Convenient Synthesis of Functionalized Unsymmetrical Vinyl Disulfides and Their Inverse Electron-Demand Hetero-Diels-Alder Reaction †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 5,5-Dimethyl-2-thioxo-2-vinylsulfanyl-[1,3,2]dioxaphosphorinane
2.2. A Typical Procedure for the Preparation of Vinyl Disulfides 2 from S-vinyl Thiotosylate and Representative Analytical Data
2.3. A Typical Procedure for the Preparation of benzo[b][1,4]thiazine disulfanyl derivatives 7 and Representative Analytical Data
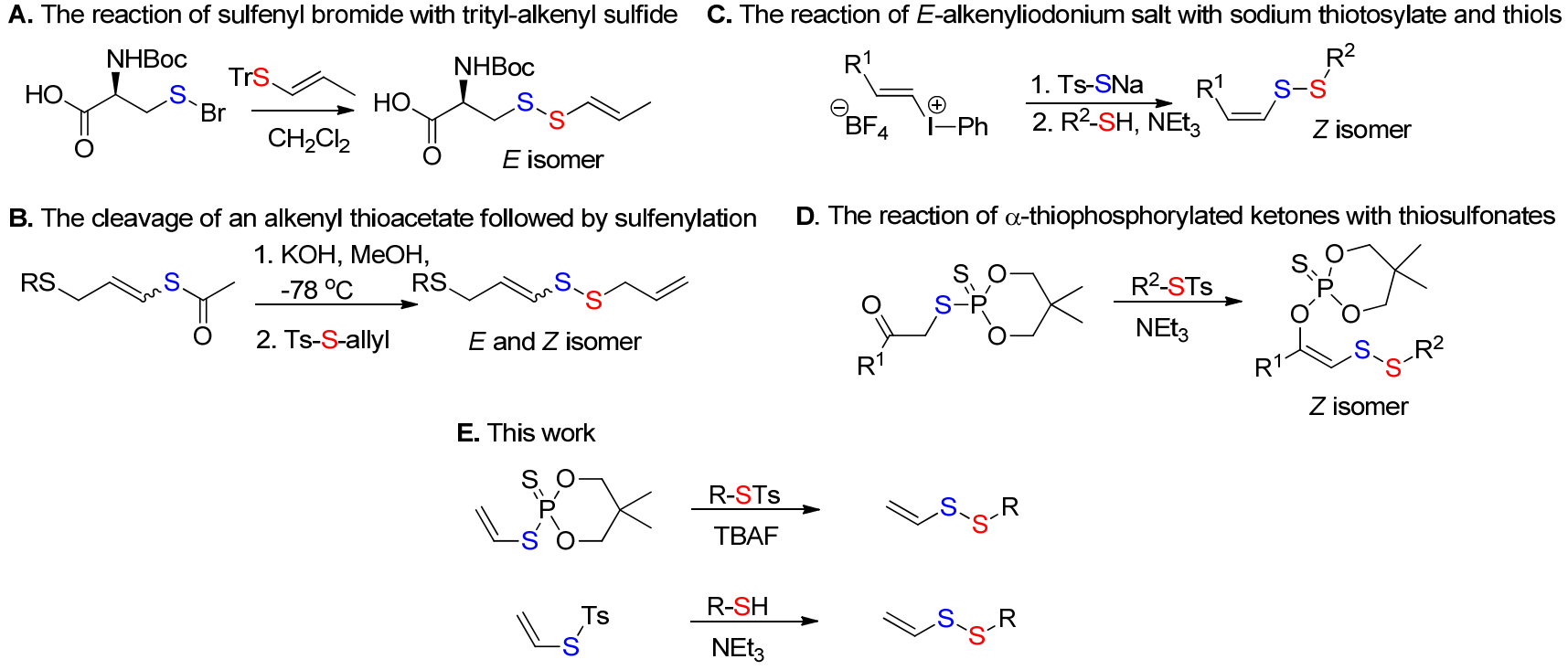
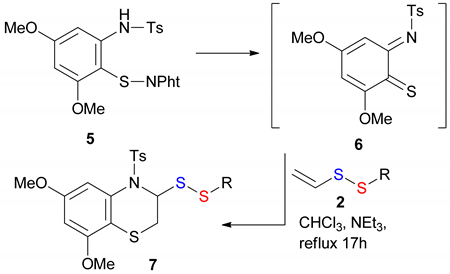
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kondo, K.; Mitsudo, T. Metal-Catalyzed Carbon−Sulfur Bond Formation. Chem. Rev. 2000, 100, 3205–3220. [Google Scholar] [CrossRef]
- Metzner, P.; Thuillier, A. Sulfur Reagents in Organic Synthesis; Elsevier BV: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Lee, M.H.; Yang, Z.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-Cleavage-Triggered Chemosensors and Their Biological Applications. Chem. Rev. 2013, 113, 5071–5109. [Google Scholar] [CrossRef]
- Ulman, A. Formation and Structure of Self-Assembled Monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Witt, D.; Klajn, R.; Barski, P.; Grzybowski, B. Applications, Properties and Synthesis of ω-Functionalized n-Alkanethiols and Disulfides—The Building Blocks of Self-Assembled Monolayers. Curr. Org. Chem. 2004, 8, 1763–1797. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Houseman, B.T.; Gawalt, E.S.; Mrksich, M. Maleimide-Functionalized Self-Assembled Monolayers for the Preparation of Peptide and Carbohydrate Biochips. Langmuir 2003, 19, 1522–1531. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Boozer, C.L.; Jiang, S. Controlled Chemical and Structural Properties of Mixed Self-Assembled Monolayers by Coadsorption of Symmetric and Asymmetric Disulfides on Au(111). J. Phys. Chem. B 2001, 105, 2975–2980. [Google Scholar] [CrossRef]
- Shon, Y.S.; Mazzitelli, C.; Murray, R.W. Unsymmetrical Disulfides and Thiol Mixtures Produce Different Mixed Monolay-er-Protected Gold Clusters. Langmuir 2001, 17, 7735–7741. [Google Scholar] [CrossRef]
- O’Brien, J.C.; Stickney, J.T.; Porter, M.D. Preparation and Characterization of Self-Assembled Double-Stranded DNA (dsDNA) Microarrays for Protein:dsDNA Screening Using Atomic Force Microscopy. Langmuir 2000, 16, 9559–9567. [Google Scholar] [CrossRef]
- Higashi, N.; Takahashi, M.; Niwa, M. Immobilization of DNA through Intercalation at Self-Assembled Monolayers on Gold. Langmuir 1999, 15, 111–115. [Google Scholar] [CrossRef]
