Diffusion of Shape Stabilized PEG-SiO2 as a Driver for Producing Thermoregulating Facing Bricks
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Bricks and Porosity
2.3. PEG-SiO2 Synthesis
2.4. Form-Stable PCM Synthesis
2.5. Characterization
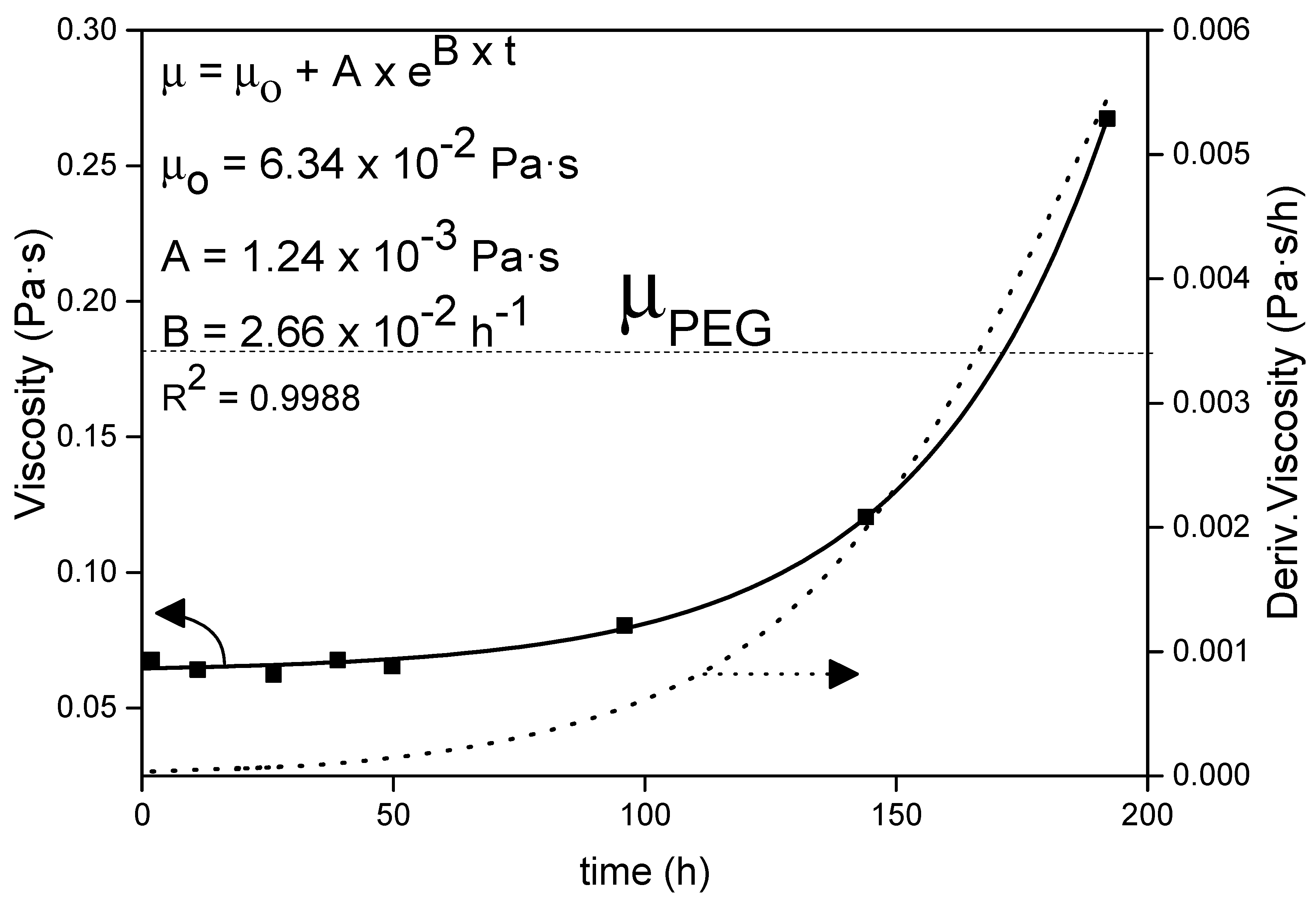
2.5.1. Sol Viscosity (Rheology)
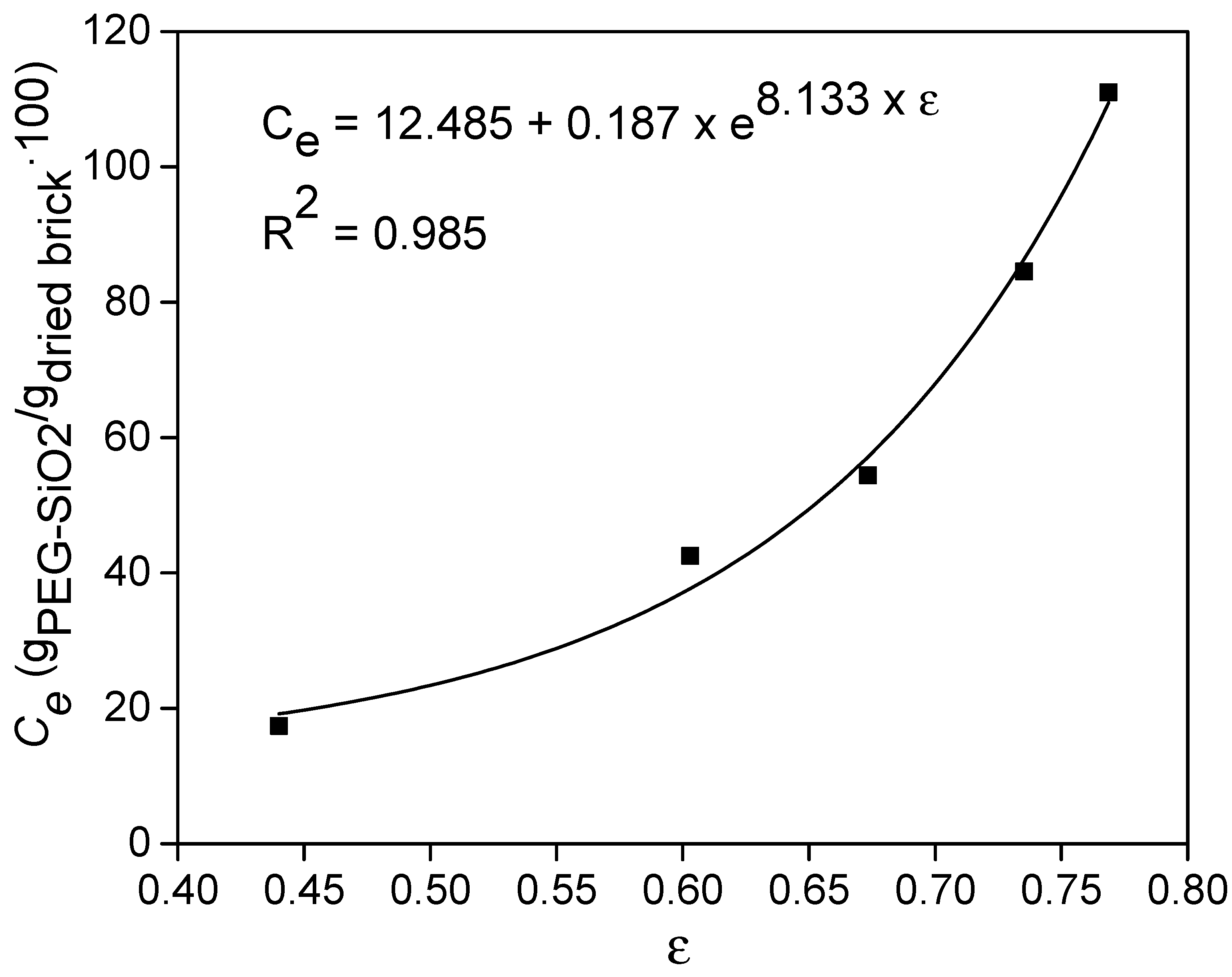
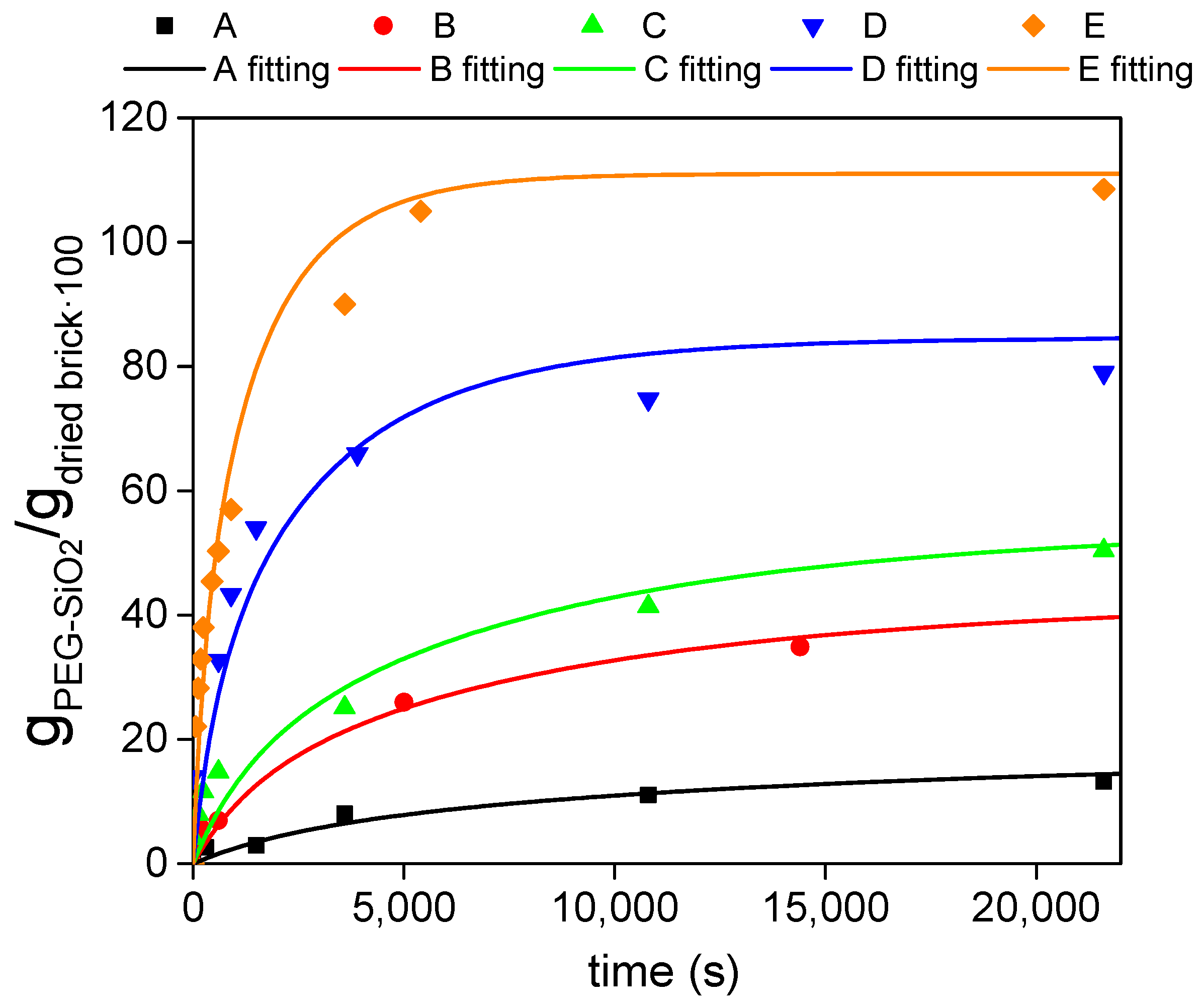
2.5.2. Adsorption Curves and Maximum Adsorption Capacity
