Glass-Forming Ability and Corrosion Resistance of Al88Y8−xFe4+x (x = 0, 1, 2 at.%) Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
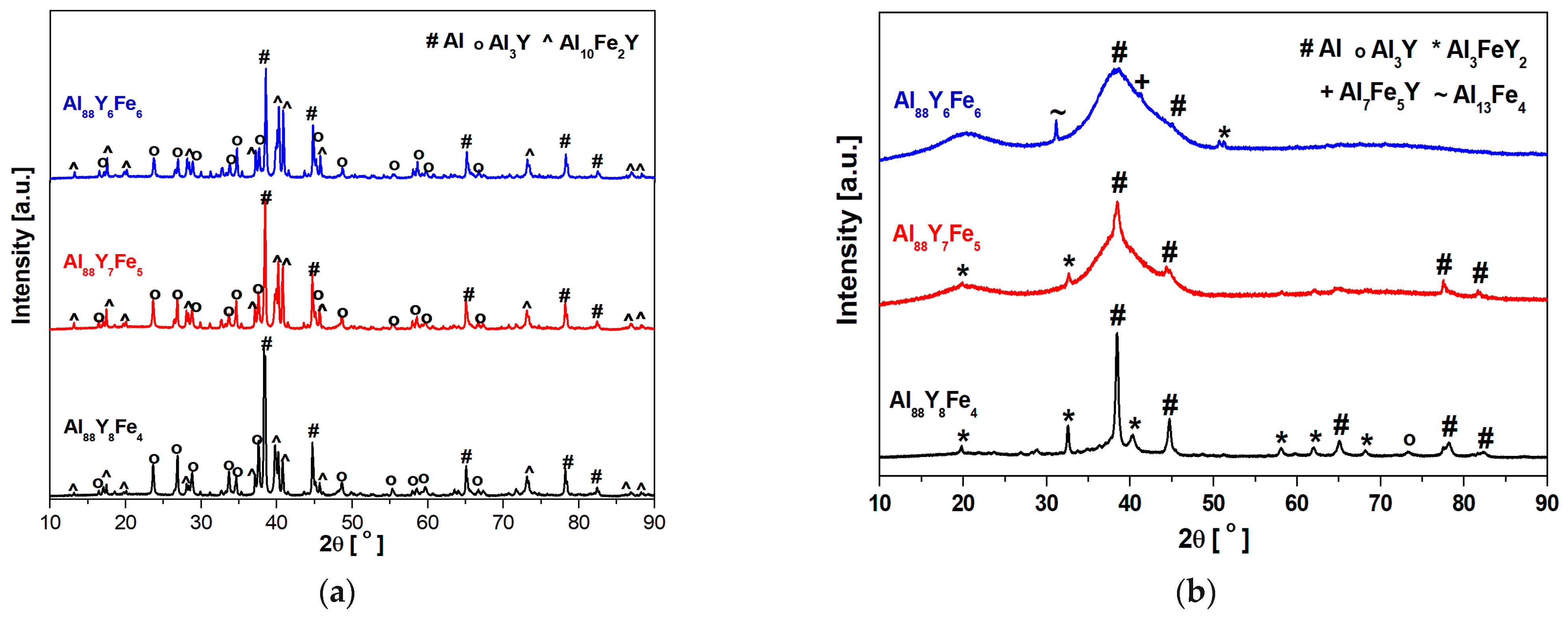
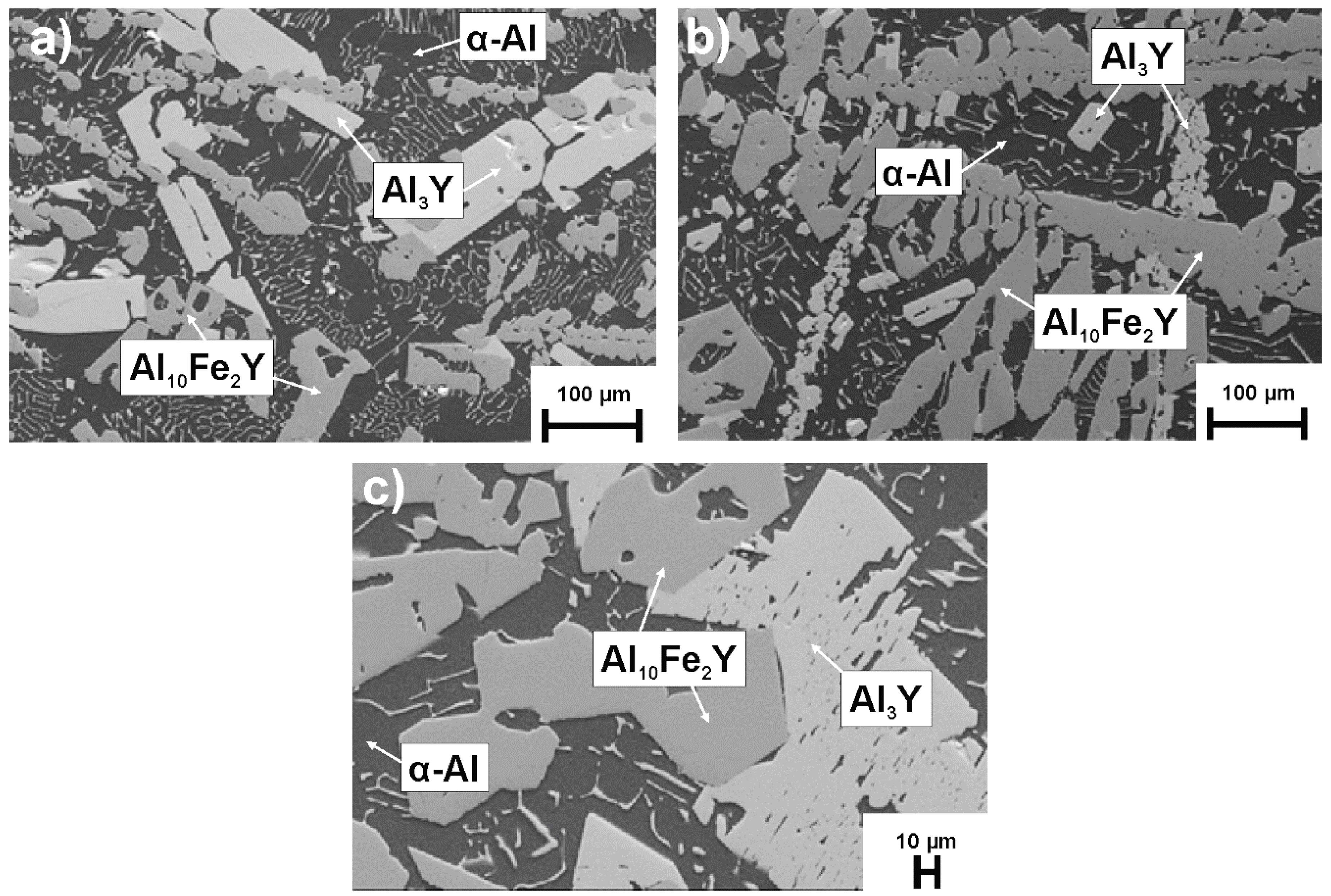
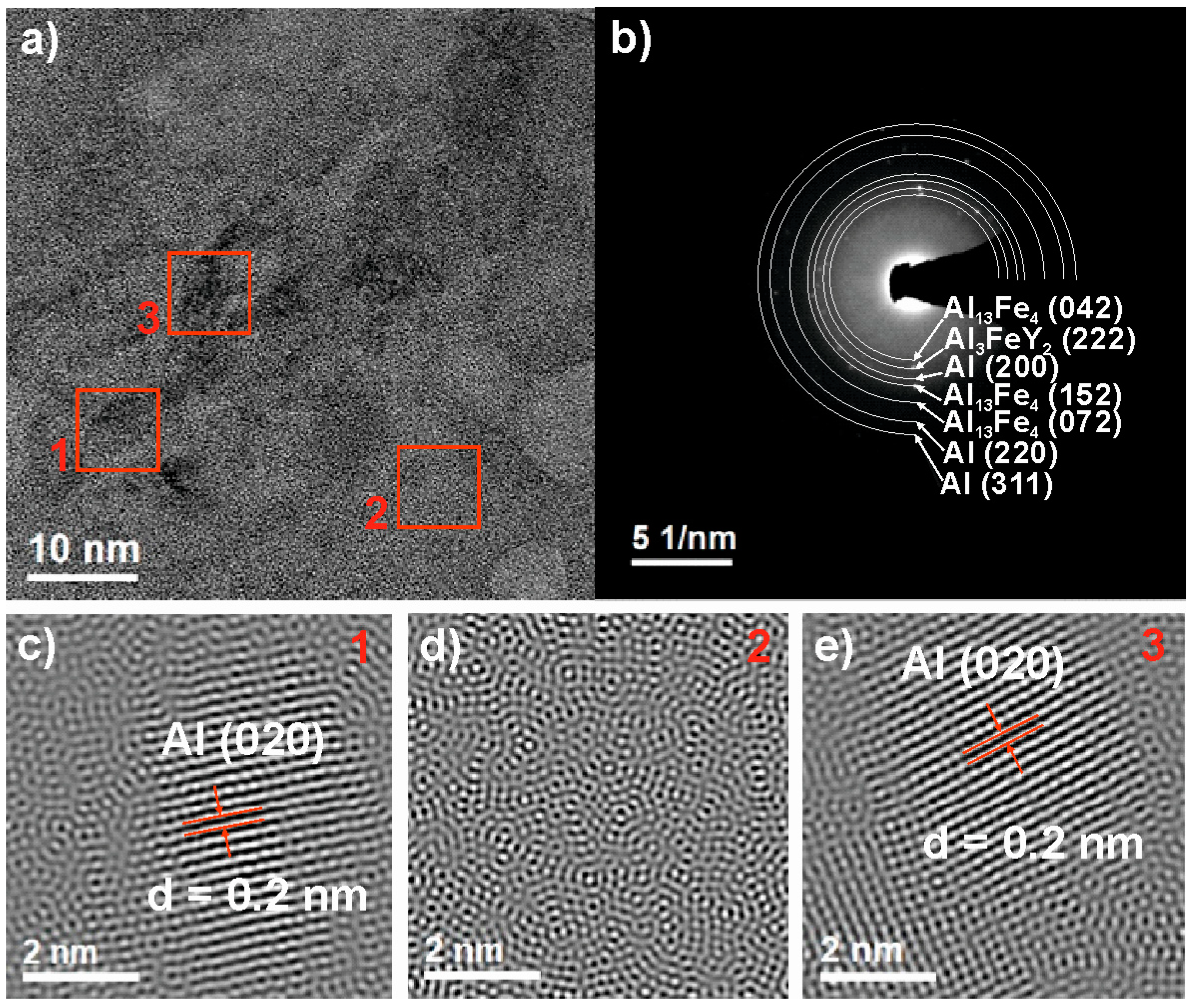
3.1. Structural Analysis
3.2. Corrosion Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Qu, R.; Scudino, S.; A Sun, B.; Prashanth, K.G.; Louzguine-Luzgin, D.V.; Chen, M.W.; Zhang, Z.F.; Eckert, J. Hybrid nanostructured aluminum alloy with super-high strength. NPG Asia Mater. 2015, 7, e229. [Google Scholar] [CrossRef]
- Perepezko, J.; Hebert, R.; Tong, W. Amorphization and nanostructure synthesis in Al alloys. Intermetallics 2002, 10, 1079–1088. [Google Scholar] [CrossRef]
- Saksl, K.; Jóvári, P.; Franz, H.; Jiang, J.Z. Atomic structure of Al88Y7Fe5 metallic glass. J. Appl. Phys. 2005, 97, 113507. [Google Scholar] [CrossRef]
- Sweitzer, J.E.; Scully, J.R.; Bley, R.A.; Hsu, J.W.P. Nanocrystalline Al87Ni8.7Y4.3 and Al90Fe5Gd5 Alloys that Retain the Localized Corrosion Resistance of the Amorphous State. Electrochem. Solid-State Lett. 1999, 2, 267–270. [Google Scholar] [CrossRef]
- Fan, C.; Yue, X.; Inoue, A.; Liu, C.-T.; Shen, X.; Liaw, P.K. Recent Topics on the Structure and Crystallization of Al-based Glassy Alloys. Mater. Res. 2019, 22, 1–15. [Google Scholar] [CrossRef]
