First-Principles Study of Mechanical and Thermodynamic Properties of Binary and Ternary CoX (X = W and Mo) Intermetallic Compounds
Abstract
:1. Introduction
2. Computational Method
3. Results and Discussion
3.1. Geometry and Structural Properties
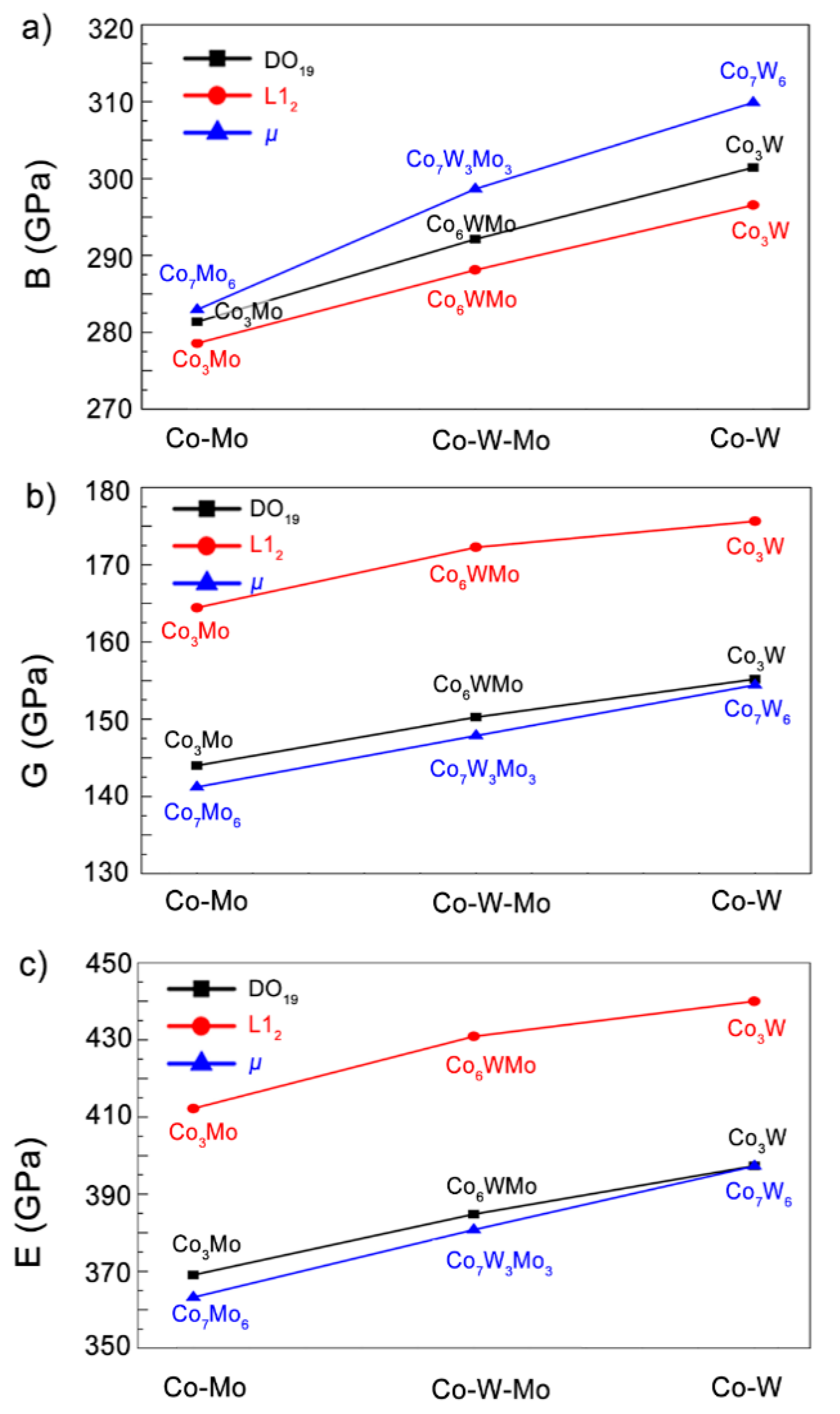
3.2. Elastic Properties
3.3. Thermodynamic Properties
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, R.C. The Superalloys: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Pollock, T.M. Alloy design for aircraft engines. Nat. Mater. 2016, 15, 809–815. [Google Scholar] [CrossRef]
- Li, N.; Yin, F.; Feng, L. Microstructure of a V-containing cobalt based alloy prepared by mechanical alloying and hot pressed sintering. Metals 2019, 9, 464. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Sawant, A.K.; Nithin, B.; Peng, Z.; Makineni, S.K.; Gault, B.; Chattopadhyay, K. On the effect of Re addition on microstructural evolution of a CoNi-based superalloy. Acta Mater. 2019, 168, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Slone, C.E.; Miao, J.; Mills, M.J. Ultra-high strength and ductility from rolling and annealing of a Ni-Cr-Co superalloy. Scr. Mater. 2018, 155, 94–98. [Google Scholar] [CrossRef]
- Sato, J.; Omori, T.; Oikawa, K.; Ohnuma, I.; Kainuma, R.; Ishida, K. Cobalt-base high-temperature alloys. Science 2006, 312, 90–91. [Google Scholar] [CrossRef]
- Ruan, J.J.; Liu, X.J.; Yang, S.Y.; Xu, W.W.; Omori, T.; Yang, T.; Deng, B.; Jiang, H.X.; Wang, C.P.; Kainuma, R.; et al. Novel Co-Ti-V-base superalloys reinforced by L12-ordered γ′ phase. Intermetallics 2018, 92, 126–132. [Google Scholar] [CrossRef]
- Cao, B.X.; Yang, T.; Fan, L.; Luan, J.H.; Jiao, Z.B.; Liu, C.T. Refractory alloying additions on the thermal stability and mechanical properties of high-entropy alloys. Mater. Sci. Eng. A 2020, 797, 140020. [Google Scholar] [CrossRef]
- Tsyntsaru, N. Electrodeposition of cobalt–tungsten alloys and their application for surface engineering. Russ. J. Electrochem. 2016, 52, 1041–1047. [Google Scholar] [CrossRef]
- Weston, D.P.; Gill, S.P.A.; Fay, M.; Harris, S.J.; Yap, G.N.; Zhang, D. Nano-structure of Co–W alloy electrodeposited from gluconate bath. Surf. Coat. Technol. 2013, 236, 75–83. [Google Scholar] [CrossRef]
- Costa, J.M.; Porto, M.B.; Amancio, R.J.; de Almeida Neto, A.F. Effects of tungsten and cobalt concentration on microstructure and anticorrosive property of cobalt-tungsten alloys. Surf. Interfaces 2020, 20, 100626. [Google Scholar] [CrossRef]