- Kalsin, A.M.; Fialkowski, M.; Paszewski, M.; Smoukov, S.K.; Bishop, K.J.M.; Grzybowski, B.A. Electrostatic Self-Assembly of Binary Nanoparticle Crystals with a Diamond-Like Lattice. Science 2006, 312, 420–424. [Google Scholar] [CrossRef] [Green Version]
- Kalsin, A.K.; Smoukov, S.K.; Kowalczyk, B.; Klajn, R.; Grzybowski, B.A. Ionic-like Behavior of Oppositely Charged Na-noparticles. J. Am. Chem. Soc. 2006, 128, 15046–15047. [Google Scholar] [CrossRef]
- Cremlyn, R.; An, J. Introduction to Organosulfur Chemistry; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Oae, S. Organic Sulfur Chemistry: Structure and Mechanism; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Vrudhula, V.M.; MacMaster, J.F.; Li, Z.; Kerr, D.E.; Senter, P.D. Reductively activated disulfide prodrugs of paclitaxel. Bioorg. Med. Chem. Lett. 2002, 12, 3591–3594. [Google Scholar] [CrossRef]
- Mu, Y.; Nodwell, M.; Pace, J.L.; Shaw, J.-P.; Judice, J. Vancomycin disulfide derivatives as antibacterial agents. Bioorg. Med. Chem. Lett. 2004, 14, 735–738. [Google Scholar] [CrossRef]
- Shcherbakova, I.; Pozharskii, A.F. Comprehensive Organic Functional Group Transformations II; Katritzky, A.R., Taylor, R., Ramsden, C., Eds.; Pergamon: Oxford, UK, 2004; Volume 2, pp. 177–187. [Google Scholar]
- Sato, R.; Kimura, T. Science of Synthesis; Kambe, N., Drabowicz, J., Molander, G.A., Eds.; Thieme: Stuttgart, Germany; New York, NY, USA, 2007; Volume 39, pp. 573–588. [Google Scholar]
- Witt, D. Recent Developments in Disulfide Bond Formation. Synthesis 2008, 2491–2509. [Google Scholar] [CrossRef]
- Mandal, B.; Basu, B. Recent advances in S–S bond formation. RSC Adv. 2014, 4, 13854–13881. [Google Scholar] [CrossRef]
- Musiejuk, M.; Witt, D. Recent Developments in the Synthesis of Unsymmetrical Disulfanes (Disulfides). A Review. Org. Prep. Proced. Int. 2015, 47, 95–131. [Google Scholar] [CrossRef]
- Harpp, D.N.; Friedlander, B.T.; Larsen, C.; Steliou, K.; Stockton, A. Organic sulfur chemistry. 29. Use of the trimethylsilyl group in synthesis. Preparation of sulfinate esters and unsymmetrical disulfides. J. Org. Chem. 1978, 43, 3481–3485. [Google Scholar] [CrossRef]
- Brown, C.; Evans, G.R. The “thio-Arbuzov” reaction of sulfenate esters with sulfenyl chlorides: Fate of the thiosulfinate product. Tetrahedron Lett. 1996, 37, 9101–9104. [Google Scholar] [CrossRef]
- Swan, J.M. Thiols, Disulphides and Thiosulphates: Some New Reactions and Possibilities in Peptide and Protein Chemistry. Nat. Cell Biol. 1957, 180, 643–645. [Google Scholar] [CrossRef]
