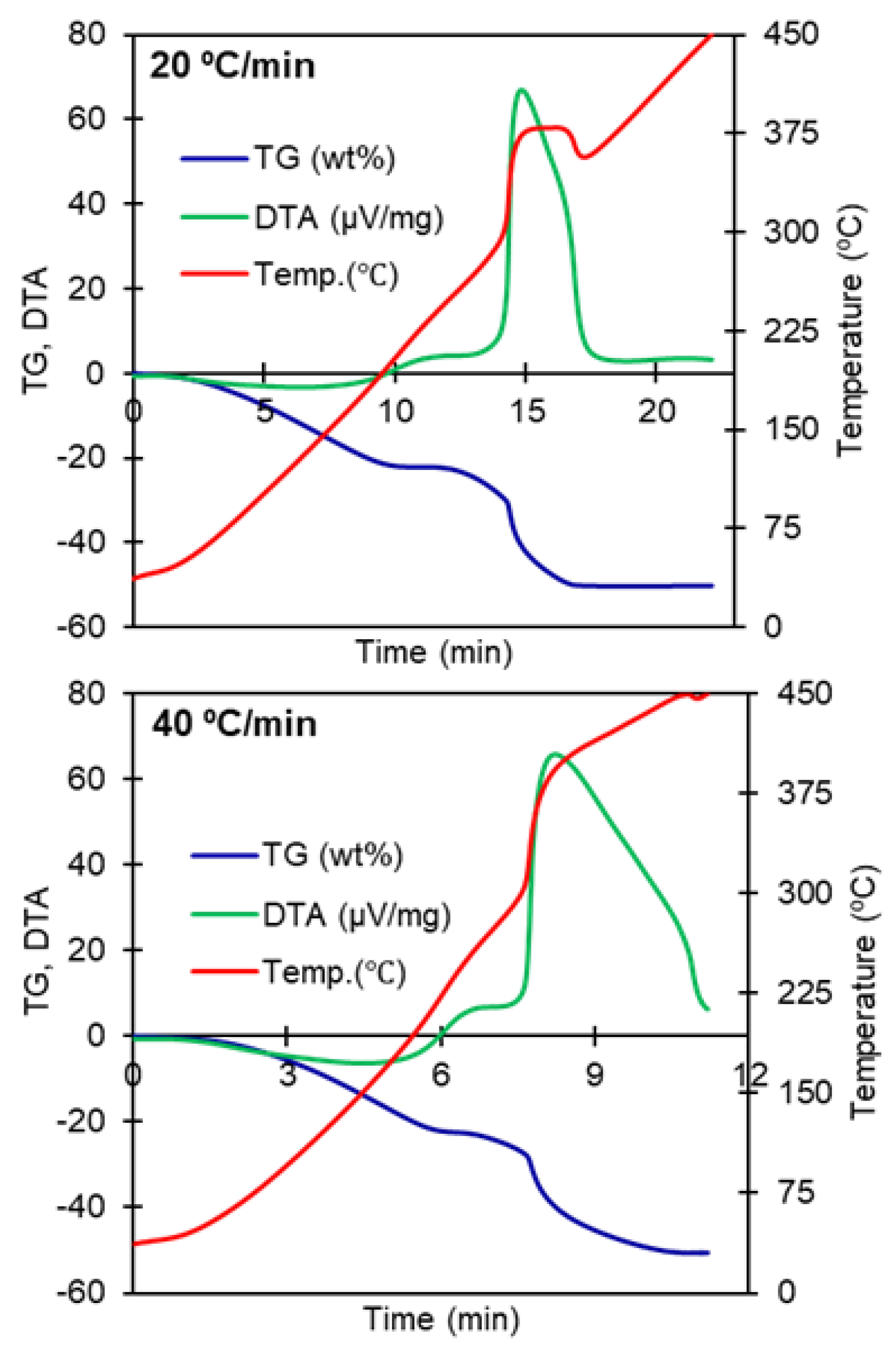
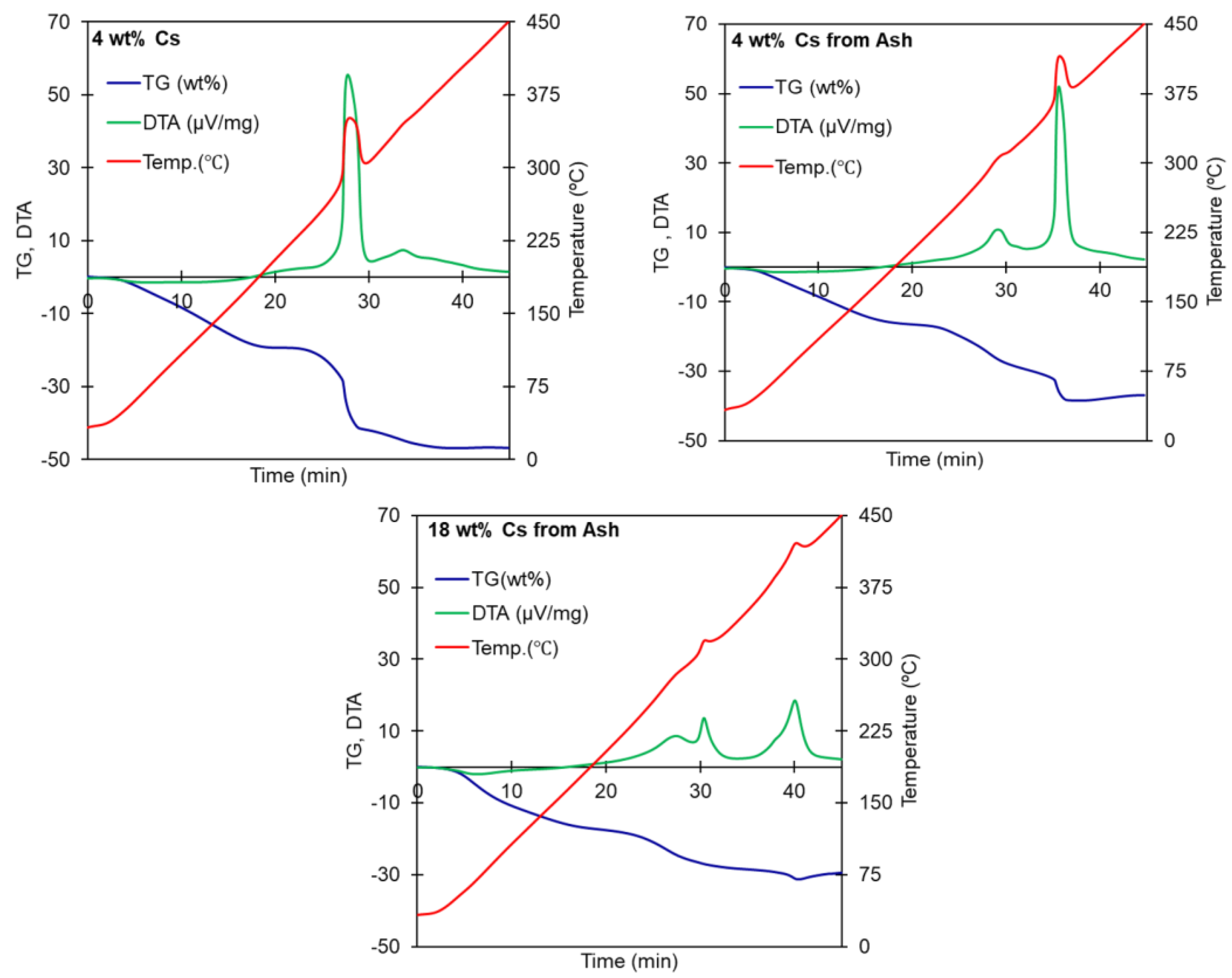
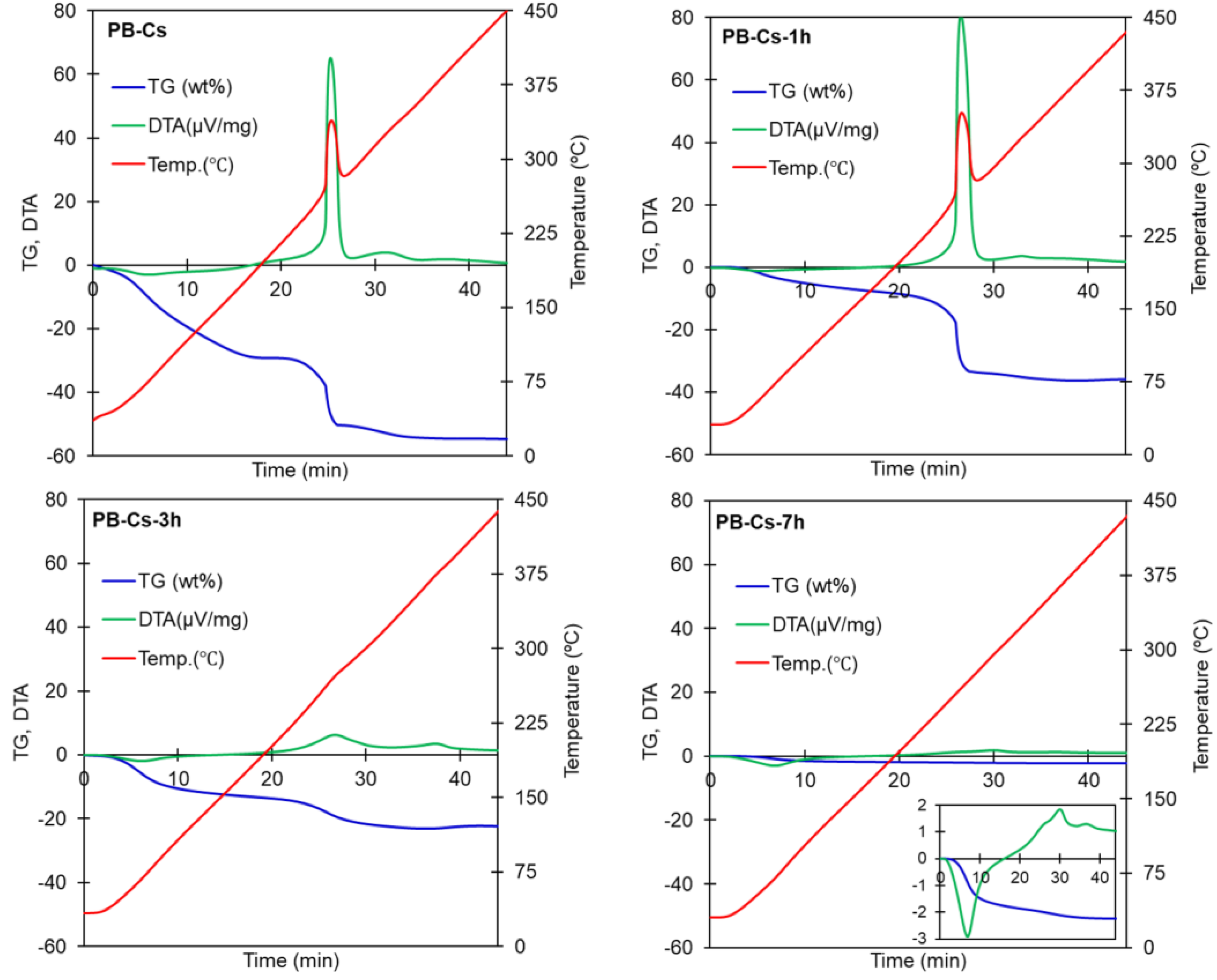

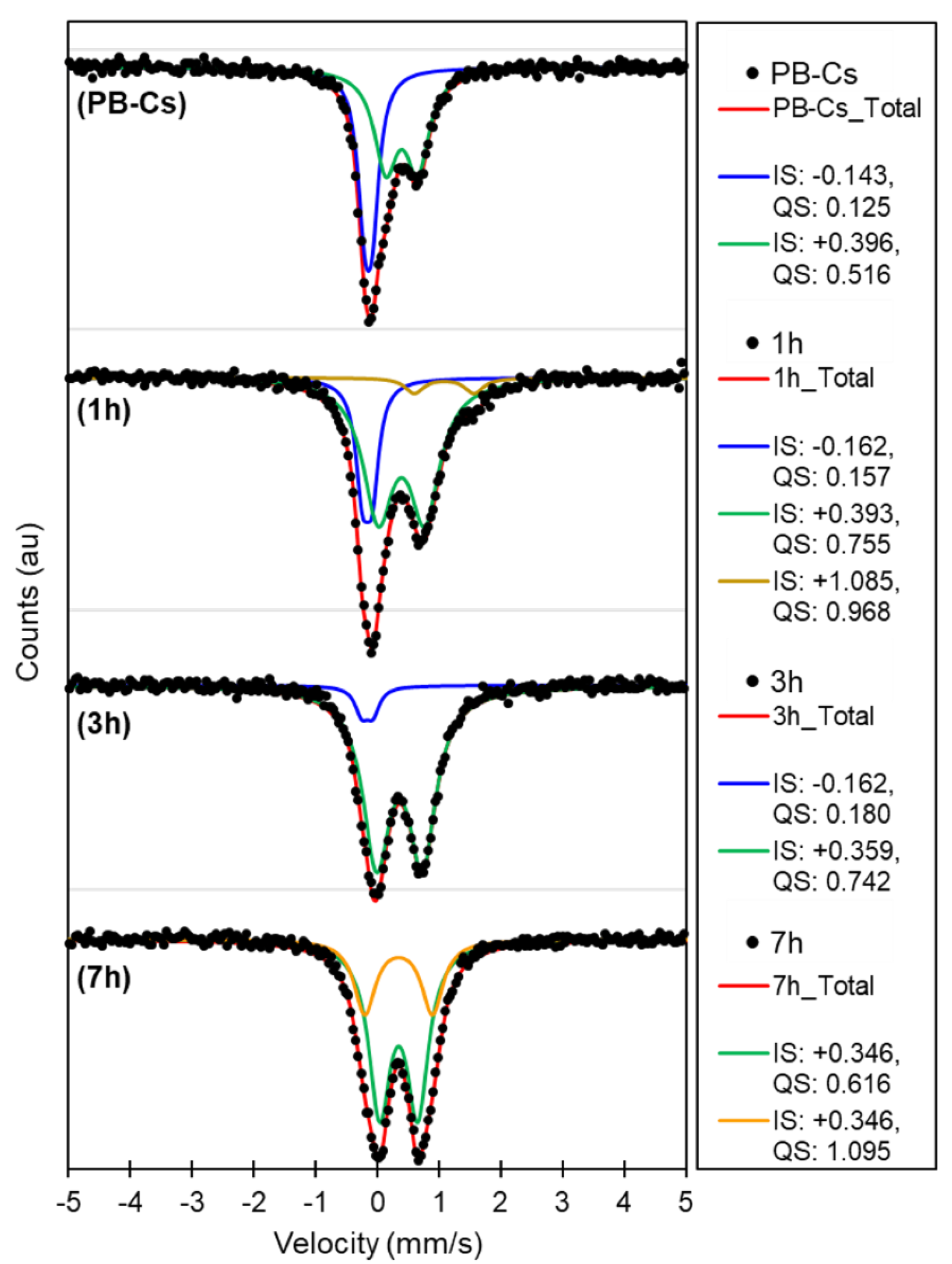
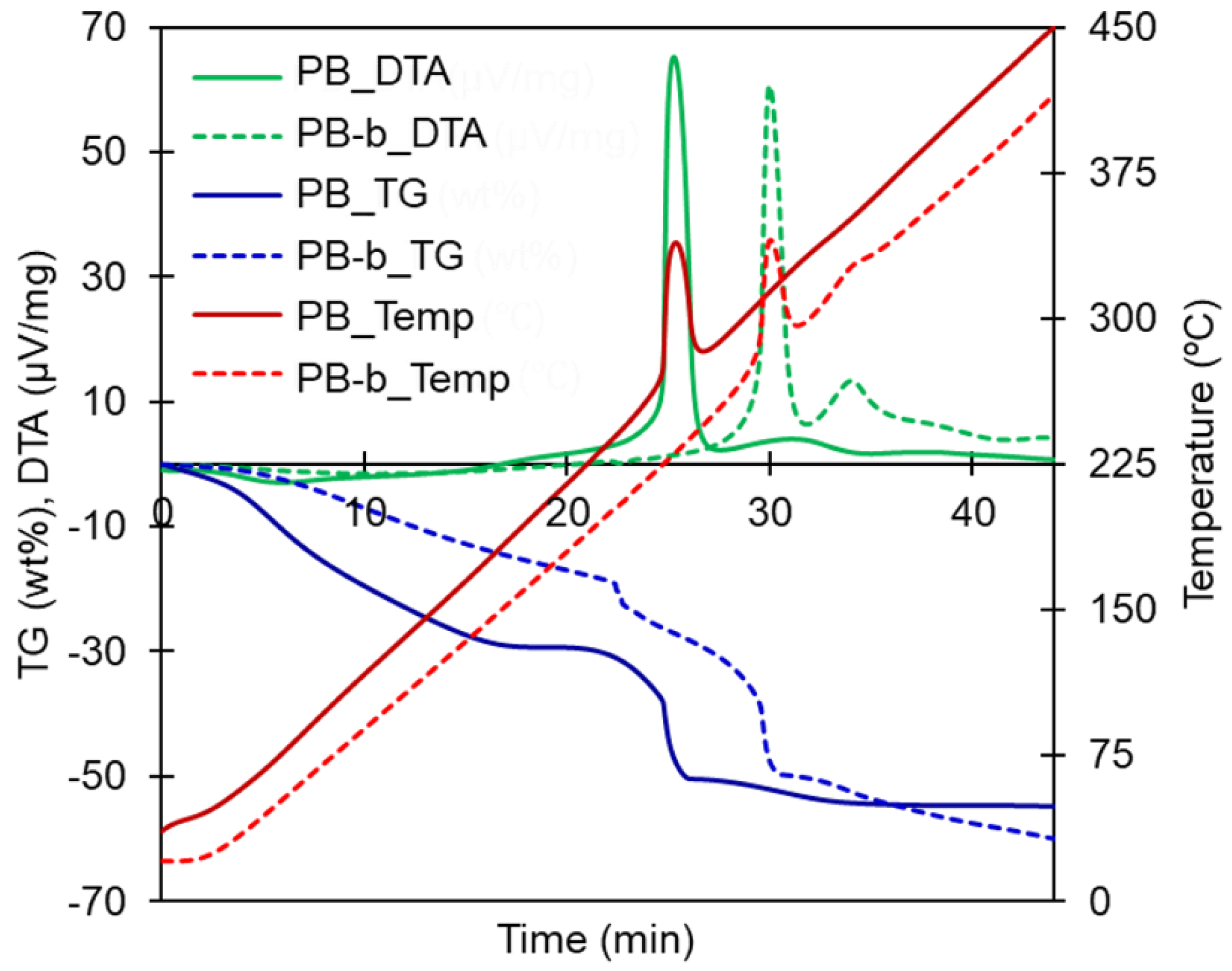

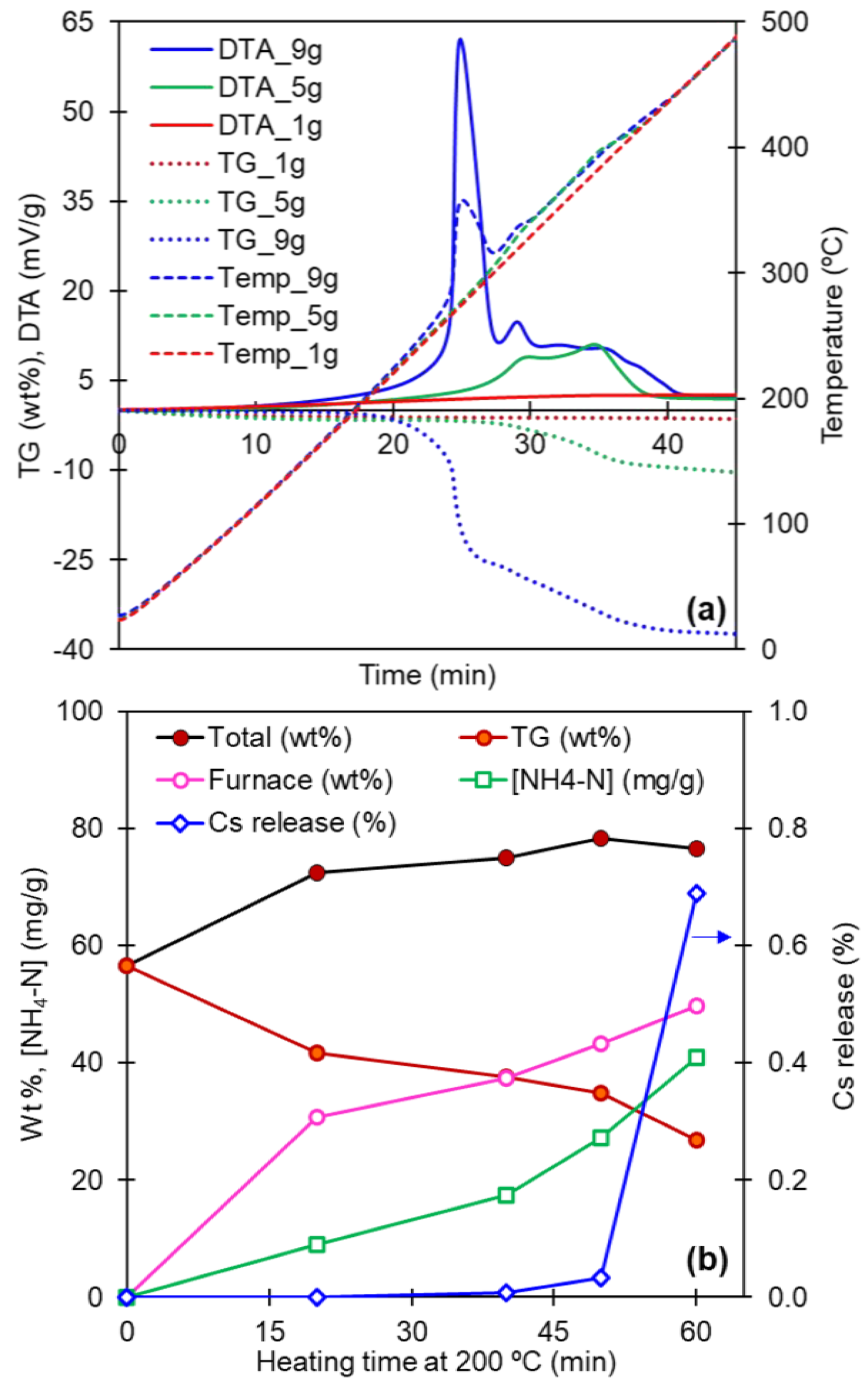
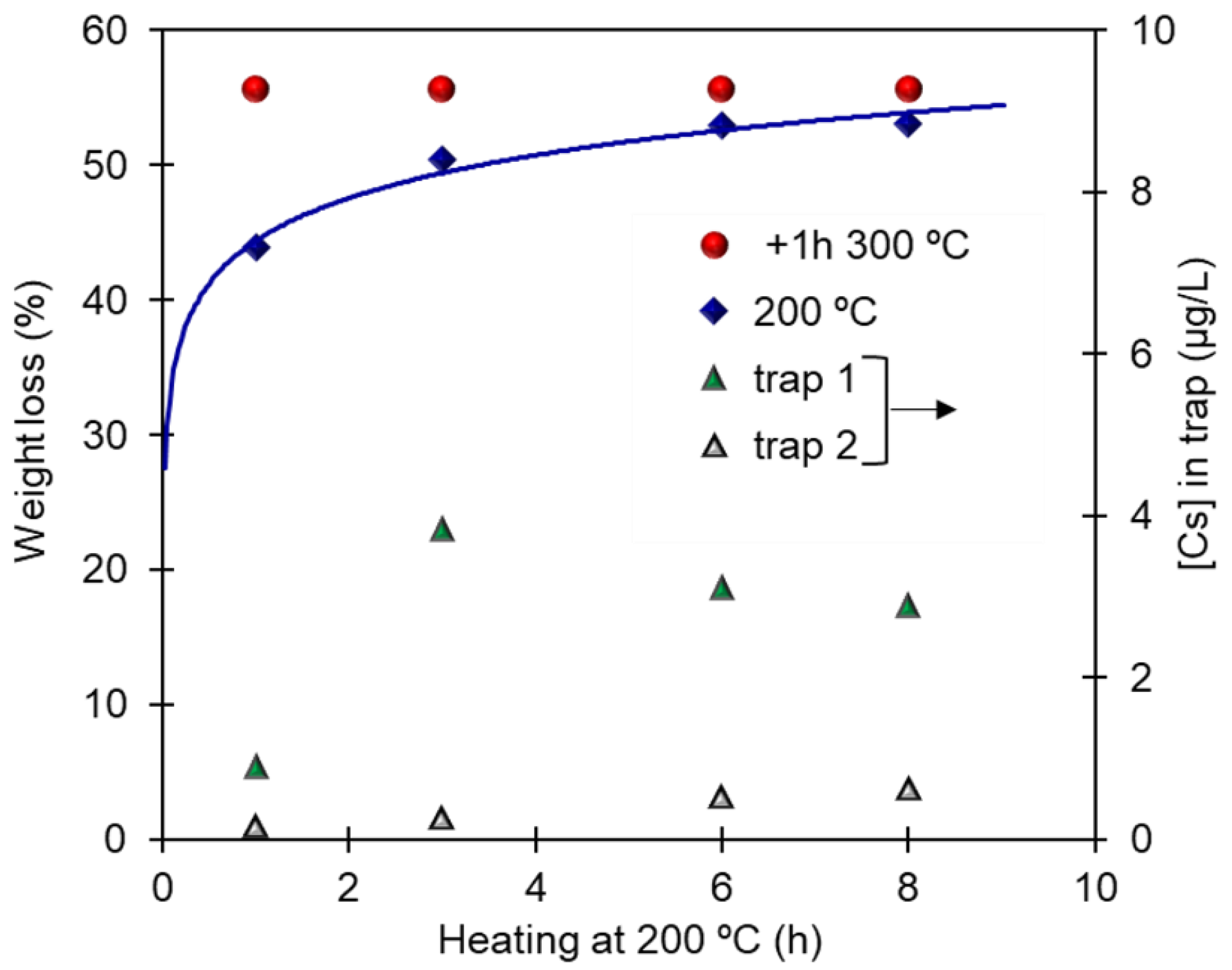
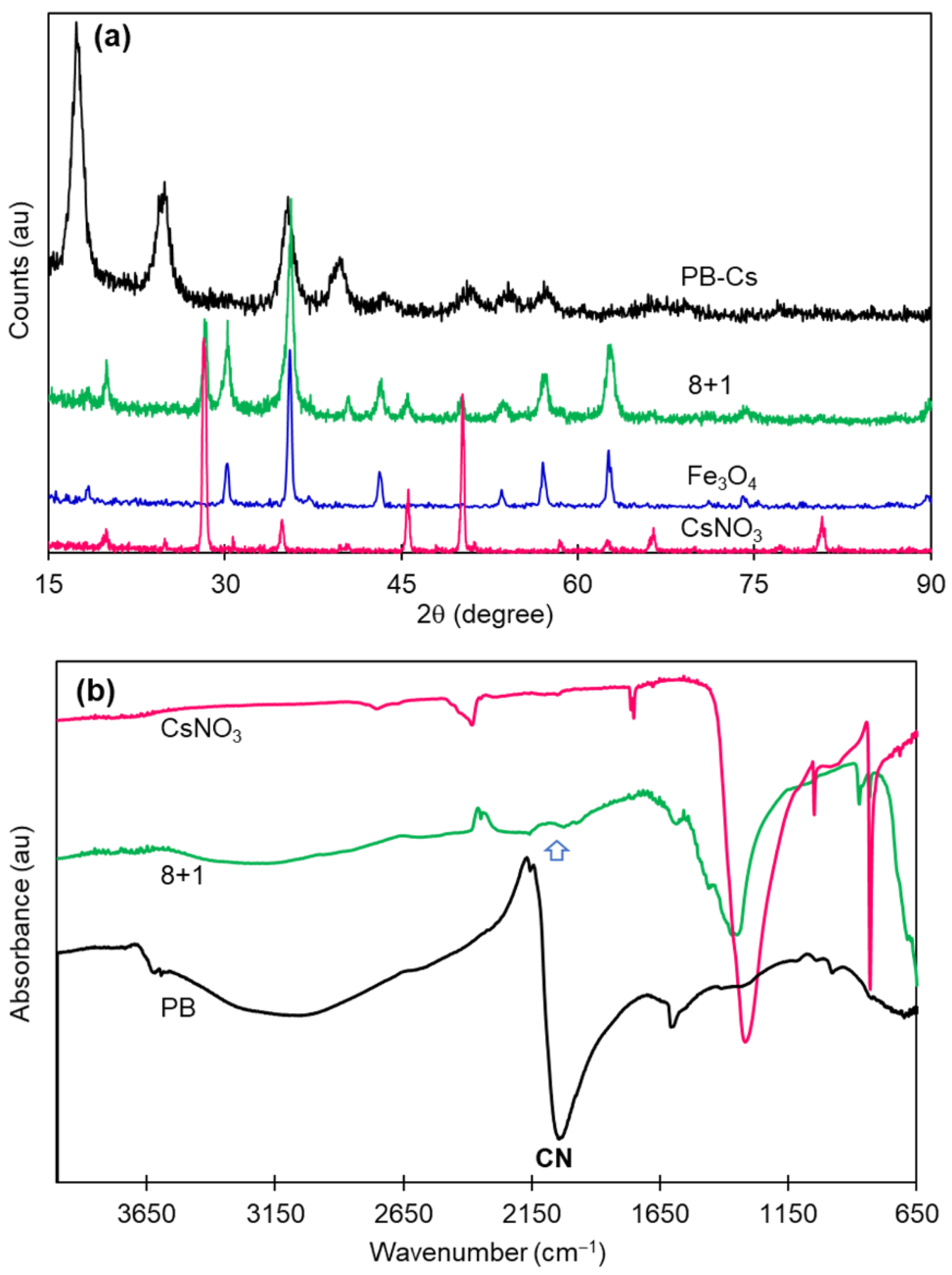
