Pluronic F-127/Silk Fibroin for Enhanced Mechanical Property and Sustained Release Drug for Tissue Engineering Biomaterial
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation SF Solution
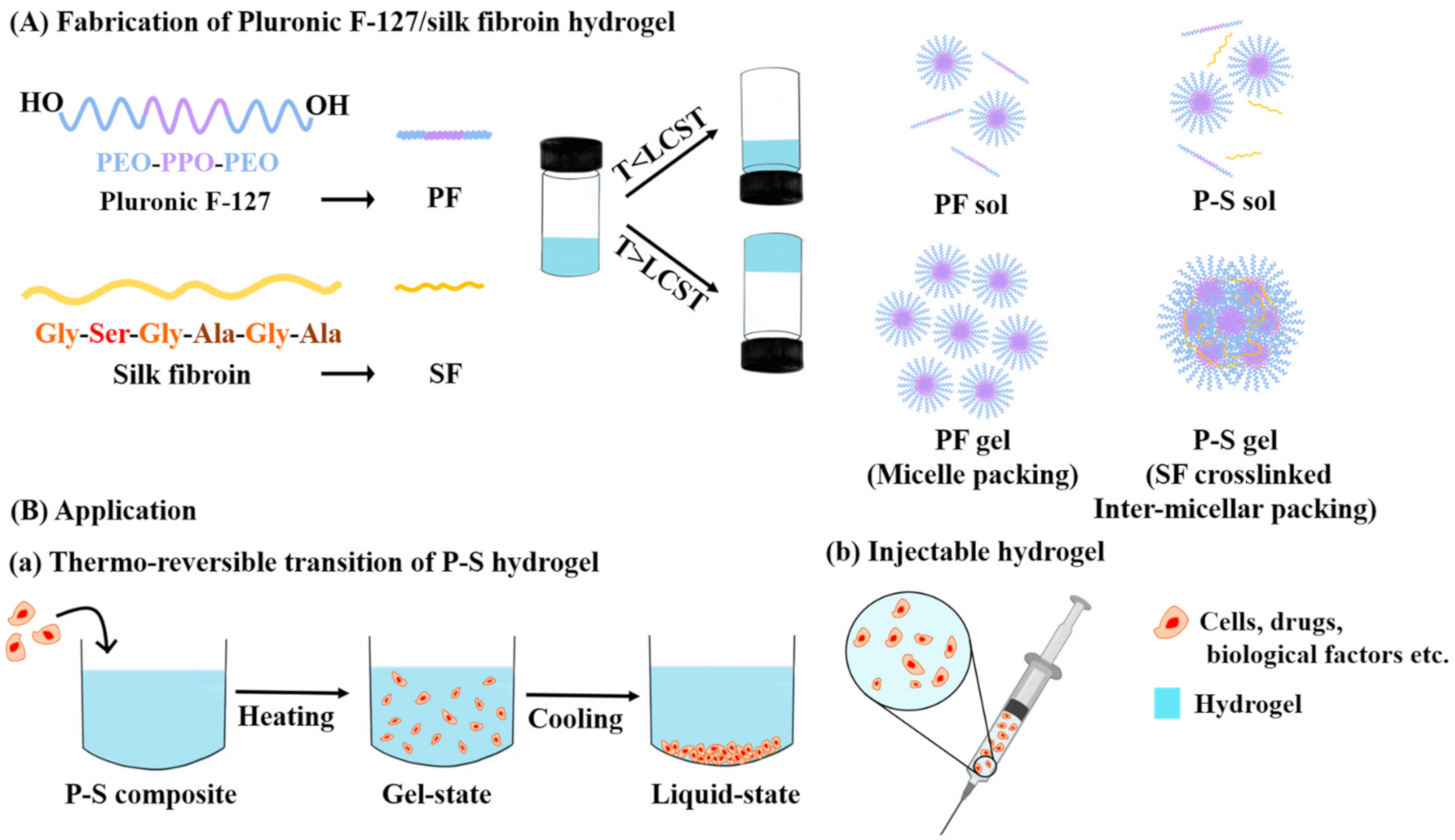
2.2. Fabrication of the Hydrogels
2.3. Characterization
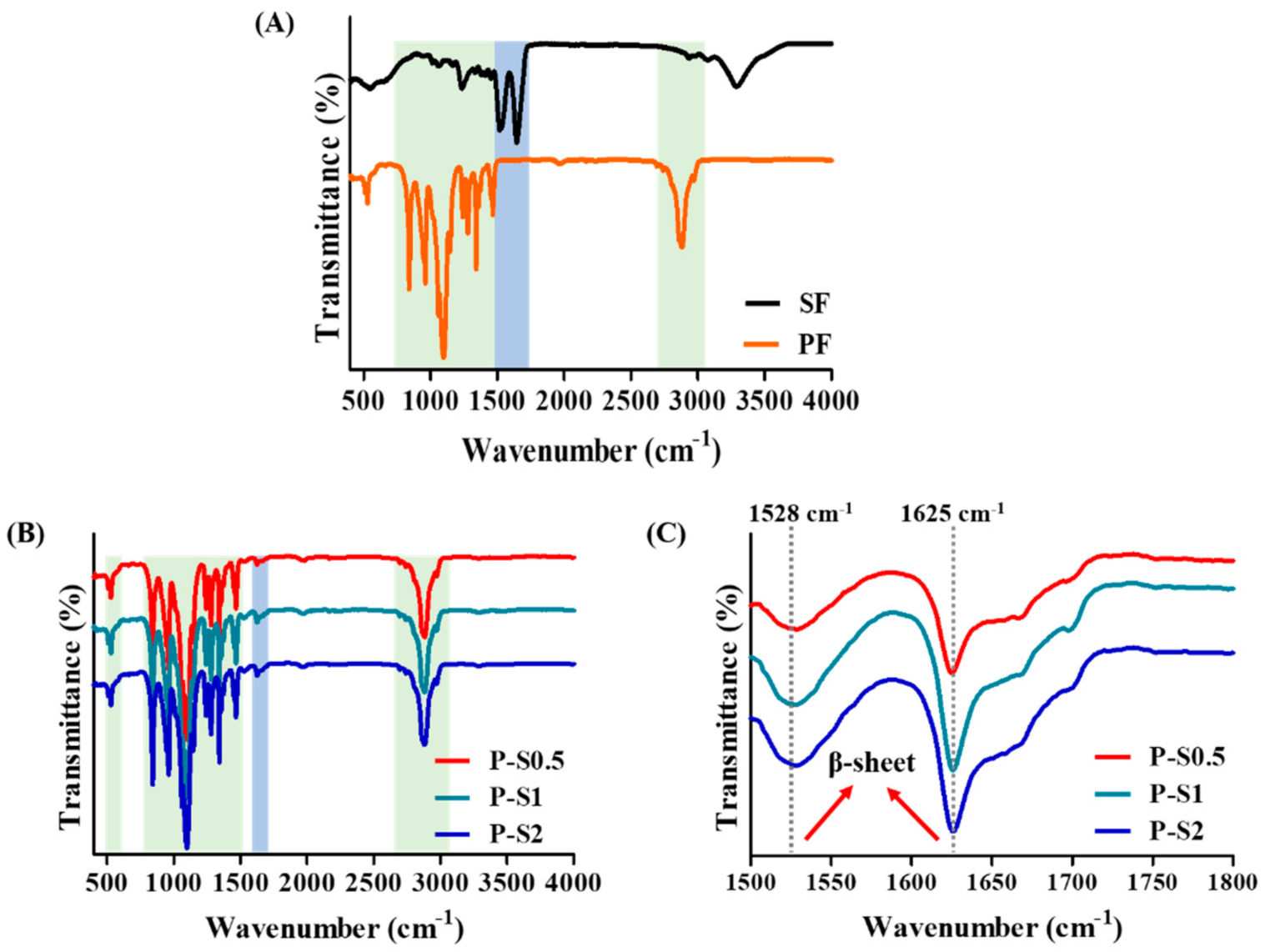
2.3.1. Chemical Structure Analysis
2.3.2. Weight Loss Ratio
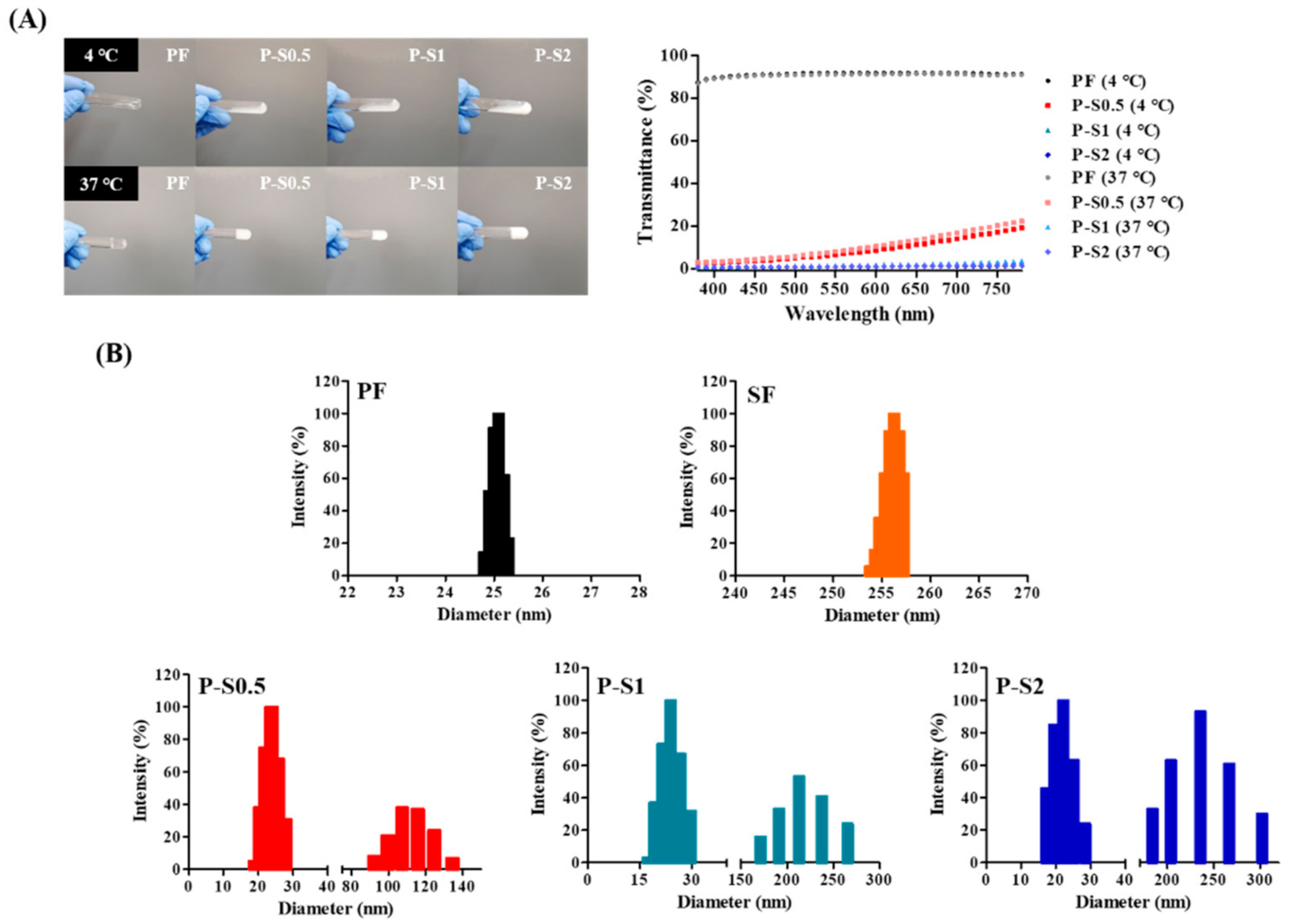
2.3.3. Optical Intensity
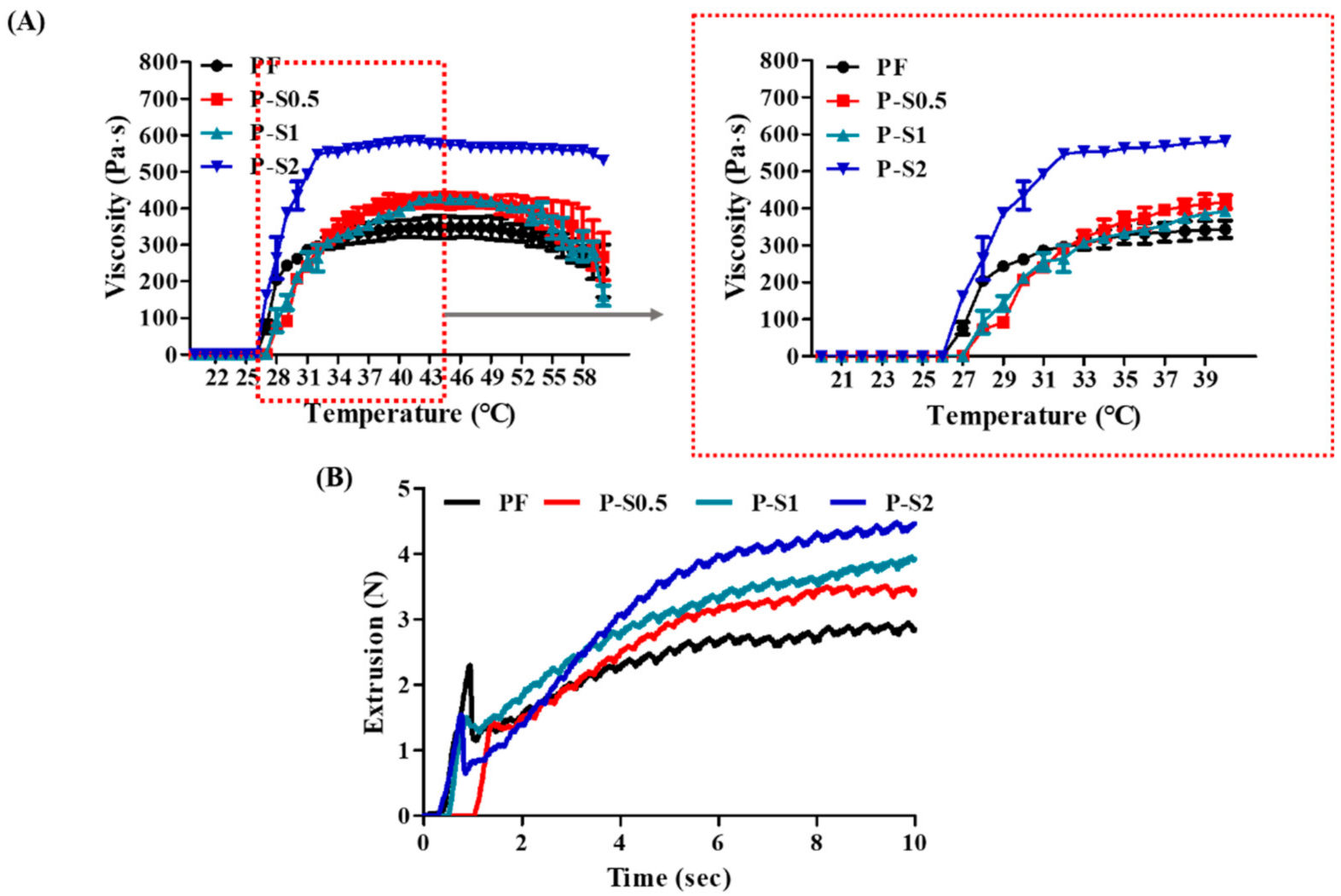
2.3.4. Viscosity Evaluation
2.3.5. Injection Force Test
2.3.6. Release Study
2.3.7. Size Analysis of P–S Hydrogel
2.4. In Vitro Study
Cytotoxicity Evaluation
3. Results and Discussion
3.1. Fabrication and Chemical Structure Evaluation
3.2. Viscosity and Injection Force Analysis
3.3. Gross Image and Transparency
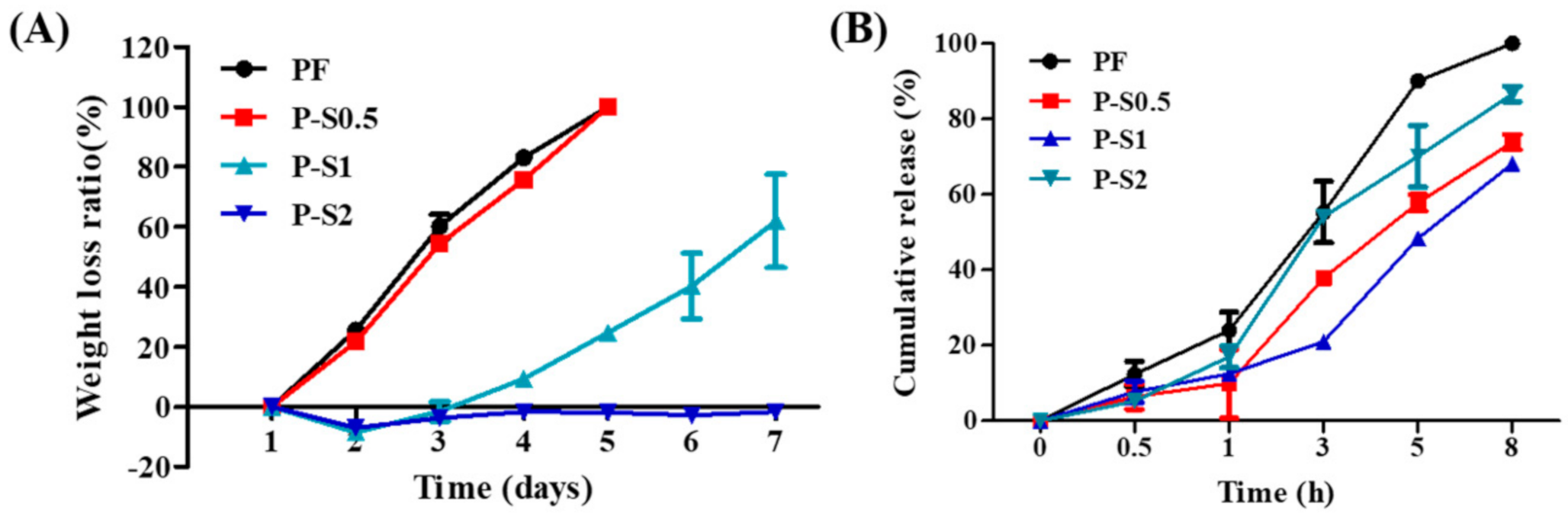
3.4. Weight Loss and Release Study
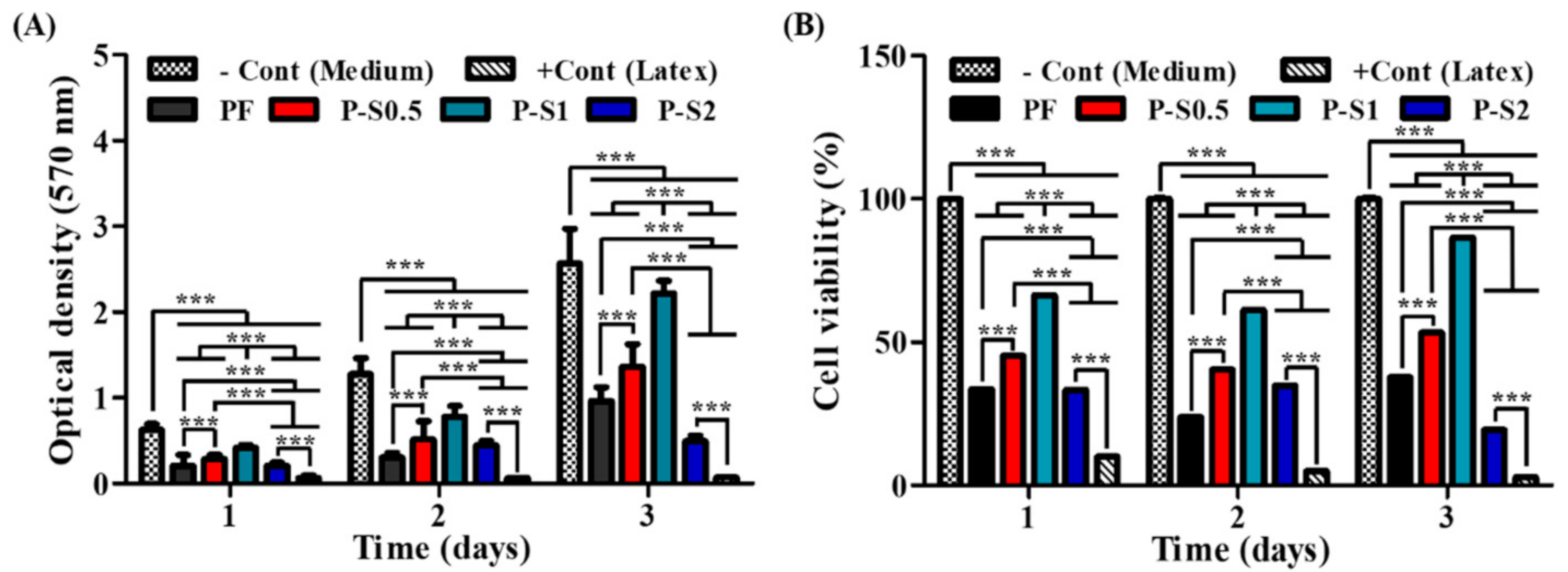
3.5. Cell Viability and Cytotoxicity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wani, S.U.D.; Gautam, S.P.; Qadrie, Z.L.; Gangadharappa, H. Silk fibroin as a natural polymeric based bio-material for tissue engineering and drug delivery systems—A review. Int. J. Biol. Macromol. 2020, 163, 2145–2161. [Google Scholar] [CrossRef]
- Cheng, A.Y.; García, A.J. Engineering the matrix microenvironment for cell delivery and engraftment for tissue repair. Curr. Opin. Biotechnol. 2013, 24, 864–871. [Google Scholar] [CrossRef]