- De Oliveira, L.A.; De Oliveira, M.C.L.; Ríos, C.T.; Antunes, R.A. Corrosion of Al85Ni9Ce6 amorphous alloy in the first hours of immersion in 3.5-wt% NaCl solution: The role of surface chemistry. Surf. Interface Anal. 2019, 52, 50–62. [Google Scholar] [CrossRef]
- Yin, P.J. Effect of Au Addition on Intermetallics Precipitation Tendency and Repassivation of Al88Fe5Y7 Glassy Alloy. Int. J. Electrochem. Sci. 2017, 12, 1288–1305. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Louzguine-Luzgin, D. Aluminum-base amorphous and nanocrystalline materials. Met. Sci. Heat Treat. 2012, 53, 472–477. [Google Scholar] [CrossRef]
- Wu, N.; Lian, J.; Wang, R.; Li, R.; Liu, W. Effect of element types on the glass forming ability of Al-TM-RE ternary metallic glasses using electron structure guiding. J. Alloys Compd. 2017, 723, 123–128. [Google Scholar] [CrossRef]
- Dunlap, R.; Lawther, D.; Beydaghyan, G.; McHenry, M.; Srinivas, V. Mössbauer Effect Studies of Amorphous Al-Fe-Rare Earth Alloys. Key Eng. Mater. 1993, 81–83, 413–418. [Google Scholar] [CrossRef]
- Yang, H.; Tan, M.-J.; Li, R.; Wang, J. Effect of Minor V Addition on Al88Y7Fe5 Amorphous Alloys. Appl. Mech. Mater. 2013, 302, 76–81. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Imhoff, S.; Chen, N.; Louzguine-Luzgin, D.; Takeuchi, A.; Chen, M.; Kato, H.; Perepezko, J.; Inoue, A. Enhance the thermal stability and glass forming ability of Al-based metallic glass by Ca minor-alloying. Intermetallics 2012, 29, 35–40. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Li, Y.; An, B.; Cao, G.; Jin, S.; Sun, Y.; Wang, W. Crystallization of Al-based Amorphous Alloys in Good Conductivity Solution. J. Mater. Sci. Technol. 2014, 30, 1262–1270. [Google Scholar] [CrossRef]
- Shen, Y.; Perepezko, J. Al-based amorphous alloys: Glass-forming ability, crystallization behavior and effects of minor alloying additions. J. Alloys Compd. 2017, 707, 3–11. [Google Scholar] [CrossRef]
- Shen, Y.; Perepezko, J. The effect of minor addition of insoluble elements on transformation kinetics in amorphous Al alloys. J. Alloys Compd. 2015, 643, S260–S264. [Google Scholar] [CrossRef]
- Presuel-Moreno, F.; Jakab, M.; Tailleart, N.; Goldman, M.; Scully, J. Corrosion-resistant metallic coatings. Mater. Today 2008, 11, 14–23. [Google Scholar] [CrossRef]
- Engin, S.; Büyük, U.; Maraşlı, N. The effects of microstructure and growth rate on microhardness, tensile strength, and electrical resistivity for directionally solidified Al–Ni–Fe alloys. J. Alloys Compd. 2016, 660, 23–31. [Google Scholar] [CrossRef]
- Mika, T.; Karolus, M.; Boichyshyn, L.; Haneczok, G.; Kotur, B.; Nosenko, V. Crystallization of Al87Y5Ni8 amorphous alloys doped with Dy and Fe. Chem. Met. Alloy. 2012, 5, 50–58. [Google Scholar] [CrossRef]