- Hiver, P.; Dicko, A.; Paquer, D. Medium effects in unsymmetrical disulfides compounds synthesis from bunte salts. Tetrahedron Lett. 1994, 35, 9569–9572. [Google Scholar] [CrossRef]
- Sirakawa, K.; Aki, O.; Tsujikawa, T.; Tsuda, T. S-Alkylthioisothioureas. I. Chem. Pharm. Bull. 1970, 18, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Ternay, A.L.; Cook, C.; Brzezinska, E. The Synthesis of Unsymmetric and Symmetric Disulfides. Phosphorus Sulfur Silicon Relat. Elem. 1994, 95, 351–352. [Google Scholar] [CrossRef]
- Ternay, A.L.; Brzezinska, E. Disulfides. 1. Syntheses Using 2,2′-Dithiobis(benzothiazole). J. Org. Chem. 1994, 59, 8239–8244. [Google Scholar]
- Hunter, R.; Caira, M.; Stellenboom, N. Inexpensive, One-Pot Synthesis of Unsymmetrical Disulfides Using 1-Chlorobenzotriazole. J. Org. Chem. 2006, 71, 8268–8271. [Google Scholar] [CrossRef] [PubMed]
- Leriverend, C.; Metzner, P. A New Mild Synthesis of Unsymmetrical Disulfides by Reaction of Dithioperoxyesters with Thiols. Synthesis 1994, 1994, 761–762. [Google Scholar] [CrossRef]
- Dai, Z.; Xiao, X.; Jiang, X. Nucleophilic disulfurating reagents for unsymmetrical disulfides construction via copper-catalyzed oxidative cross coupling. Tetrahedron 2017, 73, 3702–3706. [Google Scholar] [CrossRef]
- Dubs, P.; Stuessi, R. Eine neue Methode zur Herstellung gemischter Disulfide. Vorläufige Mitteilung. Helv. Chim. Acta 1976, 59, 1307–1311. [Google Scholar] [CrossRef]
- Barton, D.H.; Chen, C.; Wall, G.M. Synthesis of disulfides via sulfenylation of alkyl and aryldithiopyridine n-oxides. Tetrahedron 1991, 47, 6127–6138. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Hesse, R.H.; O’Sullivan, A.C.; Pechet, M.M. A new procedure for the conversion of thiols into reactive sulfenylating agents. J. Org. Chem. 1991, 56, 6697–6702. [Google Scholar] [CrossRef]
- Ohtani, M.; Narisada, N. Sulfur-sulfur bond formation reaction using bis(1-methyl-1H-tetrazol-5-yl) disulphide. J. Org. Chem. 1991, 56, 5475–5478. [Google Scholar] [CrossRef]
- Bao, M.; Shimizu, M. N -Trifluoroacetyl arenesulfenamides, effective precursors for synthesis of unsymmetrical disulfides and sulfenamides. Tetrahedron 2003, 59, 9655–9659. [Google Scholar] [CrossRef]
- Blaschette, A.; Naveke, M. Polysulfonylamides. Part 25. N-Sulfenyldimesylamines and (1-Sulfenyl-4- dimethylamino-pyridinium) Dimesylaminides. Synthesis of New Compounds and Application as Sulfenylation Reagents. Chem. Ztg. 1991, 115, 61–64. [Google Scholar]