- Ionescu, L.C.; Lee, G.C.; Sennett, B.J.; Burdick, J.A.; Mauck, R.L. An anisotropic nanofiber/microsphere composite with controlled release of biomolecules for fibrous tissue engineering. Biomaterials 2010, 31, 4113–4120. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, M.H.; Jo, G.; Kim, S.Y.; Chun, H.J.; Yang, D.H. Photo-Cured Glycol Chitosan Hydrogel for Ovarian Cancer Drug Delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Moon, Y.J.; Chun, H.J.; Yang, D.H. Doxorubicin· Hydrochloride/Cisplatin-Loaded Hydrogel/Nanosized (2-Hydroxypropyl)-Beta-Cyclodextrin Local Drug-Delivery System for Osteosarcoma Treatment In Vivo. Nanomaterials 2019, 9, 1652. [Google Scholar] [CrossRef]
- Yucel, T.; Lovett, M.L.; Kaplan, D.L. Silk-based biomaterials for sustained drug delivery. J. Control. Release 2014, 190, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Kim, H.J.; You, S.J.; Yang, D.-H.; Eun, J.; Park, H.K.; Kim, M.S.; Chun, H.J. Injectable hydrogels based on MPEG–PCL–RGD and BMSCs for bone tissue engineering. Biomater. Sci. 2020, 8, 4334–4345. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Cai, C.; Lin, J.; Chen, T. Dual-drug delivery system based on hydrogel/micelle composites. Biomaterials 2009, 30, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.S.; Nuttelman, C.R.; Collins, S.D.; Anseth, K.S. Synthesis and characterization of a fluvastatin-releasing hydrogel delivery system to modulate hMSC differentiation and function for bone regeneration. Biomaterials 2006, 27, 6102–6110. [Google Scholar] [CrossRef]
- Kim, H.J.; You, S.J.; Yang, D.H.; Chun, H.J.; Kim, M.S. Preparation of novel RGD-conjugated thermosensitive mPEG-PCL composite hydrogels and in vitro investigation of their impacts on adhesion-dependent cellular behavior. J. Ind. Eng. Chem. 2020, 84, 226–235. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, J.