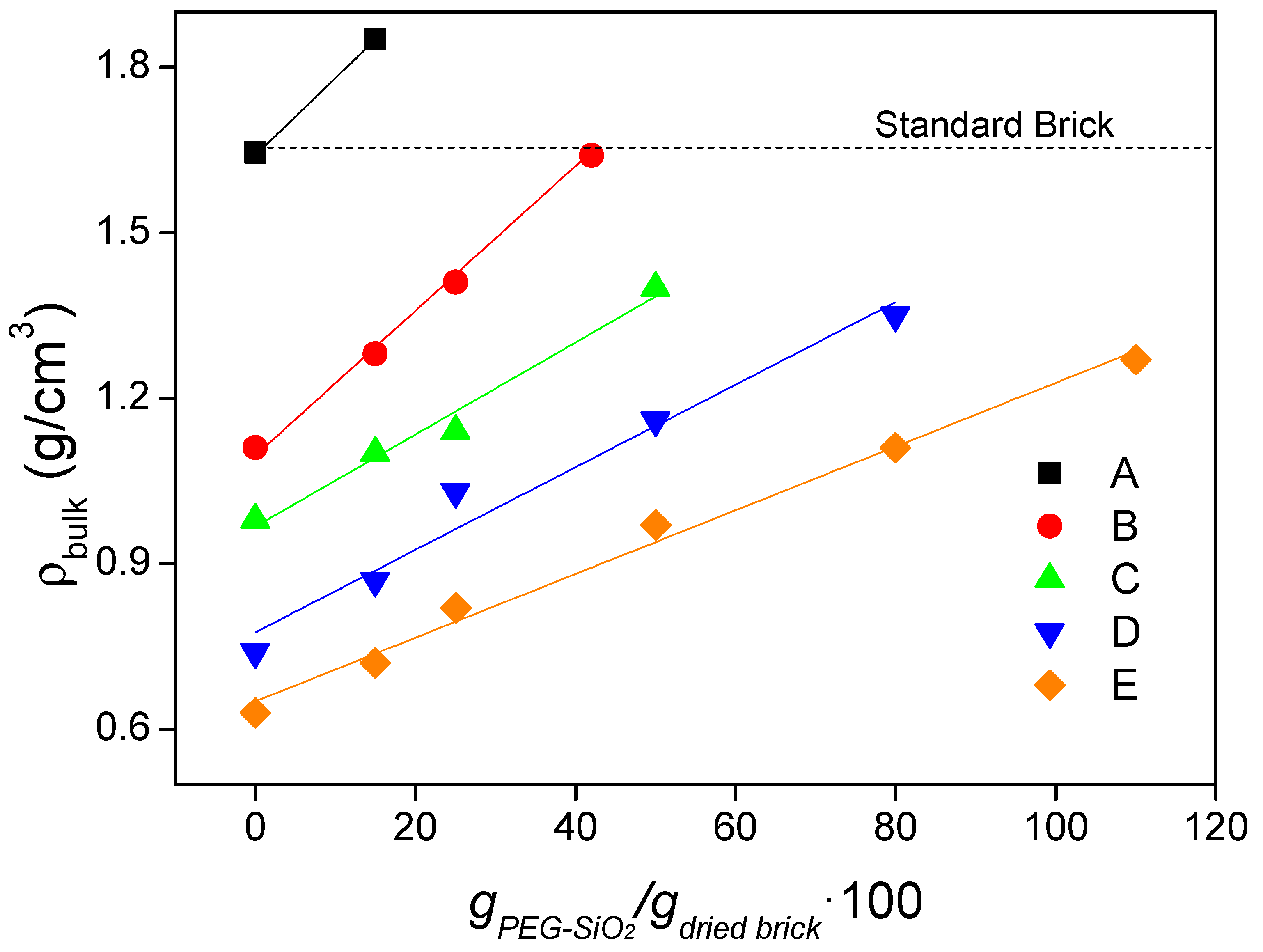
2.5.3. Bulk Density, True Density, and Porosity
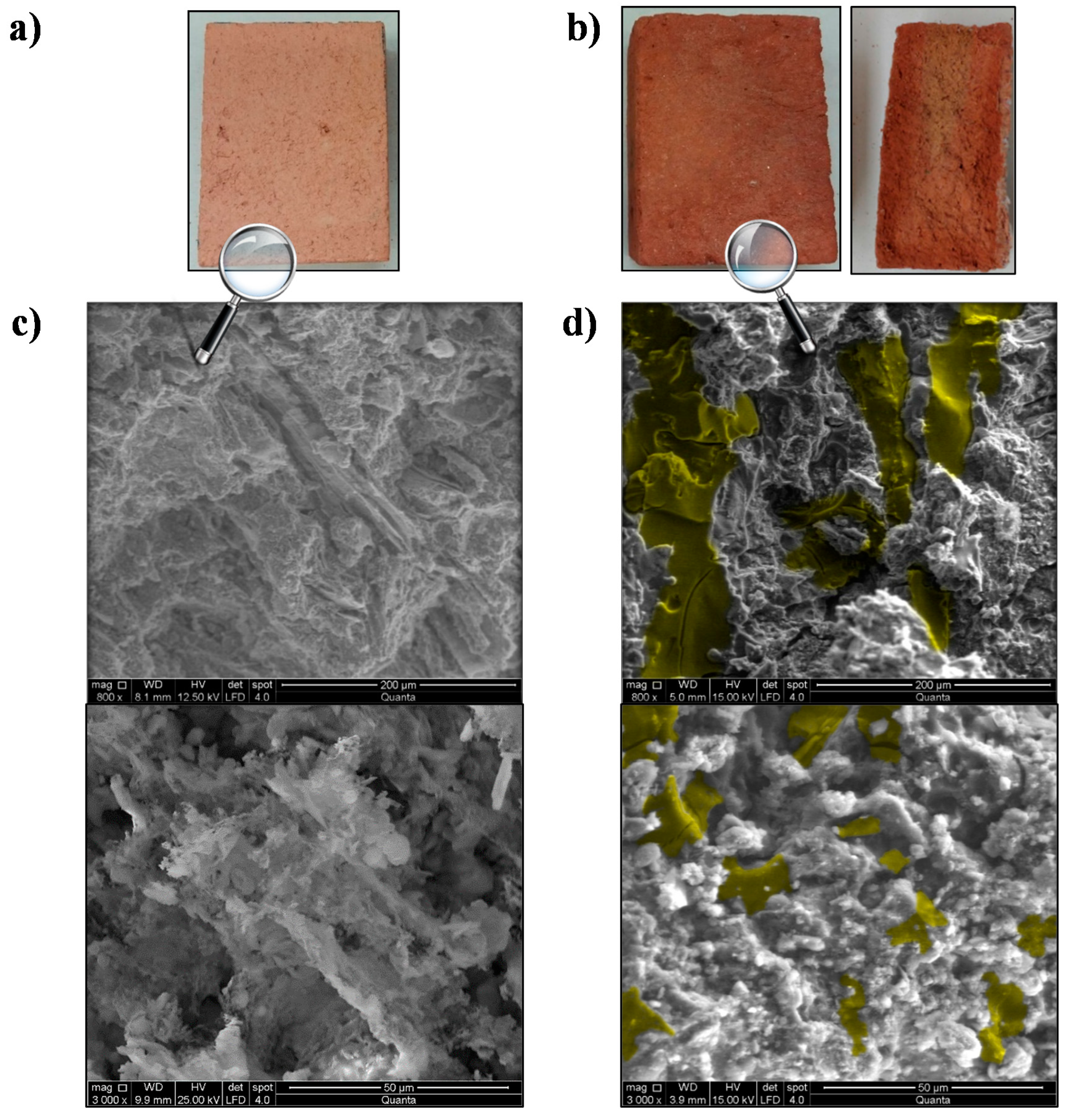
2.5.4. Scanning Electron Microscopy Analysis
2.5.5. Experimental Equipment for Thermal Behavior Tests