- Sahu, K.; Mauro, N.; Longstreth-Spoor, L.; Saha, D.; Nussinov, Z.; Miller, M.; Kelton, K. Phase separation mediated devitrification of Al88Y7Fe5 glasses. Acta Mater. 2010, 58, 4199–4206. [Google Scholar] [CrossRef]
- Bondi, K.; Gangopadhyay, A.; Marine, Z.; Kim, T.; Mukhopadhyay, A.; Goldman, A.; Buhro, W.E.; Kelton, K. Effects of microalloying with 3d transition metals on glass formation in AlYFe alloys. J. Non-Cryst. Solids 2007, 353, 4723–4731. [Google Scholar] [CrossRef]
- Gao, M.; Perepezko, J. Flash DSC determination of the delay time for primary crystallization and minor alloying effect in marginal Al-based metallic glasses. Thermochim. Acta 2019, 677, 91–98. [Google Scholar] [CrossRef]
- Xiong, X.; Yi, J.; Kong, L.; Jiang, Z.; Huang, Y.; Li, J. The atomic packing structure of Al-(TM)-Y metallic glasses. Intermetallics 2019, 111, 106505. [Google Scholar] [CrossRef]
- Sadoc, A.; Sabra, M.; Proux, O.; Hazemann, J.-L.; Bondi, K.; Kelton, K. Zr and Hf microalloying in an Al–Y–Fe amorphous alloy. Relation between local structure and glass-forming ability. Philos. Mag. 2008, 88, 2569–2582. [Google Scholar] [CrossRef]
- Babilas, R.; Łoński, W.; Młynarek, K.; Bajorek, A.; Radoń, A. Relationship between the Thermodynamic Parameters, Structure, and Anticorrosion Properties of Al-Zr-Ni-Fe-Y Alloys. Met. Mater. Trans. A 2020, 51, 4215–4227. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, M.; Chen, H.; Liang, J.; Tao, X.; Ouyang, Y.; Du, Y. Experimental Investigation on Phase Equilibria of Al-Fe-Y System at 773 K. J. Phase Equilibria Diffus. 2014, 35, 256–261. [Google Scholar] [CrossRef]