-K.; Ren, K.; Zuo, J.; Ding, J.; Chen, X. Thermosensitive Hydrogels as Scaffolds for Cartilage Tissue Engineering. Biomacromolecules 2019, 20, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ramirez, C.M.; Miljkovic, N.; Li, H.; Rubin, J.P.; Marra, K.G. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials 2009, 30, 6844–6853. [Google Scholar] [CrossRef]
- Bhatt, P.; Narvekar, P.; Lalani, R.; Chougule, M.B.; Pathak, Y.; Sutariya, V. An in vitro Assessment of Thermo-Reversible Gel Formulation Containing Sunitinib Nanoparticles for Neovascular Age-Related Macular Degeneration. AAPS PharmSciTech 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Cao, J.; Su, M.; Hasan, N.; Lee, J.; Kwak, D.; Kim, D.Y.; Kim, K.; Lee, E.H.; Jung, J.H.; Yoo, J.-W. Nitric Oxide-Releasing Thermoresponsive Pluronic F127/Alginate Hydrogel for Enhanced Antibacterial Activity and Accelerated Healing of Infected Wounds. Pharmaceutics 2020, 12, 926. [Google Scholar] [CrossRef]
- Sohn, S.S.; Revuri, V.; Nurunnabi; Kwak, K.S.; Lee, Y.-K. Biomimetic and photo crosslinked hyaluronic acid/pluronic F127 hydrogels with enhanced mechanical and elastic properties to be applied in tissue engineering. Macromol. Res. 2016, 24, 282–291. [Google Scholar] [CrossRef]
- Yoo, M.-K.; Kweon, H.Y.; Lee, K.-G.; Lee, H.-C.; Cho, C.-S. Preparation of semi-interpenetrating polymer networks composed of silk fibroin and poloxamer macromer. Int. J. Biol. Macromol. 2004, 34, 263–270. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]
- Gonçalves, L.C.; Seabra, A.B.; Pelegrino, M.T.; de Araujo, D.R.; Bernardes, J.S.; Haddad, P.S. Superparamagnetic iron oxide nanoparticles dispersed in Pluronic F127 hydrogel: Potential uses in topical applications. RSC Adv. 2017, 7, 14496–14503. [Google Scholar] [CrossRef]