3. Calculations
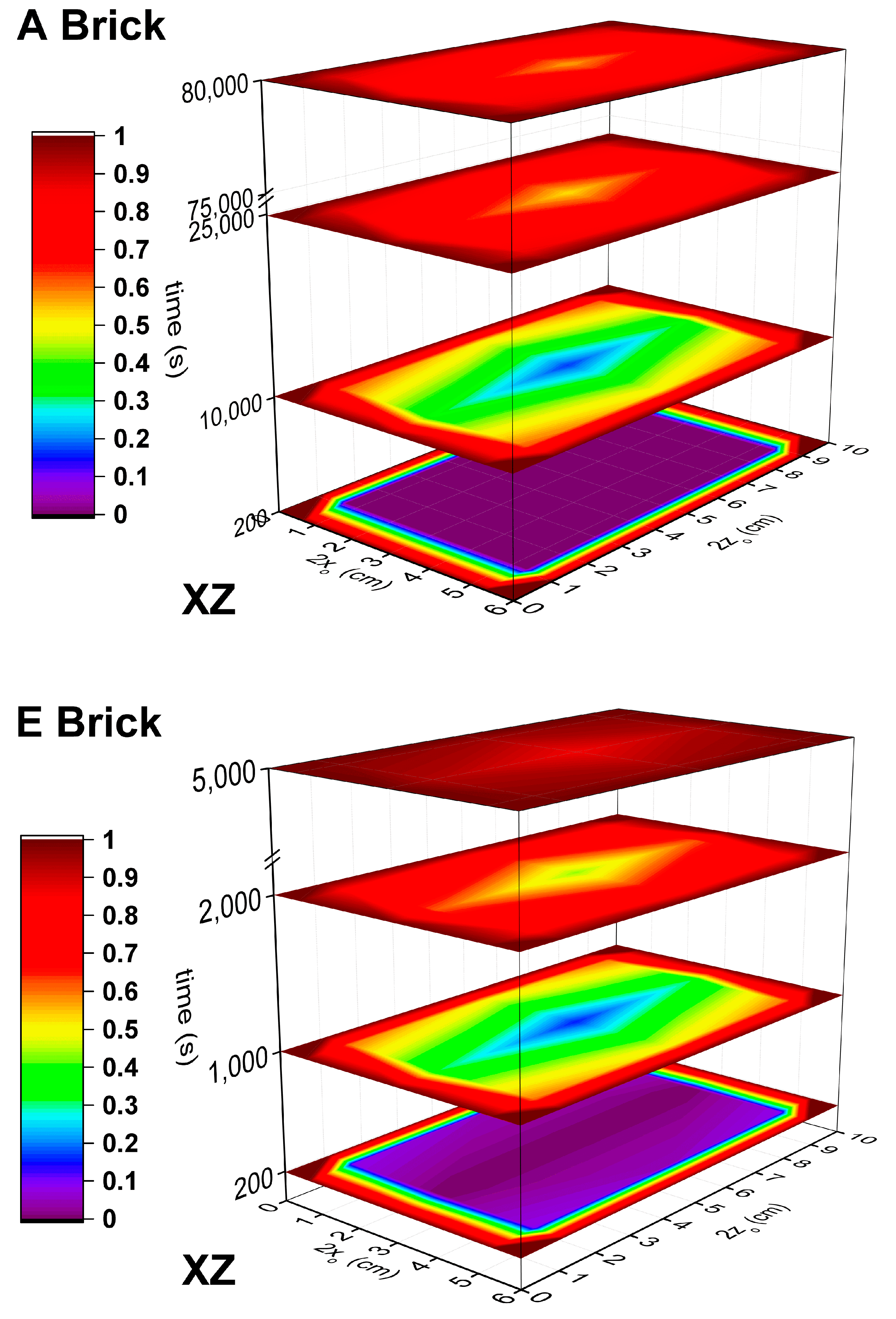
3.1. Problem Definition for Diffusion Coefficients Calculation
3.2. Orthogonal Collocation Method in Fick´s Second Law
4. Results and Discussion
4.1. PEG-SiO2 sol Rheology