- Ghaferi, Z.; Raeissi, K.; Golozar, M.A.; Edris, H. Characterization of nanocrystalline Co–W coatings on Cu substrate, electrodeposited from a citrate-ammonia bath. Surf. Coat. Technol. 2011, 206, 497–505. [Google Scholar] [CrossRef]
- Sato, J.; Oikawa, K.; Kainuma, R.; Ishida, K. Experimental verification of magnetically induced phase separation in αCo phase and thermodynamic calculations of phase equilibria in the Co–W system. Mater. Trans. 2005, 46, 1199–1207. [Google Scholar] [CrossRef] [Green Version]
- Davydov, A.; Kattner, U.R. Thermodynamic assessment of the Co-Mo system. J. Phase Equilib. 1999, 20, 5. [Google Scholar] [CrossRef]
- Ishchenko, T.V.; Meshkov, L.L.; Sokolovskaya, Y.M. On the interaction of μ phases in systems formed by transition metals. J. Less Common Met. 1984, 97, 145–150. [Google Scholar] [CrossRef]
- Wang, C.P.; Wang, J.; Guo, S.H.; Liu, X.J.; Ohnuma, I.; Kainuma, R.; Ishida, K. Experimental investigation and thermodynamic calculation of the phase equilibria in the Co–Mo–W system. Intermetallics 2009, 17, 642–650. [Google Scholar] [CrossRef]
- Ren, N.; Jianxin, Z. Site preference and alloying effect of tungsten in the μ phase of Co7Mo6. Philos. Mag. Lett. 2016, 96, 1–8. [Google Scholar] [CrossRef]
- Xu, W.W.; Han, J.J.; Wang, Z.W.; Wang, C.P.; Wen, Y.H.; Liua, X.J.; Zhu, Z.Z. Thermodynamic, structural and elastic properties of Co3X (X = Ti, Ta, W, V, Al) compounds from first-principles calculations. Intermetallics 2013, 32, 303–311. [Google Scholar] [CrossRef]
- Arikan, N. The structural, electronic, elastic and dynamic properties of Co3W in the L12 phase. AIP Conf. Proc. 2017, 1815, 050001. [Google Scholar]
- Yuan, H.K.; Cui, H.; Liu, B.; Tian, C.L.; Kuang, A.L.; Chen, H. Density functional theory calculations for magnetic properties of Co3W systems. J. Chem. Phys. 2018, 149, 014303. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Zhang, Y.; Zhang, W.; Jin, H. First-principles calculations of Co7W6 doped with Re: Site occupancy and electronic properties. Comput. Condens. Matter 2017, 13, 36–40. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Wallace, D.C. Thermodynamics of crystals. Am. J. Phys. 1972, 40, 1718–1719. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals: Their Representation by Tensors and Matrices; Oxford University Press: New York, NY, USA, 1985. [Google Scholar]