- Hiskey, R.G.; Ward, B.F. Sulfur-containing polypeptides. XII. Scope and limitations of the sulfenylthiocyanate method as a route to cystine peptides. J. Org. Chem. 1970, 35, 1118–1121. [Google Scholar] [CrossRef]
- Benati, L.; Montevecchi, P.C.; Spagnolo, P. 4′-Nitroarenesulphenanilides: Their use in the synthesis of unsymmetrical di-sulphides. Tetrahedron Lett. 1986, 27, 1739–1742. [Google Scholar] [CrossRef]
- Armitage, D.A.; Clark, M.J.; Tso, C.C. Synthesis of unsymmetrical disulphides. J. Chem. Soc. Perkin Trans. 1 1972, 1, 680–683. [Google Scholar] [CrossRef]
- Capozzi, G.; Capperucci, A.; Degl’Innocenti, A.; Del Duce, R.; Menichetti, S. Silicon in organosulphur chemistry. Part 2. Synthesis of unsymmetrical disulphides. Tetrahedron Lett. 1989, 30, 2995–2998. [Google Scholar] [CrossRef]
- Rajca, A.; Wiessler, M. Synthesis of unsymmetrical disulfides with thiolsulfonates immobilised on a polystyrene support. Tetrahedron Lett. 1990, 31, 6075–6076. [Google Scholar] [CrossRef]
- Koval, I.V. Imination of Sulfur-containing Compounds: XXXV. New Preparation Method and Oxidative Benzenesul-fonylimination of Unsymmetrical Disulfides. Russ. J. Org. Chem. 2002, 38, 232–234. [Google Scholar] [CrossRef]
- Oae, S.; Kim, Y.H.; Fukushima, D.; Shinhama, K. New syntheses of thionitrites and their chemical reactivites. J. Chem. Soc. Perkin Trans. 1 1978, 1, 913–917. [Google Scholar] [CrossRef]
- Brois, S.J.; Pilot, J.F.; Barnum, H.W. New synthetic concepts in organosulfur chemistry. I. New pathway to unsymmetrical disulfides. The thiol-induced fragmentation of sulfenyl thiocarbonates. J. Am. Chem. Soc. 1970, 92, 7629–7631. [Google Scholar] [CrossRef]
- Boustang, K.S.; Sullivan, A.B. Chemistry of sulfur compounds-VI. A novel method for the preparation of disulfides. Tetrahedron Lett. 1970, 11, 3547–3549. [Google Scholar] [CrossRef]
- Harpp, D.N.; Ash, D.K.; Back, T.G.; Gleason, J.G.; Orwig, B.A.; VanHorn, W.F.; Snyder, J.P. A new synthesis of unsymmetrical disulfides. Tetrahedron Lett. 1970, 11, 3551–3554. [Google Scholar] [CrossRef]
- Klose, J.; Reese, C.B.; Song, Q. Preparation of 2-(2-cyanoethyl)sulfanyl-1H-isoindole-1,3-(2H)-dione and related sulfur-transfer agents. Tetrahedron 1997, 53, 14411–14416. [Google Scholar] [CrossRef]
- Masui, M.; Mizuki, Y.; Sakai, K.; Ueda, C.; Ohmori, H. The reaction of Ph3P+SR with thiols: A simple, efficient synthesis of unsymmetrical disulphides. J. Chem. Soc. Chem. Commun. 1984, 843–844. [Google Scholar] [CrossRef]