- Basak, R.; Bandyopadhyay, R. Encapsulation of Hydrophobic Drugs in Pluronic F127 Micelles: Effects of Drug Hydrophobicity, Solution Temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nie, S.; Hsiao, W.L.W.; Pam, W. Thermoreversible Pluronic® F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 2011, 6, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A Review of Poloxamer 407 Pharmaceutical and Pharmacological Characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 Hydrogel Characterization and Biofabrication in Cellularized Constructs for Tissue Engineering Applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Jung, Y.-S.; Park, W.; Park, H.; Lee, D.-K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and Pluronic F-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef]
- Yeh, M.-Y.; Zhao, J.-Y.; Hsieh, Y.-R.; Lin, J.-H.; Chen, F.-Y.; Chakravarthy, R.D.; Chung, P.-C.; Lin, H.-C.; Hung, S.-C. Reverse thermo-responsive hydrogels prepared from Pluronic F127 and gelatin composite materials. RSC Adv. 2017, 7, 21252–21257. [Google Scholar] [CrossRef]
- Yap, L.-S.; Yang, M.-C. Evaluation of hydrogel composing of Pluronic F127 and carboxymethyl hexanoyl chitosan as injectable scaffold for tissue engineering applications. Colloids Surf. B Biointerfaces 2016, 146, 204–211. [Google Scholar] [CrossRef]
- Appuhamillage, G.A.; Berry, D.R.; Benjamin, C.E.; Luzuriaga, M.A.; Reagan, J.C.; Gassensmith, J.J.; Smaldone, R.A. A biopolymer-based 3D printable hydrogel for toxic metal adsorption from water. Polym. Int. 2019, 68, 964–971. [Google Scholar] [CrossRef]