- Yang, H.; Luo, L.; Shen, Y.; Li, C.; Li, R. Glass forming ability and thermal stability of Al-Y-Fe amorphous alloys. J. Shenyang Univ. Technol. 2014, 36, 498–502. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, O.T.H.; Dudina, D.V.; Le, V.V.; Kim, J.-S. Crystallization Kinetics of Al-Fe and Al-Fe-Y Amorphous Alloys Produced by Mechanical Milling. J. Nanomater. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Hebert, R.; Perepezko, J. Effect of Intense Rolling and Folding on the Phase Stability of Amorphous Al-Y-Fe Alloys. Met. Mater. Trans. A 2007, 39, 1804–1811. [Google Scholar] [CrossRef]
- Inoue, A.; Kong, F.; Zhu, S.; Liu, C.T.; Al-Marzouki, F. Development and Applications of Highly Functional Al-based Materials by Use of Metastable Phases. Mater. Res. 2015, 18, 1414–1425. [Google Scholar] [CrossRef]
- Foley, J.; Perepezko, J. The devitrification of Al-Y-Fe amorphous alloys. J. Non-Cryst. Solids 1996, 205–207, 559–562. [Google Scholar] [CrossRef]
- Waerenborgh, J.C.; Salamakha, P.; Sologub, O.; Sério, S.; Godinho, M.; Gonçalves, A.P.; Almeida, M. Y-Fe-Al ternary system: Partial isothermal section at 1070 K: Powder X-ray diffraction and Mössbauer spectroscopy study. J. Alloys Compd. 2001, 323–324, 78–82. [Google Scholar] [CrossRef]