4.2. Adsorption Capacity
4.3. Bulk Density
4.4. SEM Analysis
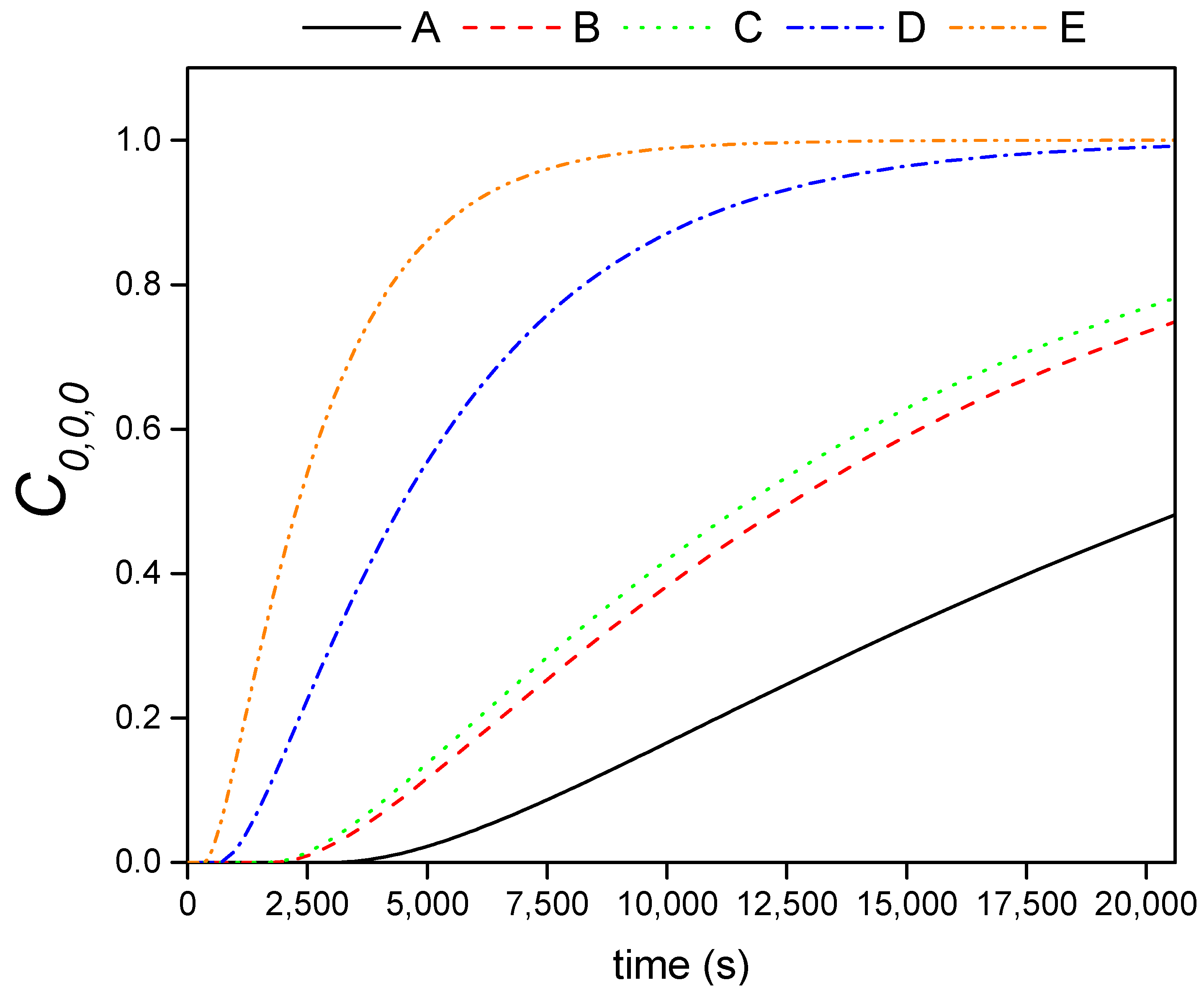
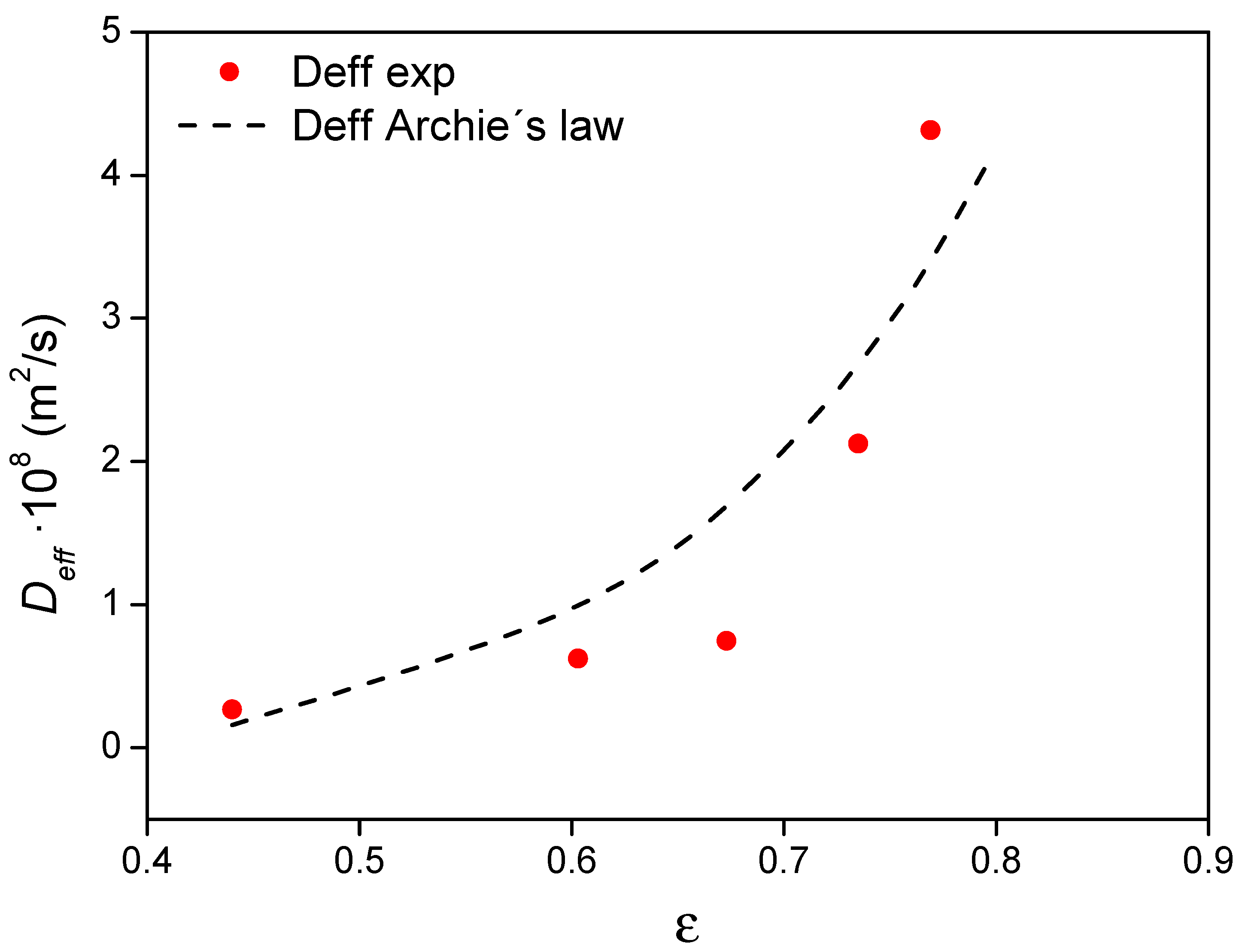
4.5. Effective Diffusion Coefficients
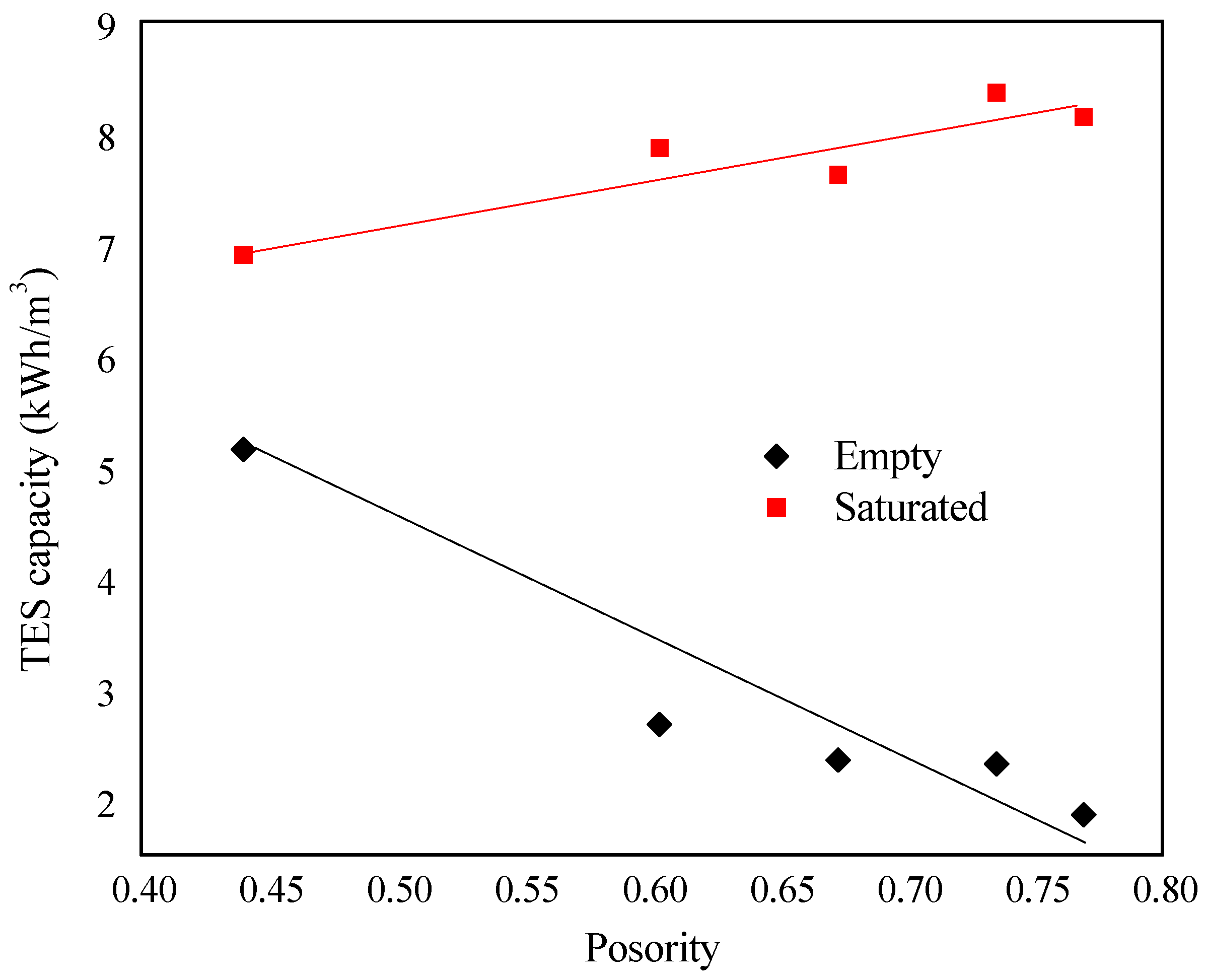
4.5.1. Influence of Porosity on Deff
4.5.2. Influence of the PEG-SiO2 Incorporation on the Composite Thermal Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament Directive 2012/27/EU. Off. J. Eur. Union 2012, 315, 1–56.