- Graber, D.R.; Morge, R.A.; Sih, J.C. Reaction of 2-(alkylsulfinyl)-, 2-(arylsulfinyl)-, and 2-(aralkylsulfinyl)benzimidazoles with thiols: A convenient synthesis of unsymmetrical disulfides. J. Org. Chem. 1987, 52, 4620–4622. [Google Scholar] [CrossRef]
- Arisawa, M.; Yamaguchi, M. Rhodium-Catalyzed Disulfide Exchange Reaction. J. Am. Chem. Soc. 2003, 125, 6624–6625. [Google Scholar] [CrossRef]
- Tanaka, K.; Ajiki, K. Phosphine-free cationic rhodium(I) complex-catalyzed disulfide exchange reaction: Convenient synthesis of unsymmetrical disulfides. Tetrahedron Lett. 2004, 45, 5677–5679. [Google Scholar] [CrossRef]
- Do, Q.T.; Elothmani, D.; Le Guillanton, G.; Simonet, J. A new electrochemical method of preparation of unsymmetrical di-sulfides. Tetrahedron Lett. 1997, 38, 3383–3384. [Google Scholar] [CrossRef]
- Sureshkumar, D.; Ganesh, V.; Vidyarini, R.S.; Chandrasekaran, S. Direct Synthesis of Functionalized Unsymmetrical β-Sulfonamido Disulfides by Tetrathiomolybdate Mediated Aziridine Ring-Opening Reactions. J. Org. Chem. 2009, 74, 7958–7961. [Google Scholar] [CrossRef]
- Sureshkumar, D.; Koutha, S.M.; Chandrasekaran, S. Chemistry of Tetrathiomolybdate: Aziridine Ring Opening Reactions and Facile Synthesis of Interesting Sulfur Heterocycles. J. Am. Chem. Soc. 2005, 127, 12760–12761. [Google Scholar] [CrossRef] [PubMed]
- Mukaiyama, T.; Takahashi, K. A convenient method for the preparation of unsymmetrical disulfides by the use of diethyl azodicarboxylate. Tetrahedron Lett. 1968, 9, 5907–5908. [Google Scholar] [CrossRef]
- Galande, A.K.; Spatola, A.F. Solid-Phase Synthesis of Disulfide Heterodimers of Peptides. Org. Lett. 2003, 5, 3431–3434. [Google Scholar] [CrossRef] [PubMed]
- Vandavasi, J.K.; Hu, W.-P.; Chen, C.-Y.; Wang, J.-J. Efficient synthesis of unsymmetrical disulfides. Tetrahedron 2011, 67, 8895–8901. [Google Scholar] [CrossRef]
- Smith, R.; Zeng, X.; Müller-Bunz, H.; Zhu, X. Synthesis of glycosyl disulfides containing an α-glycosidic linkage. Tetrahedron Lett. 2013, 54, 5348–5350. [Google Scholar] [CrossRef]
- Musiejuk, M.; Klucznik, T.; Rachon, J.; Witt, D. DDQ-mediated synthesis of functionalized unsymmetrical disulfanes. RSC Adv. 2015, 5, 31347–31351. [Google Scholar] [CrossRef]
- Dethe, D.H.; Srivastava, A.; Dherange, B.D.; Kumar, B.V. Unsymmetrical Disulfide Synthesis through Photoredox Catalysis. Adv. Synth. Catal. 2018, 360, 3020–3025. [Google Scholar] [CrossRef]
- Lach, S.; Demkowicz, S.; Witt, D. An efficient and convenient synthesis of unsymmetrical disulfides from thioacetates. Tetrahedron Lett. 2013, 54, 7021–7023. [Google Scholar] [CrossRef]