- Choi, J.H.; Choi, O.K.; Lee, J.; Noh, J.; Lee, S.; Park, A.; Rim, M.A.; Reis, R.L.; Khang, G. Evaluation of double network hydrogel of poloxamer-heparin/gellan gum for bone marrow stem cells delivery carrier. Colloids Surf. B Biointerfaces 2019, 181, 879–889. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Chen, J.-P.; Leu, Y.-L.; Wang, H.-Y. Characterization and Evaluation of Silk Protein Hydrogels for Drug Delivery. Chem. Pharm. Bull. 2006, 54, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.D.; Nahm, J.H.; Park, J.S.; Moon, J.Y.; Cho, C.S.; Yeo, J.H. Effects of poloxamer on the gelation of silk fibroin. Macromol. Rapid Commun. 2000, 21, 788–791. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Self-assembled silk sericin/poloxamer nanoparticles as nanocarriers of hydrophobic and hydrophilic drugs for targeted delivery. Nanotechnology 2009, 20, 355101. [Google Scholar] [CrossRef]
- Lee, O.J.; Lee, J.M.; Kim, J.H.; Kim, J.; Kweon, H.; Jo, Y.Y.; Park, C.H. Biodegradation behavior of silk fibroin membranes in repairing tympanic membrane perforations. J. Biomed. Mater. Res. Part A 2012, 100, 2018–2026. [Google Scholar] [CrossRef]
- Park, W.H.; Kim, M.H. Chemically cross-linked silk fibroin hydrogel with enhanced elastic properties, biodegradability, and biocompatibility. Int. J. Nanomed. 2016, 11, 2967–2978. [Google Scholar] [CrossRef]
- Chouhan, D.; Lohe, T.-U.; Samudrala, P.K.; Mandal, B.B. In Situ Forming Injectable Silk Fibroin Hydrogel Promotes Skin Regeneration in Full Thickness Burn Wounds. Adv. Heal. Mater. 2018, 7, e1801092. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Silva-Correia, J.; Gonçalves, C.; Pina, S.; Radhouani, H.; Montonen, T.; Hyttinen, J.; Roy, A.; Oliveira, A.L.; Reis, R.L.; et al. Rapidly responsive silk fibroin hydrogels as an artificial matrix for the programmed tumor cells death. PLoS ONE 2018, 13, e0194441. [Google Scholar] [CrossRef]
