Effect of Wood Biomass Ash Storage on the Properties of Cement Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
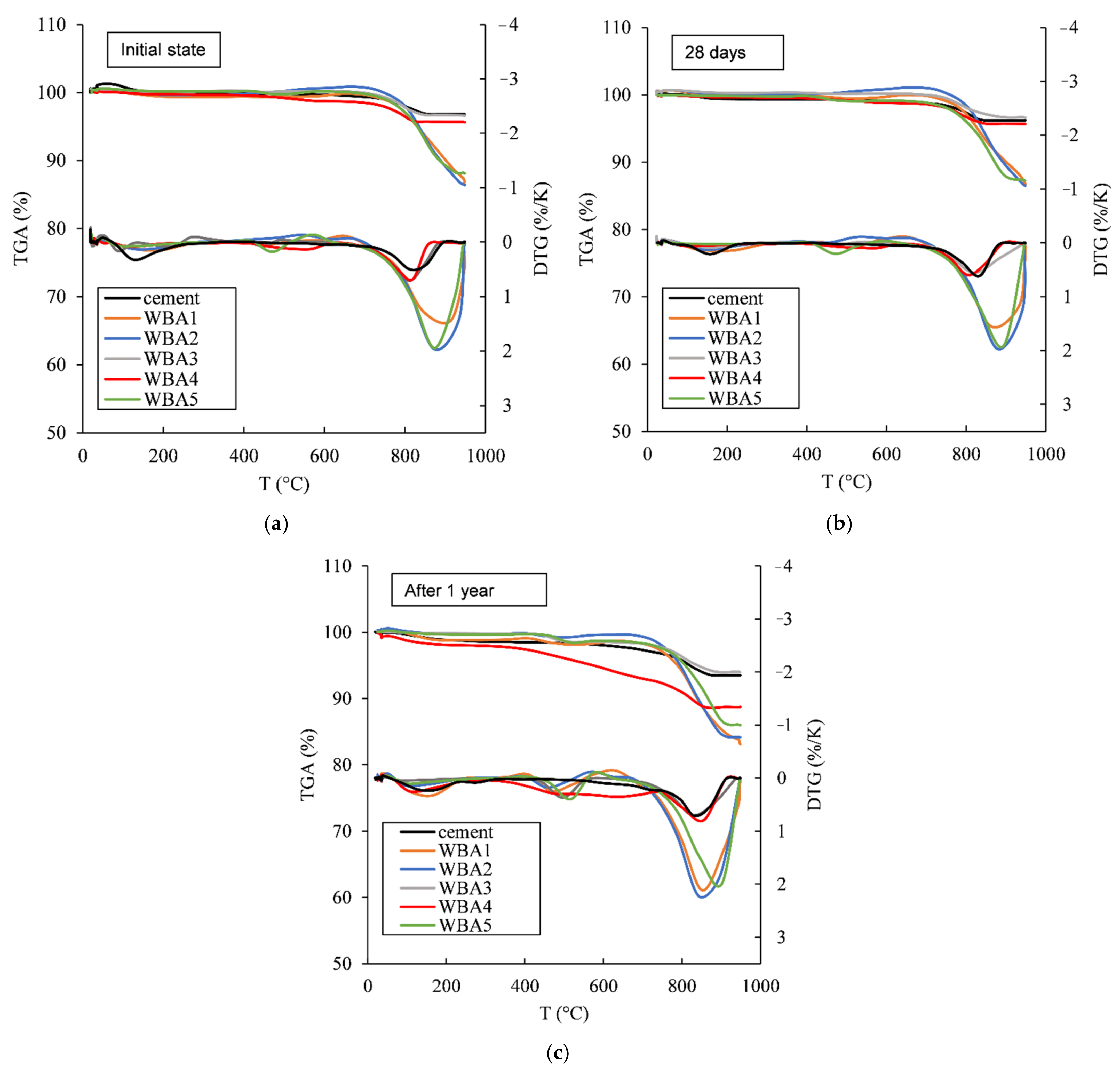
2.2. Thermogravimetric Measurement
- ∆m—mass loss between 35 to 950 °C, or mass loss between 35 to 600 °C;
- ∆m0—mass loss between 35 to 950 °C or mass loss between 35 to 600 °C for sample in the initial state;
- mi—mass of sample at 950 °C or mass at 600 °C;
- m0—mass of sample at 950 °C or mass at 600 °C in initial state.