- Antoniow, S.; Witt, D. A Novel and Efficient Synthesis of Unsymmetrical Disulfides. Synthesis 2007, 3, 363–366. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Barski, P.; Witt, D.; Grzybowski, B.A. Versatile and Efficient Synthesis of ω-Functionalized Asymmetric Di-sulfides via Sulfenyl Bromide Adducts. Langmuir 2007, 23, 2318–2321. [Google Scholar] [CrossRef]
- Szymelfejnik, M.; Demkowicz, S.; Rachon, J.; Witt, D. Functionalization of Cysteine Derivatives by Unsymmetrical Disulfide Bond Formation. Synthesis 2007, 22, 3528–3534. [Google Scholar]
- Demkowicz, S.; Rachon, J.; Witt, D. A Versatile and Convenient Preparation of Unsymmetrical Diaryl Disulfides. Synthesis 2008, 13, 2033–2038. [Google Scholar]
- Witt, D.; Okragla, E.; Demkowicz, S.; Rachón, J. A Convenient and Efficient α-Sulfenylation of Carbonyl Compounds. Synthesis 2009, 2009, 1720–1724. [Google Scholar] [CrossRef]
- Lach, S.; Witt, D. A New and Convenient Method for the Preparation of Functionalized Phosphorothioates. Synthesis 2011, 2011, 3975–3978. [Google Scholar] [CrossRef]
- Doroszuk, J.; Musiejuk, M.; Demkowicz, S.; Rachon, J.; Witt, D. Convenient and efficient synthesis of functionalized un-symmetrical alkynyl sulfides, RSC Adv. 2016, 6, 105449–105453. RSC Adv 2016, 6, 105449–105453. [Google Scholar] [CrossRef]
- Doroszuk, J.; Musiejuk, M.; Ponikiewski, Ł.; Witt, D. Convenient and Efficient Diastereoselective Preparation of Function-alized Z-Alkenyl Sulfides. Eur. J. Org. Chem. 2018, 45, 6333–6337. [Google Scholar] [CrossRef]
- Kertmen, A.; Lach, S.; Rachon, J.; Witt, D. Novel and Efficient Methods for the Synthesis of Symmetrical Trisulfides. Synthesis 2009, 9, 1459–1462. [Google Scholar]
- Lach, S.; Witt, D. TBAF Promoted Formation of Symmetrical Trisulfides. Heteroat. Chem. 2013, 25, 10–14. [Google Scholar] [CrossRef]
- Lach, S.; Sliwka-Kaszynska, M.; Witt, D. Novel and Efficient Synthesis of Unsymmetrical Trisulfides. Synlett 2010, 19, 2857–2860. [Google Scholar]
- Witt, D.; Lach, S. Efficient Synthesis of Functionalized Unsymmetrical Dialkyl Trisulfanes. Synlett 2013, 24, 1927–1930. [Google Scholar] [CrossRef]
- Block, E.; Ahmad, S.; Jain, M.K.; Crecely, R.W.; Apitz-Castro, R.; Cruz, M.R. The chemistry of alkyl thiosulfate esters. 8. (E,Z)-Ajoene: A potent antithrombotic agent from garlic. J. Am. Chem. Soc. 1984, 106, 8295–8296. [Google Scholar] [CrossRef]