- Mauney, J.R.; Nguyen, T.; Gillen, K.; Kirker-Head, C.; Gimble, J.M.; Kaplan, D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials 2007, 28, 5280–5290. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Samal, S.K.; Kaplan, D.L.; Chiellini, E. Ultrasound Sonication Effects on Silk Fibroin Protein. Macromol. Mater. Eng. 2013, 298, 1201–1208. [Google Scholar] [CrossRef]
- Lohrasbi, S.; Mirzaei, E.; Karimizade, A.; Takallu, S.; Rezaei, A. Collagen/cellulose nanofiber hydrogel scaffold: Physical, mechanical and cell biocompatibility properties. Cellulose 2019, 27, 927–940. [Google Scholar] [CrossRef]
- Chen, M.H.; Wang, L.L.; Chung, J.J.; Kim, Y.-H.; Atluri, P.; Burdick, J.A. Methods to Assess Shear-Thinning Hydrogels for Application as Injectable Biomaterials. ACS Biomater. Sci. Eng. 2017, 3, 3146–3160. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Borges, A.C.; de Oliveira, M.G.; de Farias, M.A. Visualization of supramolecular structure of Pluronic F127 micellar hydrogels using cryo-TEM. MethodsX 2020, 7, 101084. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nat. Cell Biol. 1997, 388, 860–862. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Thomas, M.; Ganaie, M.A.; Sheikh, M.U.D.; Bano, M.; Hassan, I.U.; Khan, F. Hierarchically macroporous silver monoliths using Pluronic F127: Facile synthesis, characterization and its application as an efficient biomaterial for pathogens. J. Saudi Chem. Soc. 2016, 20, 237–244. [Google Scholar] [CrossRef]