- Magneli, A.; Westgren, A. Röntgenuntersuchung von Kobalt–Wolframlegierungen. Z. Anorg. Allg. Chem. 1938, 238, 268–272. [Google Scholar] [CrossRef]
- Alte da Veiga, L. Refinement of the structure of the phase Co2Mo. Acta Crystallogr. 1965, 18, 855–857. [Google Scholar] [CrossRef]
- Forsyth, J.B.; D’Alte da Veiga, L.M. The structure of the μ-phase Co7Mo6. Acta Crystallogr. 1962, 15, 543–546. [Google Scholar] [CrossRef]
- Long, J.; Yang, L.; Wei, X. Lattice, elastic properties and Debye temperatures of ATiO3 (A = Ba, Ca, Pb, Sr) from first-principles. J. Alloys Compd. 2013, 549, 336–340. [Google Scholar] [CrossRef]
- Watt, J.P.; Peselnick, L. Clarification of the Hashin-Shtrikman bounds on the effective elastic moduli of polycrystals with hexagonal, trigonal, and tetragonal symmetries. J. Appl. Phys. 1980, 51, 1525–1531. [Google Scholar] [CrossRef]
- Hill, R. The elastic behaviour of a crystalline aggregate. Proc. Math. Phys. Eng. Sci. 1952, 65, 349. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dubl. Phil. Mag. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Yang, J.; Long, J.; Yang, L.; Li, D. First-principles investigations of the physical properties of binary uranium silicide alloys. J. Nucl. Mater. 2013, 443, 195–199. [Google Scholar] [CrossRef]
- Arya, A.; Dey, G.K.; Vasudevan, V.K.; Banerjee, S. Effect of chromium addition on the ordering behaviour of Ni–Mo alloy: Experimental results vs. electronic structure calculations. Acta Mater. 2002, 13, 3301–3315. [Google Scholar] [CrossRef]
- Chan, K.S.; Lee, Y.D.; Pan, Y.M. First-principles computations of mechanical properties of Ni2Cr and Ni2Mo. Metal. Mater. Trans. A 2006, 3, 523–537. [Google Scholar] [CrossRef]
- Schindzielorz, N.; Nowak, K.; Maisel, S.B.; Müller, S. Phase behavior and mechanical properties of Ni–W studied by first-principles calculations and ab initio based thermodynamics. Acta Mater. 2014, 75, 307–315. [Google Scholar] [CrossRef]
- Blanco, M.A.; Francisco, E.; Luana, V. GIBBS: Isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic Debye model. Comput. Phys. Commun. 2004, 158, 57–72. [Google Scholar] [CrossRef]
- Blanco, M.A.; Pendás, A.M.; Francisco, E.; Recio, J.M.; Franco, R. Thermodynamical properties of solids from microscopic theory: Applications to MgF2 and Al2O3. J. Mol. Struct. 1996, 368, 245–255. [Google Scholar] [CrossRef]