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Hasnain, S. Review on sustainable thermal energy storage technologies, Part I: Heat storage materials and techniques. Energy Convers. Manag. 1998, 39, 1127–1138. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Talavera, B.; Rodriguez, J.F.; Valverde, J.L.; Gonzalez, J.L.; Carmona, M. Enhancing the thermal comfort of fabrics for the footwear industry. Text. Res. J. 2013. [Google Scholar] [CrossRef]
- Bakan, G.; Gerislioglu, B.; Dirisaglik, F.; Jurado, Z.; Sullivan, L.; Dana, A.; Lam, C.; Gokirmak, A.; Silva, H. Extracting the temperature distribution on a phase-change memory cell during crystallization. J. Appl. Phys. 2016. [Google Scholar] [CrossRef] [Green Version]
- Gerislioglu, B.; Bakan, G.; Ahuja, R.; Adam, J.; Mishra, Y.K.; Ahmadivand, A. The role of Ge2Sb2Te5 in enhancing the performance of functional plasmonic devices. Mater. Today Phys. 2020, 12. [Google Scholar] [CrossRef]
- Borreguero, A.M.; Valverde, J.L.; Rodríguez, J.F.; Barber, A.H.; Cubillo, J.J.; Carmona, M. Synthesis and characterization of microcapsules containing Rubitherm®RT27 obtained by spray drying. Chem. Eng. J. 2011. [Google Scholar] [CrossRef]
- Oliver, A. Thermal characterization of gypsum boards with PCM included: Thermal energy storage in buildings through latent heat. Energy Build. 2012, 48, 1–7. [Google Scholar] [CrossRef]
- Athienitis, A.K.; Liu, C.; Hawes, D.; Banu, D.; Feldman, D. Investigation of the thermal performance of a passive solar test-room with wall latent heat storage. Build. Environ. 1997. [Google Scholar] [CrossRef]
- Hadjieva, M.; Stoykov, R.; Filipova, T. Composite salt-hydrate concrete system for building energy storage. Renew. Energy 2000, 19, 111–115. [Google Scholar] [CrossRef]
- Aydin, A.A.; Okutan, H. Polyurethane rigid foam composites incorporated with fatty acid ester-based phase change material. Energy Convers. Manag. 2013. [Google Scholar] [CrossRef]
- Barreneche, C.; De Gracia, A.; Serrano, S.; Elena Navarro, M.; Borreguero, A.M.; Inés Fernández, A.; Carmona, M.; Rodriguez, J.F.; Cabeza, L.F. Comparison of three different devices available in Spain to test thermal properties of building materials including phase change materials. Appl. Energy 2013. [Google Scholar] [CrossRef]
- Kreh, R.T. Masonry Skills; Cengage Learning: Boston, MA, USA, 2014; ISBN 1305177916. [Google Scholar]
- Alawadhi, E.M. Thermal analysis of a building brick containing phase change material. Energy Build. 2008. [Google Scholar] [CrossRef]
- Mukram, T.A.; Daniel, J. Building bricks with PCM inserts for Passive cooling applications. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Zhang, C.; Chen, Y.; Wu, L.; Shi, M. Thermal response of brick wall filled with phase change materials (PCM) under fluctuating outdoor temperatures. Energy Build. 2011. [Google Scholar] [CrossRef]
- Silva, T.; Vicente, R.; Soares, N.; Ferreira, V. Experimental testing and numerical modelling of masonry wall solution with PCM incorporation: A passive construction solution. Energy Build. 2012. [Google Scholar] [CrossRef]
- Mavrigiannaki, A.; Ampatzi, E. Latent heat storage in building elements: A systematic review on properties and contextual performance factors. Renew. Sustain. Energy Rev. 2016, 60, 852–866. [Google Scholar] [CrossRef] [Green Version]
- Abbasi Hattan, H.; Madhkhan, M.; Marani, A. Thermal and mechanical properties of building external walls plastered with cement mortar incorporating shape-stabilized phase change materials (SSPCMs). Constr. Build. Mater. 2021. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, Y.; Lin, K.; Xiao, W. Thermal analysis of a direct-gain room with shape-stabilized PCM plates. Renew. Energy 2008. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, Y.; Wang, X.; Lin, K.; Xiao, W. An assessment of mixed type PCM-gypsum and shape-stabilized PCM plates in a building for passive solar heating. Sol. Energy 2007. [Google Scholar] [CrossRef]
- Soares, N.; Costa, J.; Gaspar, A.; Santos, P. Review of passive PCM latent heat thermal energy storage systems towards buildings’ energy efficiency. Energy Build. 2013, 59, 82–103. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, Y.; Xu, H. Energy performance of a hybrid space-cooling system in an office building using SSPCM thermal storage and night ventilation. Sol. Energy 2011. [Google Scholar] [CrossRef]
- Serrano, A.; Borreguero, A.M.; Garrido, I.; Rodríguez, J.F.; Carmona, M. Reducing heat loss through the building envelope by using polyurethane foams containing thermoregulating microcapsules. Appl. Therm. Eng. 2016, 103. [Google Scholar] [CrossRef]
- Ling, T.-C.; Poon, C.-S. Use of phase change materials for thermal energy storage in concrete: An overview. Constr. Build. Mater. 2013, 46, 55–62. [Google Scholar] [CrossRef]
- Navarro, L.; de Gracia, A.; Niall, D.; Castell, A.; Browne, M.; McCormack, S.J.; Griffiths, P.; Cabeza, L.F. Thermal energy storage in building integrated thermal systems: A review. Part 2. Integration as passive system. Renew. Energy 2016, 85, 1334–1356. [Google Scholar] [CrossRef] [Green Version]
- Serrano, A.; Duran, M.; Dauvergne, J.L.; Doppiu, S.; Del Barrio, E.P. Tailored transition temperature plastic crystals with enhanced thermal energy storage capacity. Sol. Energy Mater. Sol. Cells 2021, 220, 110848. [Google Scholar] [CrossRef]
- Serrano, A.; Dauvergne, J.-L.; Doppiu, S.; Palomo Del Barrio, E. Neopentyl Glycol as Active Supporting Media in Shape-Stabilized PCMs. Materials 2019, 12, 3169. [Google Scholar] [CrossRef] [Green Version]