- Chakravarty, S.; Bhardwaj, N.; Mandal, B.B.; Sarma, N.S. Silk fibroin–carbon nanoparticle composite scaffolds: A cost effective supramolecular ‘turn off’ chemiresistor for nitroaromatic explosive vapours. J. Mater. Chem. C 2016, 4, 8920–8929. [Google Scholar] [CrossRef]
- Singla, P.; Chabba, S.; Mahajan, R.K. A systematic physicochemical investigation on solubilization and in vitro release of poorly water soluble oxcarbazepine drug in pluronic micelles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 479–488. [Google Scholar] [CrossRef]
- Wang, W.; Song, H.; Zhang, J.; Li, P.; Li, C.; Wang, C.; Kong, D.; Zhao, Q. An injectable, thermosensitive and multicompartment hydrogel for simultaneous encapsulation and independent release of a drug cocktail as an effective combination therapy platform. J. Control. Release 2015, 203, 57–66. [Google Scholar] [CrossRef]
- Vannozzi, L.; Ricotti, L.; Filippeschi, C.; Sartini, S.; Coviello, V.; Piazza, V.; Pingue, P.; la Motta, C.; Dario, P.; Menciassi, A. Nanostructured ultra-thin patches for ultrasound-modulated delivery of anti-restenotic drug. Int. J. Nanomed. 2015, 11, 69–92. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Vannozzi, L.; Yasa, I.C.; Ceylan, H.; Menciassi, A.; Ricotti, L.; Sitti, M. Self-Folded Hydrogel Tubes for Implantable Muscular Tissue Scaffolds. Macromol. Biosci. 2018, 18, e1700377. [Google Scholar] [CrossRef] [PubMed]
- Srisuwan, Y.; Srihanam, P.; Baimark, Y. Preparation of Silk Fibroin Microspheres and Its Application to Protein Adsorption. J. Macromol. Sci. Part A 2009, 46, 521–525. [Google Scholar] [CrossRef]