- Sitek, J.; Degmová, J. Aluminium-based amorphous and nanocrystalline alloys with Fe impurity. Czechoslov. J. Phys. 2006, 56, E17–E22. [Google Scholar] [CrossRef]
- Dunlap, R.; Yewondwossen, M.; Lawther, D. Mössbauer effect studies of amorphous Al-Y-Ni-Fe alloys. J. Non-Cryst. Solids 1993, 156–158, 192–195. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Kirtay, S. Improvement of Oxidation Resistance of Mild Steel by SiO2-Al2O3 Sol Gel Coating. Acta Phys. Pol. A 2015, 128, 90–92. [Google Scholar] [CrossRef]
- Jinhong, P.; Ye, P.; Jili, W.; Xiancong, H. Influence of Minor Addition of in on Corrosion Resistance of Cu-Based Bulk Metallic Glasses in 3.5% NaCl Solution. Rare Met. Mater. Eng. 2014, 43, 32–35. [Google Scholar] [CrossRef]
- Lin, J.; Xu, J.; Wang, W.; Li, W. Electrochemical behavior of partially crystallized amorphous Al86Ni9La5 alloys. Mater. Sci. Eng. B 2011, 176, 49–52. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Ma, A.; Hu, H.; Zheng, Y.; Yang, B.; Wang, J. Thermally induced structure evolution on the corrosion behavior of Al-Ni-Y amorphous alloys. Corros. Sci. 2018, 144, 172–183. [Google Scholar] [CrossRef]
- Seikh, A.H.; Baig, M.; Singh, J.K.; Mohammed, J.A.; Luqman, M.; Abdo, H.S.; Khan, A.R.; Alharthi, N.H. Microstructural and Corrosion Characteristics of Al-Fe Alloys Produced by High-Frequency Induction-Sintering Process. Coatings 2019, 9, 686. [Google Scholar] [CrossRef]