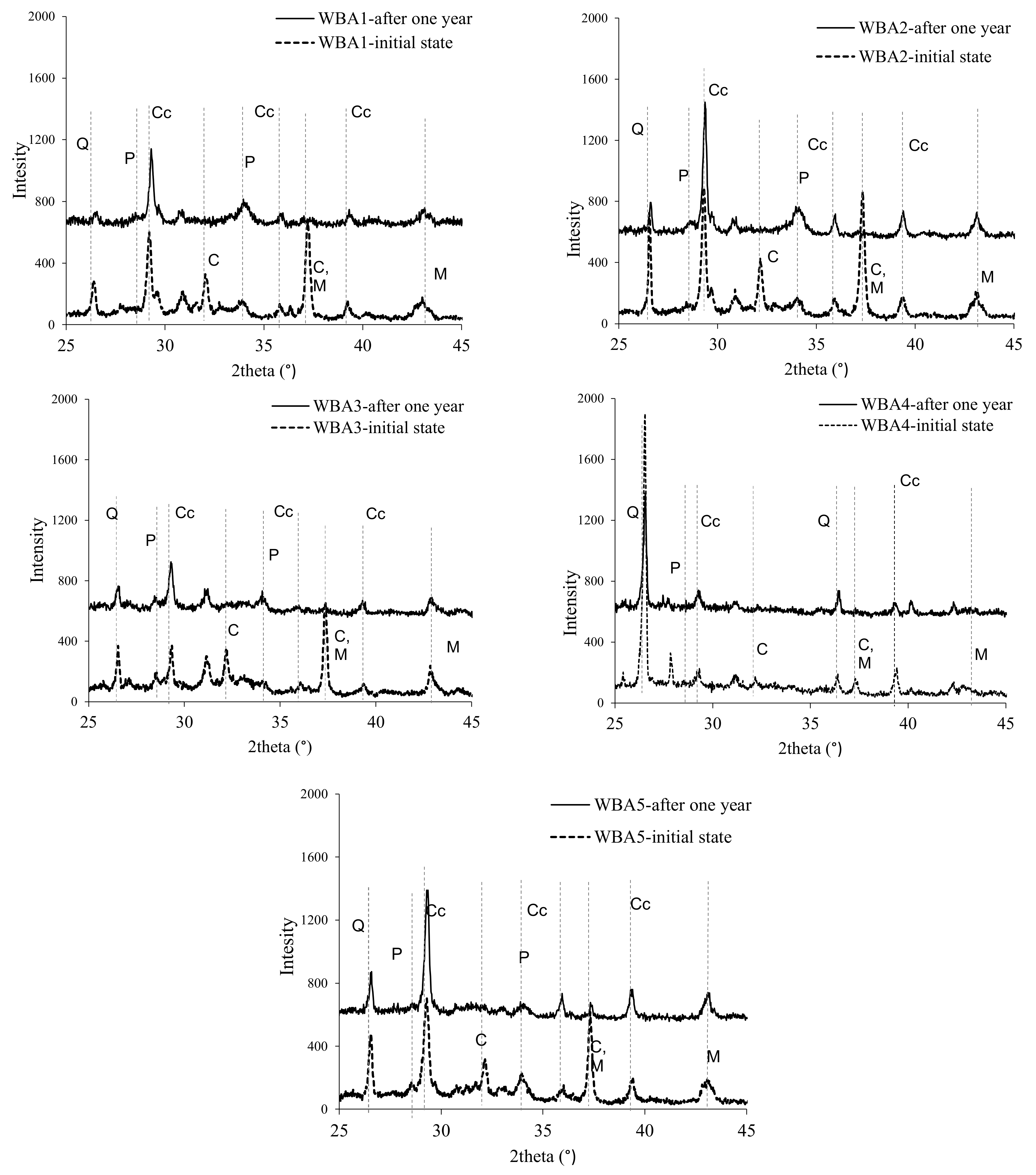
2.3. XRD Analysis
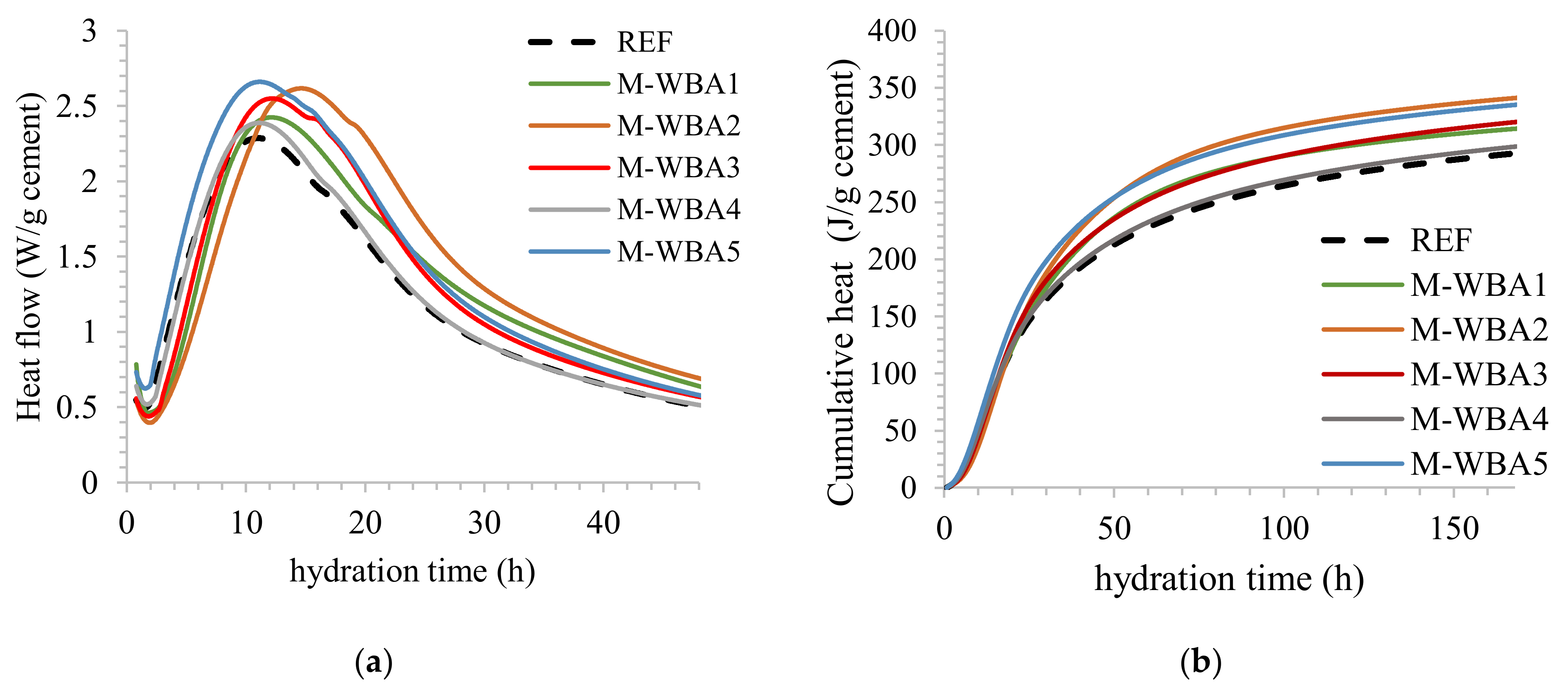
2.4. Isothermal Calorimetry
2.5. Mix Design
3. Results and Discussion
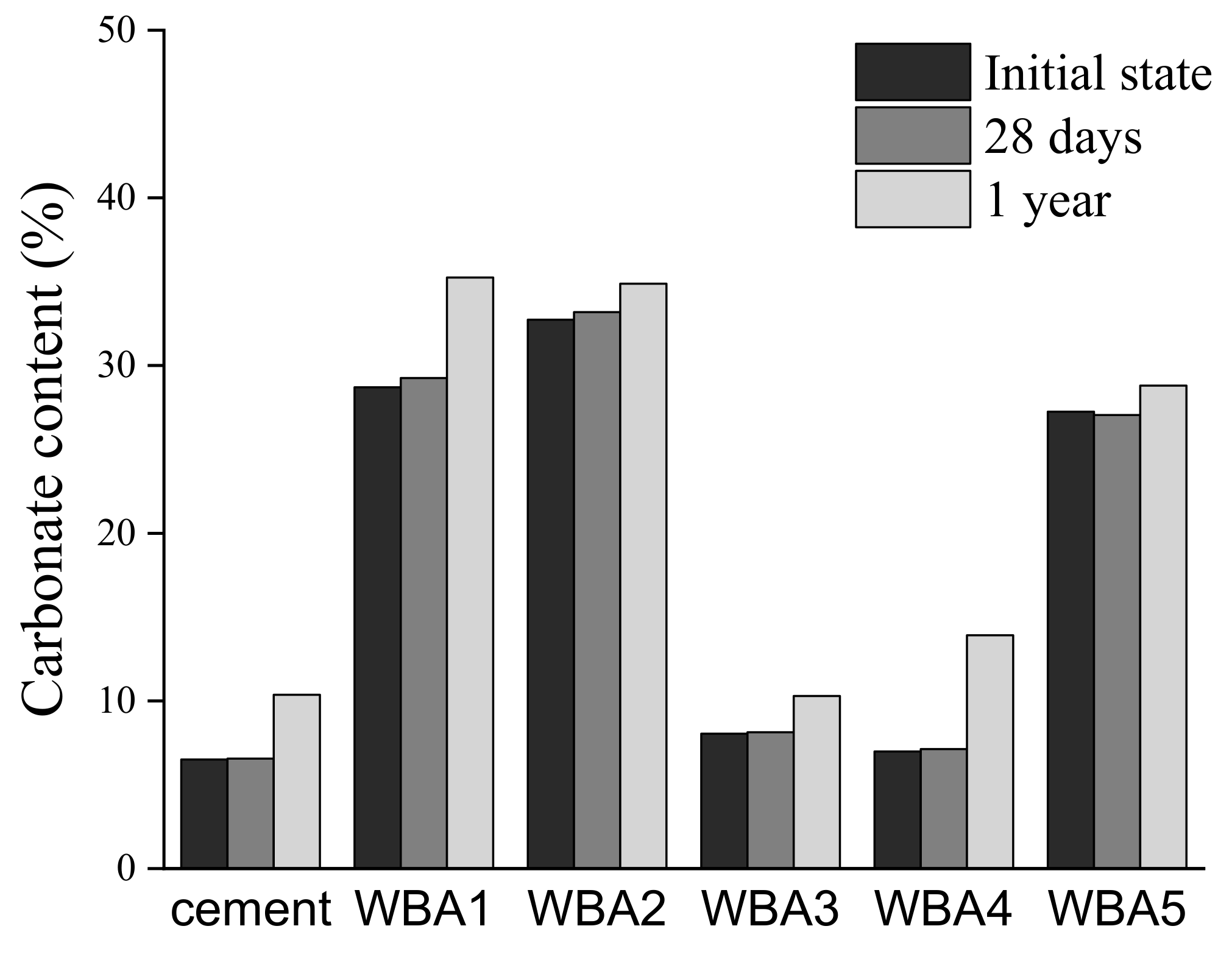