- Flórez, M.; Recio, J.M.; Francisco, E.; Blanco, M.A.; Pendás, A.M. First-principles study of the rocksalt–cesium chloride relative phase stability in alkali halides. Phys. Rev. B 2002, 66, 144112. [Google Scholar] [CrossRef]
- Francisco, E.; Recio, J.M.; Blanco, M.A.; Pendás, A.M.; Costales, A. Quantum-mechanical study of thermodynamic and bonding properties of MgF2. J. Phys. Chem. A 1998, 102, 1595–1601. [Google Scholar] [CrossRef]
- Francisco, E.; Blanco, M.A.; Sanjurjo, G. Atomistic simulation of SrF2 polymorphs. Phys. Rev. B 2001, 63, 094107. [Google Scholar] [CrossRef]
- Kittel, C.; McEuen, P.; McEuen, P. Introduction to Solid State Physics; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Barin, I. Thermochemical Data of Pure Substances; VCH Verlagsgesellschaft mbH: Weinheim, Germany, 1989. [Google Scholar]




| Compound | Structure | Calculated (Å) | Experimental (Å) | ΔE | ΔH | ||
|---|---|---|---|---|---|---|---|
| a | b | c | d | (eV/atom) | (eV/atom) | ||
| Co3W | DO19 | 5.120 | 4.115 | 5.120 [30] | 4.116 [30] | −7.682 | −0.238 |
| Co3W | L12 | 3.590 | 3.590 | −7.640 | −0.195 | ||
| Co3Mo | DO19 | 5.097 | 4.076 | 5.125 [31] | 4.113 [31] | −7.486 | −0.196 |
| Co3Mo | L12 | 3.585 | 3.585 | −7.427 | −0.138 | ||
| Co6WMo | DO19 | 5.101 | 4.082 | −7.585 | −0.218 | ||
| Co6WMo | L12 | 3.589 | −7.533 | −0.166 | |||
| Co7W6 | μ | 4.743 | 25.590 | 4.751 [30] | 25.617 [30] | −8.554 | −0.146 |
| Co7Mo6 | μ | 4.737 | 25.417 | 4.762 [32] | 25.617 [32] | −8.219 | −0.096 |
| Co7W3Mo3 | μ | 4.745 | 25.476 | −8.399 | −0.134 | ||
| Structures | Co3W | Co3Mo | Co6WMo | Co3W | Co3Mo | Co6WMo | Co7W6 | Co7Mo6 | Co7W3Mo3 |
|---|---|---|---|---|---|---|---|---|---|
| DO19 | DO19 | DO19 | L12 | L12 | L12 | μ | μ | μ | |
| C11(GPa) | 501.463 | 462.259 | 484.533 | 430.166 | 408.132 | 418.138 | 516.285 | 472.228 | 490.915 |
| C22(GPa) | 501.463 | 462.259 | 484.533 | 430.166 | 408.132 | 418.138 | 516.285 | 472.228 | 490.915 |
| C33(GPa) | 539.047 | 505.988 | 522.428 | 430.166 | 408.132 | 418.635 | 571.091 | 504.111 | 537.801 |
| C44(GPa) | 116.850 | 109.096 | 113.547 | 185.551 | 170.768 | 182.684 | 110.768 | 102.862 | 107.542 |
| C55(GPa) | 116.850 | 109.096 | 113.547 | 185.551 | 170.768 | 182.684 | 110.768 | 102.862 | 107.542 |
| C66(GPa) | 144.633 | 134.472 | 139.718 | 185.551 | 170.768 | 182.256 | 147.279 | 135.152 | 143.475 |