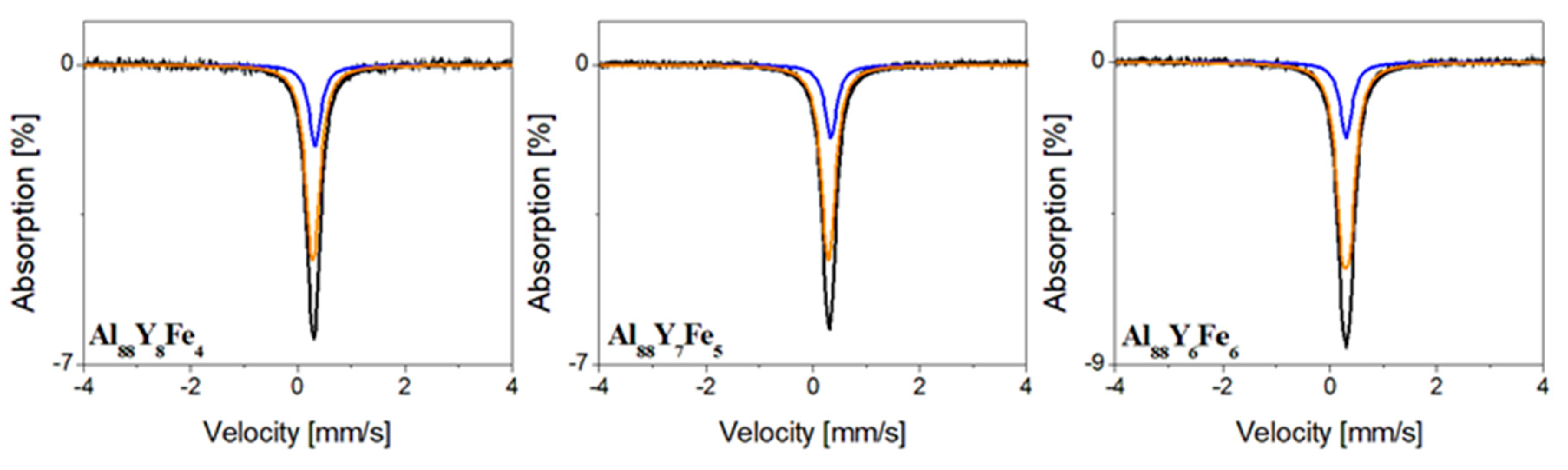
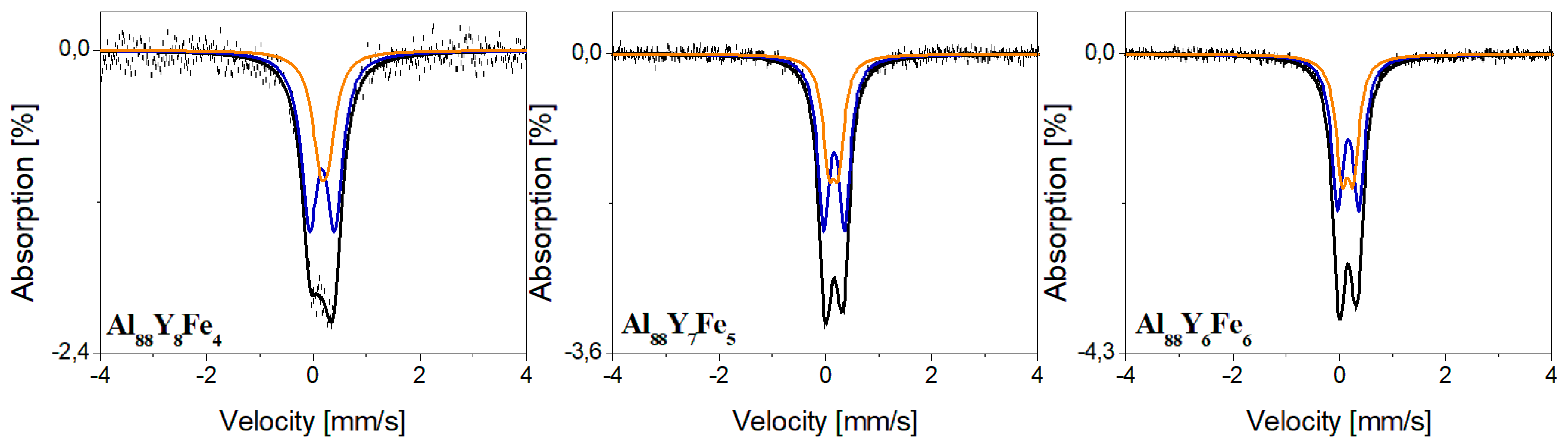

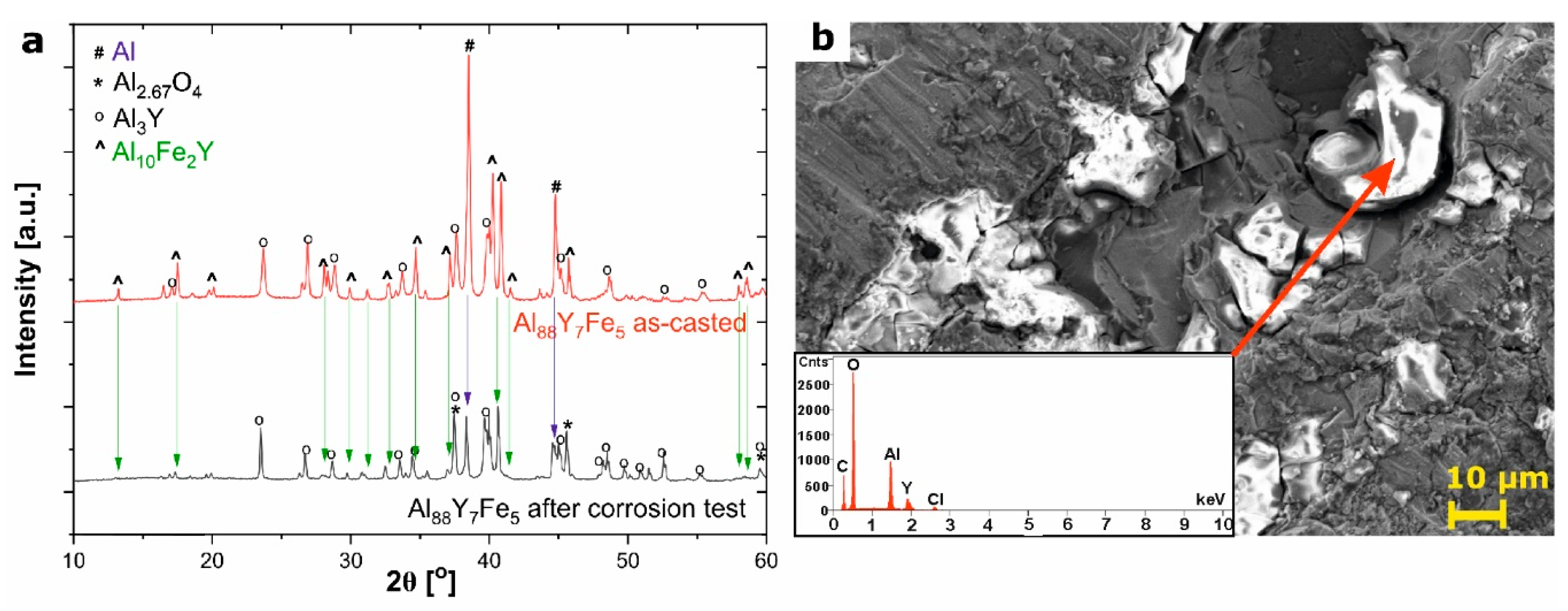
| Component | IS (mm/s) | QS (mm/s) | FWHM (mm/s) | A (%) | Compound |
|---|---|---|---|---|---|
| Al88Y6Fe6 | |||||
| Singlet | 0.31 | - | 0.27 | 22 | Al(Fe) |
| Doublet | 0.30 | 0.15 | 78 | Al10Fe2Y [32] | |
| Al88Y7Fe5 | |||||
| Singlet | 0.34 | - | 0.27 | 22 | Al(Fe) |
| Doublet | 0.29 | 0.09 | 78 | Al10Fe2Y [32] | |
| Al88Y8Fe4 | |||||
| Singlet | 0.34 | - | 0.27 | 26 | Al(Fe) |
| Doublet | 0.29 | 0.10 | 74 | Al10Fe2Y [32] | |
| Component | IS (mm/s) | QS (mm/s) | FWHM (mm/s) | A (%) | Compound |
|---|---|---|---|---|---|
| Al88Y6Fe6 | |||||
| Doublet 1 | 0.17 | 0.40 | 0.27 | 58 | Al(Fe,Y) |
| Doublet 2 | 0.15 | 0.22 | 42 | Al(Fe) | |
| Al88Y7Fe5 | |||||
| Doublet 1 | 0.16 | 0.39 | 0.27 | 63 | Al(Fe,Y) |
| Doublet 2 | 0.15 | 0.18 | 37 | Al(Fe) | |
| Al88Y8Fe4 | |||||
| Doublet 1 | 0.17 | 0.47 | 0.36 | 67 | Al(Fe,Y) |
| Doublet 2 | 0.20 | 0.16 | 33 | Al(Fe) | |
| Alloy | EOCP (Mv) | Ecorr (mV) | |βa| (mV/dec) | |βc| (mV/dec) | Rp (kΩ∙cm2) | jcorr (μA/cm2) |
|---|---|---|---|---|---|---|
| Ingots | ||||||
| Al88Y8Fe4 | −888 | −921 | 167 | 219 | 15.2 | 2.70 |
| Al88Y7Fe5 | −1041 | −987 | 62 | 87 | 7.9 | 1.99 |