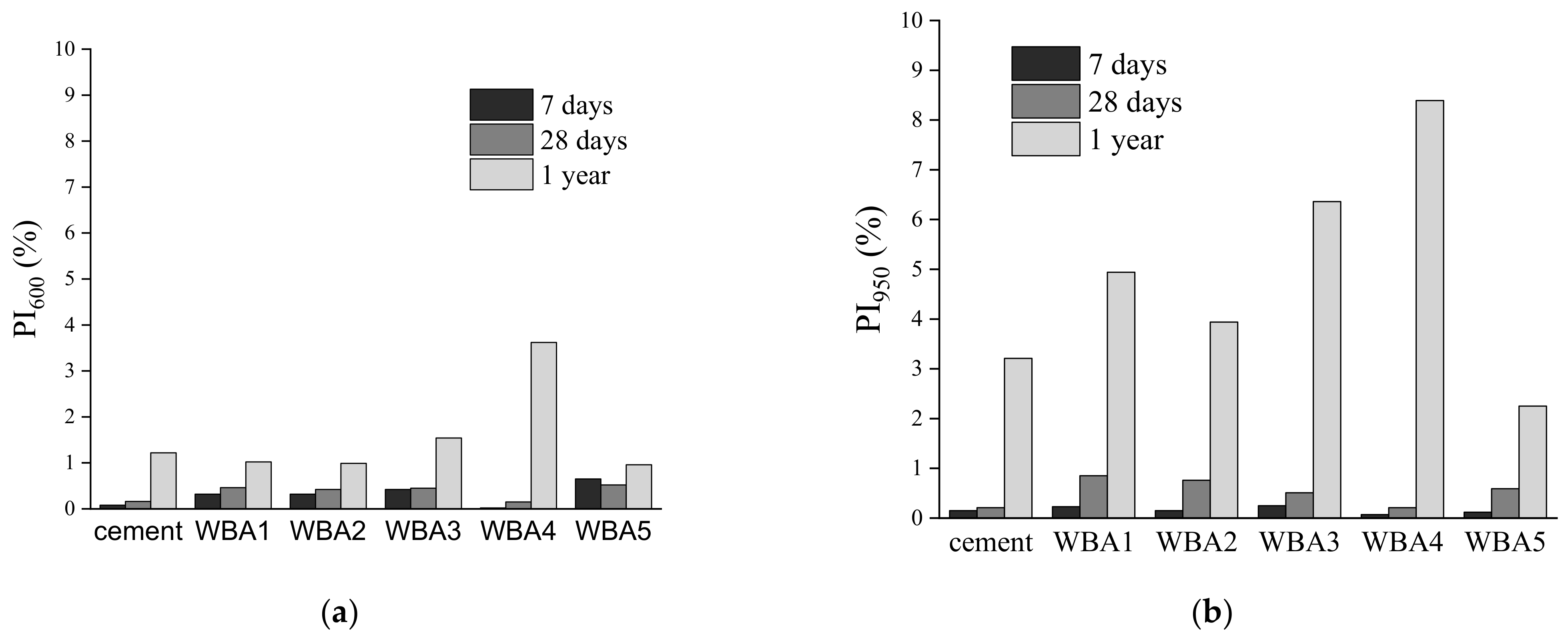
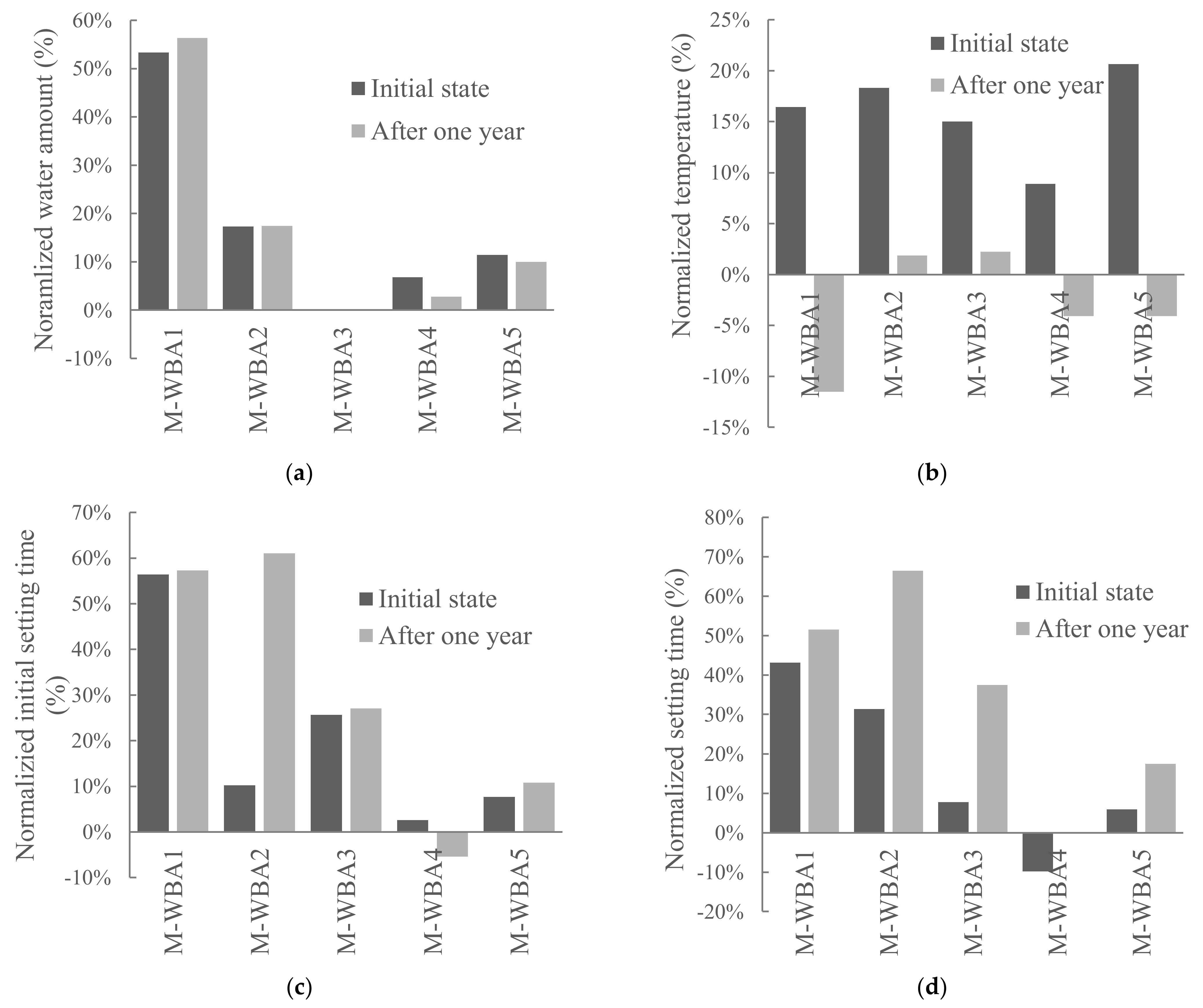
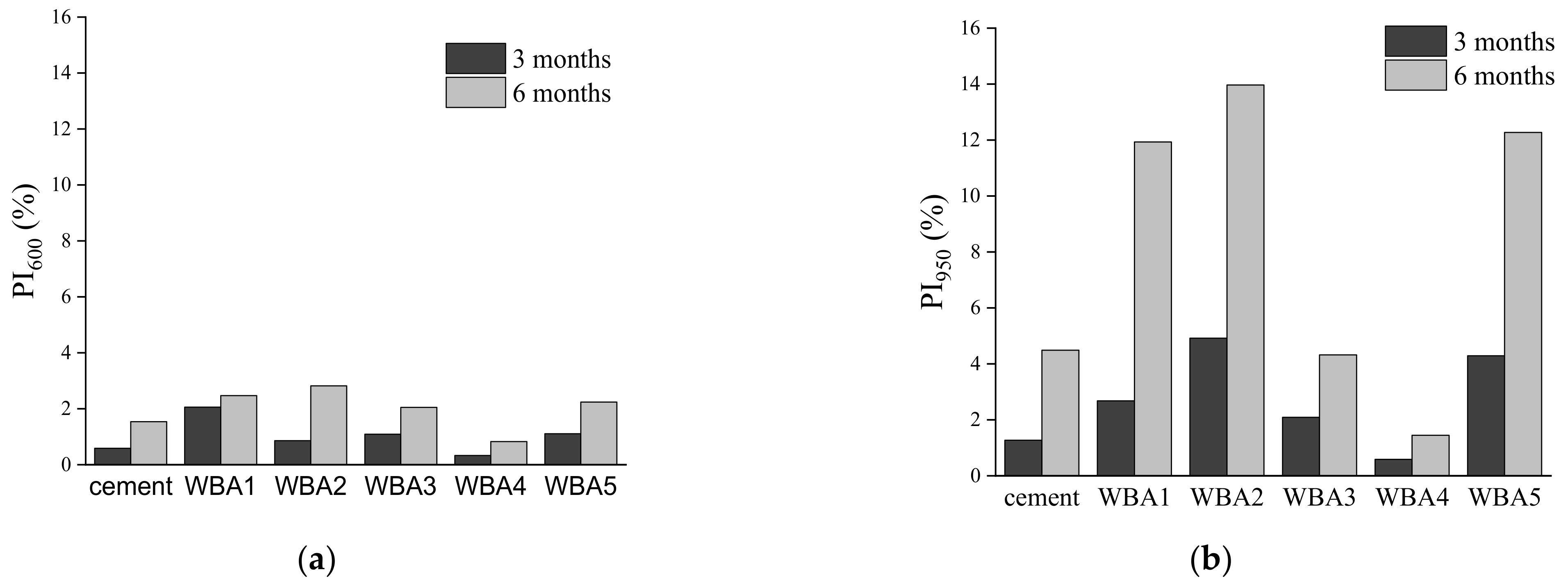
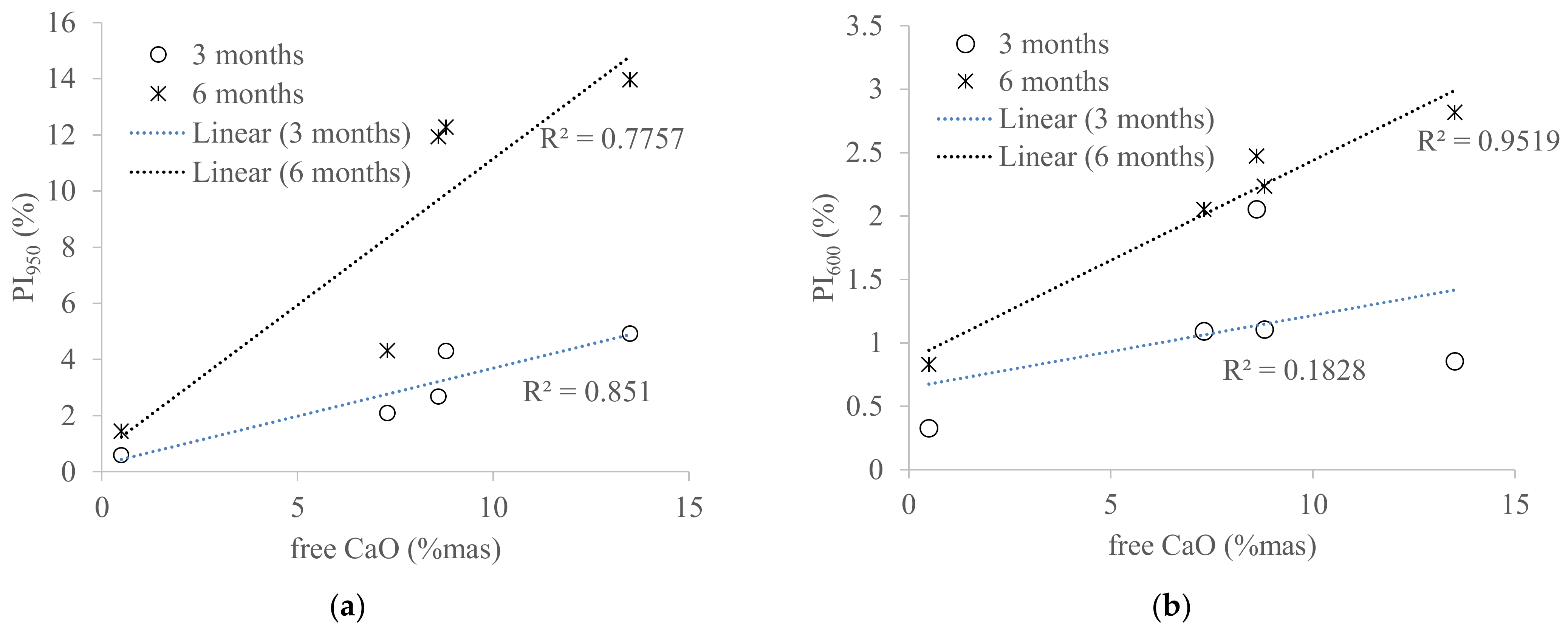
3.1. Aging of WBA Samples Stored in Closed Containers
3.2. Aging of WBA Samples Stored in Opened Containers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderón, C.; Gauthier, G.; Jossart, J.-M. AEBIOM Statistical Report; European Bioenergy Outlook: Brussels, Belgium, 2016. [Google Scholar]
- Eurostat Statistics Explained Renewable Energy Statistics. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Renewable_energy_statistics (accessed on 1 July 2017).