- Block, E.; Ahmad, S.; Catalfamo, J.L.; Jain, M.K.; Apitz-Castro, R. The chemistry of alkyl thiosulfinate esters. 9. Antithrombotic organosulfur compounds from garlic: Structural, mechanistic, and synthetic studies. J. Am. Chem. Soc. 1986, 108, 7045–7055. [Google Scholar] [CrossRef]
- Li, M.; Ciu, J.-R.; Ye, Y.; Min, J.-M.; Zhang, L.-H.; Wang, K.; Gares, M.; Cros, J.; Wright, M.; Leung-Tack, J. Antitumor activity of Z-ajoene, a natural compound purified from garlic: Antimitotic and microtubule-interaction properties. Carcinog 2002, 23, 573–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Min, J.-M.; Cui, J.-R.; Zhang, L.-H.; Wang, K.; Valette, A.; Davrinche, C.; Wright, M.; Leung-Tack, J. Z-Ajoene Induces Apoptosis of HL-60 Cells: Involvement of Bcl-2 Cleavage. Nutr. Cancer 2002, 42, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Parkin, K.L. S-Alk(en)ylmercaptocysteine: Chemical Synthesis, Biological Activities, and Redox-Related Mechanism. J. Agric. Food Chem. 2013, 61, 1896–1903. [Google Scholar] [CrossRef]
- Hunter, R.; Kaschula, C.H.; Parker, I.M.; Caira, M.R.; Richards, P.; Travis, S.; Taute, F.; Qwebani, T. Substituted ajoenes as novel anti-cancer agents. Bioorg. Med. Chem. Lett. 2008, 18, 5277–5279. [Google Scholar] [CrossRef] [PubMed]
- Kaschula, C.H.; Hunter, R.; Stellenboom, N.; Caira, M.R.; Winks, S.; Ogunleye, T.; Richards, P.; Cotton, J.; Zilbeyaz, K.; Wang, Y.; et al. Structure–activity studies on the anti-proliferation activity of ajoene analogues in WHCO1 oesophageal cancer cells. Eur. J. Med. Chem. 2012, 50, 236–254. [Google Scholar] [CrossRef]
- Silva, F.; Khokhar, S.S.; Williams, D.M.; Saunders, R.; Evans, G.J.S.; Graz, M.; Wirth, T. Short Total Synthesis of Ajoene. Angew. Chem. Int. Ed. 2018, 57, 12290–12293. [Google Scholar] [CrossRef]
- Musiejuk, M.; Doroszuk, J.; Witt, D. Convenient and efficient synthesis of functionalized unsymmetrical Z-alkenyl disulfanes. RSC Adv. 2018, 8, 9718–9722. [Google Scholar] [CrossRef] [Green Version]
- Musiejuk, M.; Doroszuk, J.; Jędrzejewski, B.; Nieto, G.O.; Navarro, M.M.; Witt, D. Diastereoselective Synthesis of Z-Alkenyl Disulfides from α-Thiophosphorylated Ketones and Thiosulfonates. Adv. Synth. Catal. 2020, 362, 618–626. [Google Scholar] [CrossRef]
- Rathore, B.S.; Kumar, M. Synthesis of 7-chloro-5-trifluoromethyl/7-fluoro/7-trifluoromethyl-4H-1,4-benzothiazines as antimicrobial agents. Bioorg. Med. Chem. 2006, 14, 5678–5682. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, G.-F. Microwave-assisted, one-pot syntheses and fungicidal activity of polyfluorinated 2-benzylthiobenzothiazoles. Bioorg. Med. Chem. 2006, 14, 8280–8285. [Google Scholar] [CrossRef] [PubMed]