| Material | Heating Rate (°C/min) | wt loss (%) Until 450 °C | Temperature Surge | Corresponding DTA Peaks |
|---|---|---|---|---|
| PB (before adsorption) | 2 | 55.7 | not observed | 227 °C (low energy broad) |
| 5 | 55 | twice, small | 271 and 353 °C (low energy twin peaks) | |
| 10 | 50.4 | once, high, sharp | 281 °C (major, sharp) 320 °C (minor) | |
| 20 | 50.2 | once, high, broad | 293 to 360 °C (major, broad) | |
| 40 | 51 | once, high, continued | 298 to 450 °C (major, broad) | |
| PB-Cs (heating rate = 10 °C/min) | 4 wt.% Cs, pure water solution | 47 | once, high, sharp | 288 °C (major) 325 °C (minor) |
| 4 wt.% Cs, ash extract | 37 | twice, small then sharp | 292 °C (minor) 360 °C (major) | |
| 18 wt.% Cs, ash extract | 29.6 | twice, small | 315 °C and 360 °C (low energy peaks) |
| Values in % | PB-Cs | PB-Cs-1 h | PB-Cs-3 h | PB-Cs-7 h |
|---|---|---|---|---|
| Data from TG-DTA | ||||
| Water | 29 | 8 | 12 | 17 |
| PB-Cs except water | 71 | 92 | 88 | 83 |
| Weight change after heating | ||||
| Wt loss | 32.2 | 46.5 | 51.1 | |
| Loss except water | 3.2 | 17.5 | 22.1 | |
| Forms of Fe calculated from Mossbauer spectra | ||||
| FeII | 44 | 29 | 7 | 0 |