- European Statistics Energy from Renewable Sources. Available online: https://ec.europa.eu/eurostat/web/energy/data/shares (accessed on 15 May 2020).
- Sikkema, R.; Proskurina, S.; Banja, M.; Vakkilainen, E. How can solid biomass contribute to the EU’s renewable energy targets in 2020, 2030 and what are the GHG drivers and safeguards in energy- and forestry sectors? Renew. Energy 2021, 165, 758–772. [Google Scholar] [CrossRef]
- Obernberger, I.; Supancic, K. Possibilities of Ash Utilisation from Biomass Combustion Plants. In Proceedings of the 17th European Biomass Conference & Exhibition, Hamburg, Germany, 29 June–3 July 2009. [Google Scholar]
- Doudart de la Grée, G.C.H.; Florea, M.V.A.; Keulen, A.; Brouwers, H.J.H. Contaminated biomass fly ashes—Characterization and treatment optimization for reuse as building materials. Waste Manag. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Carević, I.; Serdar, M.; Štirmer, N.; Ukrainczyk, N. Preliminary screening of wood biomass ashes for partial resources replacements in cementitious materials. J. Clean. Prod. 2019, 229, 1045–1064. [Google Scholar] [CrossRef]
- Sigvardsen, N.M.; Kirkelund, G.M.; Jensen, P.E.; Geiker, M.R.; Ottosen, L.M. Impact of production parameters on physiochemical characteristics of wood ash for possible utilisation in cement-based materials. Resour. Conserv. Recycl. 2019, 145, 230–240. [Google Scholar] [CrossRef]
- Zagvozda, M.; Dimter, S.; Rukavina, T.; Grubeša, I.N. Possibilities of bioash application in road building. Građevinar 2018, 70, 393–402. [Google Scholar]
- Ukrainczyk, N.; Koenders, E.A.B.; Stirmer, N. Transformation of Wood Ash Waste into Construction Materials. In Proceedings of the 1st International Conference on Construction Materials for Sustainable Future, Zadar, Croatia, 19–21 April 2017; pp. 101–108. [Google Scholar]
- Omran, A.; Soliman, N.; Xie, A.; Davidenko, T.; Tagnit-hamou, A. Field trials with concrete incorporating biomass-fly ash. Constr. Build. Mater. 2018, 186, 660–669. [Google Scholar] [CrossRef]
- Biedermann, F.; Obernberger, I. Ash-related Problems during Biomass Combustion and Possibilities for a Sustainable Ash Utilisation. In Proceedings of the International Conference “World Renewable Energy Congress” (WREC), Birmingham, UK, 28 February–2 March 2005; Elsevier BV: Oxford, UK, 2005; pp. 120–124. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash.: Part 2. Potential utilisation, technological and ecological advantages and challenges. Fuel 2013, 105, 19–39. [Google Scholar] [CrossRef]
- Jensen, O.M.; Hansen, P.F.; Lachowski, E.E.; Glasser, F.P. Clinker mineral hydration at reduced relative humidities. Cem. Concr. Res. 1999, 29, 1505–1512. [Google Scholar] [CrossRef]
- Dubina, E.; Korat, L.; Black, L.; Strupi-šuput, J.; Plank, J. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Influence of water vapour and carbon dioxide on free lime during storage at 80 °C, studied by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 111, 299–303. [Google Scholar] [CrossRef]
- Getachew, B.; Adugna, T.; Assefa, S. Investigation on the effect of storage time dueto moisture absorption of cement on the properties of the concrete. Int. J. Dev. Res. 2018, 8, 18558–18561. [Google Scholar]
- Whittaker, M.; Dubina, E.; Arkless, L.; Plank, J.; Black, L. The Effects of Prehydration on Cement Performance. In Proceedings of the 30th Cement and Concrete Science Conference, Birmingham, UK, 13–15 September 2010. [Google Scholar]
- Ramge, P.; Schmidt, W. Effect of the storage of cement on early properties of cementitious systems. In Proceedings of the International Conference on Advances in Cement and Concrete Technology in Africa, Johannesburg, South Africa, 28–30 January 2013. [Google Scholar]
- Whittaker, M.; Al-Mutawa, F.; Black, L.; Plank, J.; Dubina, E.; Arkless, L. The effect of prehydration on the engineering properties of CEM I Portland cement. Adv. Cem. Res. 2013, 25. [Google Scholar] [CrossRef] [Green Version]
- Winnefeld, F. Influence of cement ageing and addition time on the performance of superplasticizers. ZKG Int. 2008, 61, 68–77. [Google Scholar]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials, 1st ed.; CRC Press: Boca Ranton, FL, USA, 2017; ISBN 9781138747234. [Google Scholar]
- Akinyemi, S.A.; Akinlua, A.; Gitari, W.M.; Khuse, N.; Eze, P.; Akinyeye, R.O.; Petrik, L.F. Natural weathering in dry disposed ash dump: Insight from chemical, mineralogical and geochemical analysis of fresh and unsaturated drilled cores. J. Environ. Manag. 2012, 102, 96–107. [Google Scholar] [CrossRef]