| C12(GPa) | 212.196 | 198.316 | 205.097 | 229.722 | 213.797 | 223.863 | 225.810 | 203.879 | 210.551 |
| C13(GPa) | 186.658 | 176.421 | 181.877 | 229.722 | 213.797 | 222.571 | 183.424 | 172.516 | 186.904 |
| C23(GPa) | 186.658 | 176.421 | 181.877 | 229.722 | 213.797 | 222.569 | 183.424 | 172.516 | 186.904 |
| S11(MPa) | 2.584 | 2.824 | 2.677 | 3.701 | 3.829 | 3.807 | 2.522 | 2.759 | 2.658 |
| S22(MPa) | 2.584 | 2.824 | 2.677 | 3.701 | 3.829 | 3.807 | 2.522 | 2.759 | 2.658 |
| S33(MPa) | 2.265 | 2.429 | 2.345 | 3.701 | 3.829 | 3.783 | 2.082 | 2.403 | 2.282 |
| S44(MPa) | 8.558 | 9.166 | 8.807 | 5.389 | 5.856 | 5.474 | 9.028 | 9.722 | 9.299 |
| S55(MPa) | 8.558 | 9.166 | 8.807 | 5.389 | 5.856 | 5.474 | 9.028 | 9.722 | 9.299 |
| S66(MPa) | 6.914 | 7.436 | 7.157 | 5.389 | 5.856 | 5.487 | 6.790 | 7.399 | 6.970 |
| S12(MPa) | −0.873 | −0.964 | −0.901 | −1.288 | −1.316 | −1.340 | −0.920 | −0.967 | −0.909 |
| S13(MPa) | −0.593 | −0.649 | −0.618 | −1.288 | −1.316 | −1.312 | −0.514 | −0.613 | −0.608 |
| S23(MPa) | −0.593 | −0.649 | −0.618 | −1.288 | −1.316 | −1.312 | −0.514 | −0.613 | −0.608 |
| B(GPa) | 301.429 | 281.370 | 292.109 | 296.537 | 278.575 | 288.102 | 309.871 | 282.932 | 298.647 |
| G(GPa) | 155.180 | 144.002 | 150.249 | 175.623 | 164.428 | 172.261 | 154.418 | 141.217 | 147.852 |
| E(GPa) | 397.352 | 369.047 | 384.777 | 440.004 | 412.187 | 430.902 | 397.264 | 363.220 | 380.726 |
| σ | 0.280 | 0.281 | 0.280 | 0.253 | 0.253 | 0.251 | 0.286 | 0.286 | 0.288 |
| B/G | 1.942 | 1.954 | 1.944 | 1.688 | 1.694 | 1.672 | 2.007 | 2.004 | 2.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, C.; Sun, J.; Li, S.; Liu, W.; Wu, H.; Wang, J. First-Principles Study of Mechanical and Thermodynamic Properties of Binary and Ternary CoX (X = W and Mo) Intermetallic Compounds. Materials 2021, 14, 1404. https://doi.org/10.3390/ma14061404
Yang Y, Wang C, Sun J, Li S, Liu W, Wu H, Wang J. First-Principles Study of Mechanical and Thermodynamic Properties of Binary and Ternary CoX (X = W and Mo) Intermetallic Compounds. Materials. 2021; 14(6):1404. https://doi.org/10.3390/ma14061404
Chicago/Turabian StyleYang, Yunfei, Changhao Wang, Junhao Sun, Shilei Li, Wei Liu, Hao Wu, and Jinshu Wang. 2021. "First-Principles Study of Mechanical and Thermodynamic Properties of Binary and Ternary CoX (X = W and Mo) Intermetallic Compounds" Materials 14, no. 6: 1404. https://doi.org/10.3390/ma14061404