- Viglianisi, C.; Bonaccorsi, P.M.; Simone, L.; Nassini, L.; Menichetti, S. Copper-Mediated One-Pot Access to Benzo[b][1,4]thiazines from 2-N-Sulfonylaminoaryl Disulfides. Eur. J. Org. Chem. 2012, 2012, 1707–1711. [Google Scholar] [CrossRef]

| Entry 1 | R | Yield (%) 2 | Yield (%) 2 |
|---|---|---|---|
| 1 | –n–C12H25 1a | 93 2a | - |
| 2 | –(CH2)9CH=CH2 1b | 82 2b | - |
| 3 | –(CH2)10COOMe 1c | 73 2c | - |
| 4 | –(CH2)11OMe 1d | 62 2d | - |
| 5 | –(CH2)11SAc 1e | 85 2e | - |
| 6 | –(CH2)2NHBoc 1f | 75 2f | - |
| 7 | –(CH2)2C6H4–4–CH3 1g | 76 2g | - |
| 8 | –(CH2)2–3–indyl 1h | 75 2h | - |
| 9 | –(CH2)2C6H4–4–CF3 1i | 65 2i | - |
| 10 | –(CH2)2C6H4–4–F 1j | - | 100 3j |
| 11 | –C6H4–4–CH3 1k | - | 100 3k |
| 12 | –CH2–2–naphthyl 1l | - | 80 3l |
| 13 | –CH2C6H4–4–NO2 1m | - | 70 3m |
| 14 | –CH2C6H4–4–OMe 1n | - | 85 3n |
| 15 | –CH2C6H4–4–CN 1o | - | 75 3o |
| 16 | –(CH2)2C6H4–4–OMe 1p | - | 86 3p |
| 17 | –CH2Ph 1r | - | 76 3r |

| Entry 1 | R 1 | Yield (%) 2 |
|---|---|---|
| 1 | –n–C12H25 4a | 97 2a |
| 2 | –(CH2)10COOMe 4c | 88 2c |
| 3 | –(CH2)2C6H4–4–F 4j | 90 2j |
| 4 | –C6H4–4–CH3 4k | 96 2k |
| 5 | –CH2–2–naphthyl 4l | 92 2l |
| 6 | –CH2C6H4–4–NO2 4m | 80 2m |
| 7 | –CH2C6H4–4–OMe 4n | 87 2n |
| 8 | –CH2C6H4–4–CN 4o | 89 2o |
| 9 | –CH2Ph 4r | 98 2r |
| 10 | –(CH2)10COOH 4s | 84 2s |
| 11 | –(CH2)11OH 4t | 91 2t |
| Entry 1 | R | Yield (%) 2 | Recovered 2 (%) 2 |
|---|---|---|---|
| 1 | –n–C12H25 2a | 50 7a | 35 2a |
| 2 | –(CH2)10COOMe 2c | 30 7c | 42 2c |
| 3 | –CH2C6H4–4–NO2 2m | 29 7m | 46 2m |
| 4 | –CH2C6H4–4–OMe 2n | 27 7n | 44 2n |
| 5 | –CH2Ph 2r | 25 7r | 52 2r |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jędrzejewski, B.; Musiejuk, M.; Doroszuk, J.; Witt, D. Convenient Synthesis of Functionalized Unsymmetrical Vinyl Disulfides and Their Inverse Electron-Demand Hetero-Diels-Alder Reaction. Materials 2021, 14, 1342. https://doi.org/10.3390/ma14061342
Jędrzejewski B, Musiejuk M, Doroszuk J, Witt D. Convenient Synthesis of Functionalized Unsymmetrical Vinyl Disulfides and Their Inverse Electron-Demand Hetero-Diels-Alder Reaction. Materials. 2021; 14(6):1342. https://doi.org/10.3390/ma14061342
Chicago/Turabian StyleJędrzejewski, Bartosz, Mateusz Musiejuk, Justyna Doroszuk, and Dariusz Witt. 2021. "Convenient Synthesis of Functionalized Unsymmetrical Vinyl Disulfides and Their Inverse Electron-Demand Hetero-Diels-Alder Reaction" Materials 14, no. 6: 1342. https://doi.org/10.3390/ma14061342