- Gori, M.; Bergfeldt, B.; Pfrang-stotz, G.; Reichelt, J.; Sirini, P. Effect of short-term natural weathering on MSWI and wood waste bottom ash leaching behaviour. J. Hazard. Mater. 2011, 189, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Saffarzadeh, A.; Shimaoka, T.; Wei, Y.; Gardner, K.H.; Musselman, C.N. Impacts of natural weathering on the transformation / neoformation processes in landfilled MSWI bottom ash: A geoenvironmental perspective. Waste Manag. 2011, 31, 2440–2454. [Google Scholar] [CrossRef] [PubMed]
- Meima, J.A.; Comans, R.N.J. The leaching of trace elements from municipal solid waste incinerator bottom ash at different stages of weathering. Appl. Geochem. 1999, 14, 159–171. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R. The leaching behavior of incinerator bottom ash as affected by accelerated ageing. J. Hazard. Mater. 2004, 113, 209–215. [Google Scholar] [CrossRef]
- Steenari, B.M.; Lindqvist, O. Stabilisation of biofuel ashes for recycling to forest soil. Biomass-Bioenergy 1997, 13, 39–50. [Google Scholar] [CrossRef]
- Supancic, K.; Obernberger, I.; Kienzl, N.; Arich, A. Conversion and leaching characteristics of biomass ashes during outdoor storage—Results of laboratory tests. Biomass-Bioenergy 2014, 61, 211–226. [Google Scholar] [CrossRef]
- Steenari, B.; Karlsson, L.G.; Lindqvist, O. Evaluation of the leaching characteristics of wood ash and the influence of ash agglomeration. Biomass-Bioenergy 1999, 16, 119–136. [Google Scholar] [CrossRef]
- Karltun, E.; Saarsalmi, A.; Ingerslev, M.; Mandre, M.; Andersson, S.; Gaitnieks, T.; Ozolinčius, R.; Varnagiryte-Kabasinskiene, I. Wood Ash Recycling—Possibilities and Risks. In Sustainable Use of Forest Biomass for Energy; Managing Forest Ecosystems; Springer: Dordrecht, The Netherlands, 2008; pp. 79–108. [Google Scholar] [CrossRef]
- Ukrainczyk, N. Reuse of Woody Biomass Ash Waste in Cementitious Materials. Chem. Biochem. Eng. Q. 2016, 30, 137–148. [Google Scholar] [CrossRef]
- Ohenoja, K.; Tanskanen, P.; Wigren, V.; Kinnunen, P.; Körkkö, M.; Peltosaari, O.; Österbacka, J.; Illikainen, M. Self-hardening of fly ashes from a bubbling fluidized bed combustion of peat, forest industry residuals, and wastes. Fuel 2016, 165, 440–446. [Google Scholar] [CrossRef]
- Tishmack, J.K.; Olek, J.; Diamond, S. Characterization of High-Calcium Fly Ashes and Their Potential Influence on Ettringite Formation in Cementitious Systems. Cem. Concr. Aggregates 1999, 21, 82–92. [Google Scholar] [CrossRef]
- Holmberg, S.L.; Claesson, T. Mineralogy of granulated wood ash from a heating plant in Kalmar, Sweden. Environ. Earth Sci. 2001, 40, 820–828. [Google Scholar] [CrossRef]
- Milovanović, B.; Štirmer, N.; Carević, I.; Baričević, A. Wood biomass ash as a raw material in concrete industry. J. Croat. Assoc. Civ. 2019, 71, 505–514. [Google Scholar] [CrossRef]
- Carević, I.; Baričević, A.; Štirmer, N.; Bajto Šantek, J. Correlation between physical and chemical properties of wood biomass ash and cement composites performances. Constr. Build. Mater. 2020, 256, 119450. [Google Scholar] [CrossRef]
- Stoian, J.; Oey, T.; Bullard, J.W.; Huang, J.; Kumar, A.; Balonis, M.; Neithalath, N.; Sant, G. The Prehydration of Cement and Its Mitigation. In Proceedings of the 2nd International Congress on Durability of Concrete, New Delhi, India, 4–6 December 2014. [Google Scholar]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Matrix-flushing method for quantitative multicomponent analysis. J. Appl. Crystallogr. 1974, 7, 519–525. [Google Scholar] [CrossRef]
- The European Committee for Standardization. Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness (EN 196-3:2016); The European Committee for Standardization (CEN): Brussels, Belgium, 2016. [Google Scholar]
- Medina, J.M.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I.; Medina, C. Durability of new blended cements additioned with recycled biomass bottom ASH from electric power plants. Constr. Build. Mater. 2019, 225, 429–440. [Google Scholar] [CrossRef]
- Ukrainczyk, N.; Ukrainczyk, M.; Sipusic, J. XRD and TGA investigation of hardened cement paste. In Proceedings of the MATRIB 2006 International Conference on Materials, Tribology, Recycling, Vela Luka, Croatia, 22–24 June 2006. [Google Scholar]
- Deboucha, W.; Leklou, N.; Khelidj, A.; Oudjit, M.N. Hydration development of mineral additives blended cement using thermogravimetric analysis (TGA): Methodology of calculating the degree of hydration. Constr. Build. Mater. 2017, 146, 687–701. [Google Scholar] [CrossRef]