| FeIII | 56 | 71 | 93 | 100 |
| CHN analysis | ||||
| N in total wt | 19.6 | 21.1 | 7.31 | 1.11 |
| N except water | 27.6 | 22.9 | 8.31 | 1.34 |
| N released during heating | 17.0 | 69.9 | 95.1 | |
| C in total wt | 17.1 | 16.6 | 6.6 | 1.68 |
| C except water | 24.1 | 18.0 | 7.56 | 2.02 |
| C released during heating | 25.3 | 68.6 | 91.6 | |
| Heating Step | Heating Time (h) | PB-b Mass (g) | Total Mass Loss (%) |
|---|---|---|---|
| Initial | 0 | 129.7 | - |
| 220 °C | 4 | 79.9 | 38.4 |
| 300 °C_1st | 5 | 70.6 | 45.6 |
| 300 °C_2nd | 5 | 68.6 | 47.1 |
| 300 °C_3rd | 5 | 67.4 | 48.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parajuli, D.; Tanaka, H.; Sakurai, K.; Hakuta, Y.; Kawamoto, T. Thermal Decomposition Behavior of Prussian Blue in Various Conditions. Materials 2021, 14, 1151. https://doi.org/10.3390/ma14051151
Parajuli D, Tanaka H, Sakurai K, Hakuta Y, Kawamoto T. Thermal Decomposition Behavior of Prussian Blue in Various Conditions. Materials. 2021; 14(5):1151. https://doi.org/10.3390/ma14051151
Chicago/Turabian StyleParajuli, Durga, Hisashi Tanaka, Koji Sakurai, Yukiya Hakuta, and Tohru Kawamoto. 2021. "Thermal Decomposition Behavior of Prussian Blue in Various Conditions" Materials 14, no. 5: 1151. https://doi.org/10.3390/ma14051151
APA StyleParajuli, D., Tanaka, H., Sakurai, K., Hakuta, Y., & Kawamoto, T. (2021). Thermal Decomposition Behavior of Prussian Blue in Various Conditions. Materials, 14(5), 1151. https://doi.org/10.3390/ma14051151