- Memon, S.A. Phase change materials integrated in building walls: A state of the art review. Renew. Sustain. Energy Rev. 2014, 31, 870–906. [Google Scholar] [CrossRef]
- Karaman, S.; Karaipekli, A.; Sar, A.; Biçer, A. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of capric acid/expanded perlite composite for thermal energy storage. Mater. Chem. Phys. 2008. [Google Scholar] [CrossRef]
- Sari, A.; Karaipekli, A.; Alkan, C. Preparation, characterization and thermal properties of lauric acid/expanded perlite as novel form-stable composite phase change material. Chem. Eng. J. 2009. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sari, A. Preparation and characterization of fatty acid ester/building material composites for thermal energy storage in buildings. Energy Build. 2011. [Google Scholar] [CrossRef]
- Serrano, A.; Martín del Campo, J.; Peco, N.; Rodriguez, J.F.; Carmona, M. Influence of gelation step for preparing PEG–SiO2 shape-stabilized phase change materials by sol–gel method. J. Sol-Gel Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Xu, J.; Yang, T.; Xu, X.; Guo, X.; Cao, J. Processing solid wood into a composite phase change material for thermal energy storage by introducing silica-stabilized polyethylene glycol. Compos. Part A Appl. Sci. Manuf. 2020. [Google Scholar] [CrossRef]
- Iglesias, I.; Serrano, Á.; Acosta, A. Fabricación de un soporte cerámico poroso a partir de arcillas y biomasa para el desarrollo de un material termorregulador. XXIV Reun. la Soc. Española Arcillas 2016, 65, 57. [Google Scholar]
- Borreguero, A.M.; Luz Sánchez, M.; Valverde, J.L.; Carmona, M.; Rodríguez, J.F. Thermal testing and numerical simulation of gypsum wallboards incorporated with different PCMs content. Appl. Energy 2011. [Google Scholar] [CrossRef]
- Treybal, R.E.; García Rodríguez, A. Operaciones de Transferencia de Masa; McGraw Hill: New York, NY, USA, 1988; ISBN 9686046348. [Google Scholar]
- Soliman, M.; Al-Zeghayer, Y.; Ajbar, A. A modified orthogonal collocation method for reaction diffusion problems. Braz. J. Chem. Eng. 2014. [Google Scholar] [CrossRef] [Green Version]
- Finlayson, B.A. The Method of Weighted Residuals and Variational Principles; University of Washington: Seattle, WA, USA, 2013. [Google Scholar]
- Finlayson, B.A. Orthogonal Collocation in Chemical Reaction Engineering. Catal. Rev. 1974. [Google Scholar] [CrossRef]
- Brinker, C.J.; Keefer, K.D.; Schaefer, D.W.; Ashley, C.S. Sol-gel transition in simple silicates. J. Non. Cryst. Solids 1982. [Google Scholar] [CrossRef]
- Brinker, C.J. Hydrolysis and condensation of silicates: Effects on structure. J. Non. Cryst. Solids 1988. [Google Scholar] [CrossRef] [Green Version]
- Valverde, J.L.; De Lucas, A.; González, M.; Rodríguez, J.F. Equilibrium data for the exchange of Cu2+, Cd2+, and Zn2+ ions for H+ on the cationic exchanger Amberlite IR-120. J. Chem. Eng. Data 2002. [Google Scholar] [CrossRef]
- Iglesias, I.; García-Romero, E.; Acosta, A. Influence of dolomite microcrystals on the technological properties of Santa Cruz de Mudela clays used for building ceramics. Appl. Clay Sci. 2014. [Google Scholar] [CrossRef]
- Villadsen, J.V.; Stewart, W.E. Solution of boundary-value problems by orthogonal collocation. Chem. Eng. Sci. 1967. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in Fortran 90; Cambridge University Press: Cambridge, UK, 2010; ISBN 9780521880688. [Google Scholar]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963. [Google Scholar] [CrossRef]
- Archie, G.E. The Electrical Resistivity Log as an Aid in Determining Some Reservoir Characteristics. Trans. AIME 1942, 146, 54–62. [Google Scholar] [CrossRef]
- Navarre-Sitchler, A.; Steefel, C.I.; Yang, L.; Tomutsa, L.; Brantley, S.L. Evolution of porosity and diffusivity associated with chemical weathering of a basalt clast. J. Geophys. Res. Earth Surf. 2009. [Google Scholar] [CrossRef] [Green Version]
- Van der Zanden, A.J.J.; de Wit, M.H. A Procedure to Measure the Diffusion Coefficient of Water in Brick as a Function of the Water Concentration. Dry. Technol. 2012. [Google Scholar] [CrossRef]



| Sample | A | B | C | D | E |
|---|---|---|---|---|---|
| ε | 0.440 | 0.603 | 0.673 | 0.735 | 0.769 |
| Roots of Polynomials | Bm ij | ||||
|---|---|---|---|---|---|
| 0.0000 | 84.0000 | −122.0631 | 58.6666 | −44.6035 | 24.0000 |
| 0.1127 | 53.2379 | −73.3333 | 26.6667 | 13.3333 | 6.7621 |
| 0.5000 | −6.0000 | 16.6667 | −21.3333 | 16.6667 | −6.0000 |
| 0.8872 | 6.7621 | −13.3333 | 26.6666 | 73.3333 | 53.2379 |
| 1.0000 | 24.0000 | −44.6035 | 58.6666 | −122.0632 | 84.0000 |
| Sample | Deff/ε (m2/s) |
|---|---|
| A | 6.086 × 10−9 ± 2.343 × 10−9 |
| B | 1.030 × 10−8 ± 4.314 × 10−9 |
| C | 1.109 × 10−8 ± 5.165 × 10−9 |
| D | 2.887 × 10−8 ± 8.359 × 10−9 |
| E | 5.609 × 10−8 ± 2.202 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano, A.; Borreguero, A.M.; Iglesias, I.; Acosta, A.; Rodríguez, J.F.; Carmona, M. Diffusion of Shape Stabilized PEG-SiO2 as a Driver for Producing Thermoregulating Facing Bricks. Materials 2021, 14, 1395. https://doi.org/10.3390/ma14061395
Serrano A, Borreguero AM, Iglesias I, Acosta A, Rodríguez JF, Carmona M. Diffusion of Shape Stabilized PEG-SiO2 as a Driver for Producing Thermoregulating Facing Bricks. Materials. 2021; 14(6):1395. https://doi.org/10.3390/ma14061395
Chicago/Turabian StyleSerrano, Angel, Ana M. Borreguero, Isabel Iglesias, Anselmo Acosta, Juan F. Rodríguez, and Manuel Carmona. 2021. "Diffusion of Shape Stabilized PEG-SiO2 as a Driver for Producing Thermoregulating Facing Bricks" Materials 14, no. 6: 1395. https://doi.org/10.3390/ma14061395