- Khattak, S.F.; Bhatia, S.R.; Roberts, S.C. Pluronic F127 as a Cell Encapsulation Material: Utilization of Membrane-Stabilizing Agents. Tissue Eng. 2005, 11, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Singh-Joy, S.D.; McLain, V.C. Safety Assessment of Poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, Poloxamer 105 Benzoate, and Poloxamer 182 Dibenzoate as Used in Cosmetics. Int. J. Toxicol. 2008, 27, 93–128. [Google Scholar] [CrossRef]
- Azadi, M.; Teimouri, A.; Mehranzadeh, G. Preparation, characterization and biocompatible properties of β-chitin/silk fibroin/nanohydroxyapatite composite scaffolds prepared using a freeze-drying method. RSC Adv. 2015, 6, 7048–7060. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youn, J.; Choi, J.H.; Lee, S.; Lee, S.W.; Moon, B.K.; Song, J.E.; Khang, G. Pluronic F-127/Silk Fibroin for Enhanced Mechanical Property and Sustained Release Drug for Tissue Engineering Biomaterial. Materials 2021, 14, 1287. https://doi.org/10.3390/ma14051287
Youn J, Choi JH, Lee S, Lee SW, Moon BK, Song JE, Khang G. Pluronic F-127/Silk Fibroin for Enhanced Mechanical Property and Sustained Release Drug for Tissue Engineering Biomaterial. Materials. 2021; 14(5):1287. https://doi.org/10.3390/ma14051287
Chicago/Turabian StyleYoun, Jina, Joo Hee Choi, Sumi Lee, Seong Won Lee, Byung Kwan Moon, Jeong Eun Song, and Gilson Khang. 2021. "Pluronic F-127/Silk Fibroin for Enhanced Mechanical Property and Sustained Release Drug for Tissue Engineering Biomaterial" Materials 14, no. 5: 1287. https://doi.org/10.3390/ma14051287
APA StyleYoun, J., Choi, J. H., Lee, S., Lee, S. W., Moon, B. K., Song, J. E., & Khang, G. (2021). Pluronic F-127/Silk Fibroin for Enhanced Mechanical Property and Sustained Release Drug for Tissue Engineering Biomaterial. Materials, 14(5), 1287. https://doi.org/10.3390/ma14051287