| Al88Y6Fe6 | −732 | −707 | 76 | 10 | 1.4 | 2.67 |
| Ribbons | ||||||
| Al88Y8Fe4 | −779 | −653 | 242 | 4 | 12.2 | 0.14 |
| Al88Y7Fe5 | −731 | −691 | 197 | 23 | 96.7 | 0.09 |
| Al88Y6Fe6 | −729 | −648 | 7 | 11 | 14.9 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babilas, R.; Spilka, M.; Młynarek, K.; Łoński, W.; Łukowiec, D.; Radoń, A.; Kądziołka-Gaweł, M.; Gębara, P. Glass-Forming Ability and Corrosion Resistance of Al88Y8−xFe4+x (x = 0, 1, 2 at.%) Alloys. Materials 2021, 14, 1581. https://doi.org/10.3390/ma14071581
Babilas R, Spilka M, Młynarek K, Łoński W, Łukowiec D, Radoń A, Kądziołka-Gaweł M, Gębara P. Glass-Forming Ability and Corrosion Resistance of Al88Y8−xFe4+x (x = 0, 1, 2 at.%) Alloys. Materials. 2021; 14(7):1581. https://doi.org/10.3390/ma14071581
Chicago/Turabian StyleBabilas, Rafał, Monika Spilka, Katarzyna Młynarek, Wojciech Łoński, Dariusz Łukowiec, Adrian Radoń, Mariola Kądziołka-Gaweł, and Piotr Gębara. 2021. "Glass-Forming Ability and Corrosion Resistance of Al88Y8−xFe4+x (x = 0, 1, 2 at.%) Alloys" Materials 14, no. 7: 1581. https://doi.org/10.3390/ma14071581
APA StyleBabilas, R., Spilka, M., Młynarek, K., Łoński, W., Łukowiec, D., Radoń, A., Kądziołka-Gaweł, M., & Gębara, P. (2021). Glass-Forming Ability and Corrosion Resistance of Al88Y8−xFe4+x (x = 0, 1, 2 at.%) Alloys. Materials, 14(7), 1581. https://doi.org/10.3390/ma14071581