- Misra, M.K.; Ragland, K.W.; Bakert, A.J. Wood ash compostion as a function of furnace temperature. Biomass-Bioenergy 1993, 4, 103–116. [Google Scholar] [CrossRef]
- Straka, P.; Náhunková, J.; Margit, Z. Analysis of unburned carbon in industrial ashes from biomass combustion by thermogravimetric method using Boudouard reaction. Thermochim. Acta 2014, 575, 188–194. [Google Scholar] [CrossRef]
- Mohebbi, M.; Rajabipour, F.; Scheetz, B.E. Evaluation of two-atmosphere thermogravimetric analysis for determining the unburned carbon content in fly ash. Adv. Civ. Eng. Mater. 2017, 6, 258–279. [Google Scholar] [CrossRef]
- Bernal, S.A.; Juenger, M.C.G.; Ke, X.; Matthes, W.; Lothenbach, B.; Belie, N. De Provis, J.L. Characterization of supplementary cementitious materials by thermal analysis. Mater. Struct. 2017, 50, 1–13. [Google Scholar] [CrossRef]
- The European Committee for Standardization. Fly Ash for Concrete—Part 1: Definition, Specifications and Conformity Criteria; British Standards Institution: Lodon, UK, 2012. [Google Scholar]
- Sigvardsen, N.M.; Geiker, M.R.; Ottosen, L.M. Phase development and mechanical response of low-level cement replacements with wood ash and washed wood ash. Constr. Build. Mater. 2020, 269, 121234. [Google Scholar] [CrossRef]
- Chatterji, S. Mechanism of expansion of concrete due to the presence of dead-burnt CaO and MgO. Cem. Concr. Res. 1995, 25, 51–56. [Google Scholar] [CrossRef]
- Arjunan, P.; Kumar, A. Rapid techniques for determination of free lime and free magnesia in cement clinker and portlandite in hydrates. Cem. Concr. Res. 1994, 24, 343–352. [Google Scholar] [CrossRef]
- Pielert, J.; Lamond, J.H. (Eds.) Significance of Tests and Properties of Concrete and Concrete-Making Materials, STP 169D. Lamond; ASTM International: Conshohocken, PA, USA, 2006. [Google Scholar]
- Lagerlund, J. Long Term Effects on Stored Calcium Rich Fly Ash as Relevant to Cement Replacement. In Proceedings of the Ash Utilisation 2012, Ashes in a Sustainable Society, Stockholm, Sweden, 25–27 January 2012. [Google Scholar]
- Janz, M.; Jihansson, S.-E. The Function of Different Binding Agents in Deep Stabilization; Swedish Deep Stabilization Research Centre: Linköping, Sweden, 2002; pp. 1–50. [Google Scholar]
- Tepsri, P.; Chumphu, A.; Yoriya, S. High-calcium fly ash recovery from wet-stored condition and its properties High-calcium fl y ash recovery from wet-stored condition and its properties. Mater. Res. Express 2018, 5, 115506. [Google Scholar] [CrossRef] [Green Version]
- Korat, L. Characterization of the Cement Composities with Mineral Additives (in Slovenian). Ph.D. Thesis, Faculty of Natural Science and Engineering University of Ljubljana, Ljubljana, Slovenia, 2015. [Google Scholar]
- Yeheyis, M.B.; Shang, J.Q.; Yanful, E.K. Chemical and Mineralogical Transformations of Coal Fly Ash after Landfilling. In Proceedings of the 2009 World of Coal Ash (WOCA) Conference, Lexington, KY, USA, 4–7 May 2009; pp. 1–13. [Google Scholar]
- Berra, M.; Mangialardi, T.; Paolini, A.E. Reuse of woody biomass fly ash in cement-based materials. Constr. Build. Mater. 2015, 76, 286–296. [Google Scholar] [CrossRef]
- Rajamma, R.; Senff, L.; Ribeiro, M.J.; Labrincha, J.A.; Ball, R.J.; Allen, G.C.; Ferreira, V.M. Biomass fly ash effect on fresh and hardened state properties of cement based materials. Compos. Part B Eng. 2015, 77, 1–9. [Google Scholar] [CrossRef]
- Skibsted, J.; Snellings, R. Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem. Concr. Res. 2019, 124, 1190–1199. [Google Scholar] [CrossRef]
- Dubina, E. The Phenomenon of Cement Ageing on Moist Air: Surface Chemistry, Mechanisms and Effects on Admixture Performance. Ph.D. Thesis, Technical University of Munich, Munich, Germany, 2013. [Google Scholar]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- Shafigh, P.; Hashemi, M.; Nam, B.H.; Asadi, I.; Lumpur, K.; Shafigh, P.; Hashemi, M.; Nam, B.H.; Asadi, I.; Hashemi, M.; et al. Laboratory comparison of roller-compacted concrete and ordinary vibrated concrete for pavement structures. Građevinar 2020, 72, 127–137. [Google Scholar]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mishra, M.; Suganya, O. The incorporation of wood waste ash as a partial cement replacement material for making structural grade concrete: An overview. Ain Shams Eng. J. 2015, 6, 429–437. [Google Scholar] [CrossRef] [Green Version]
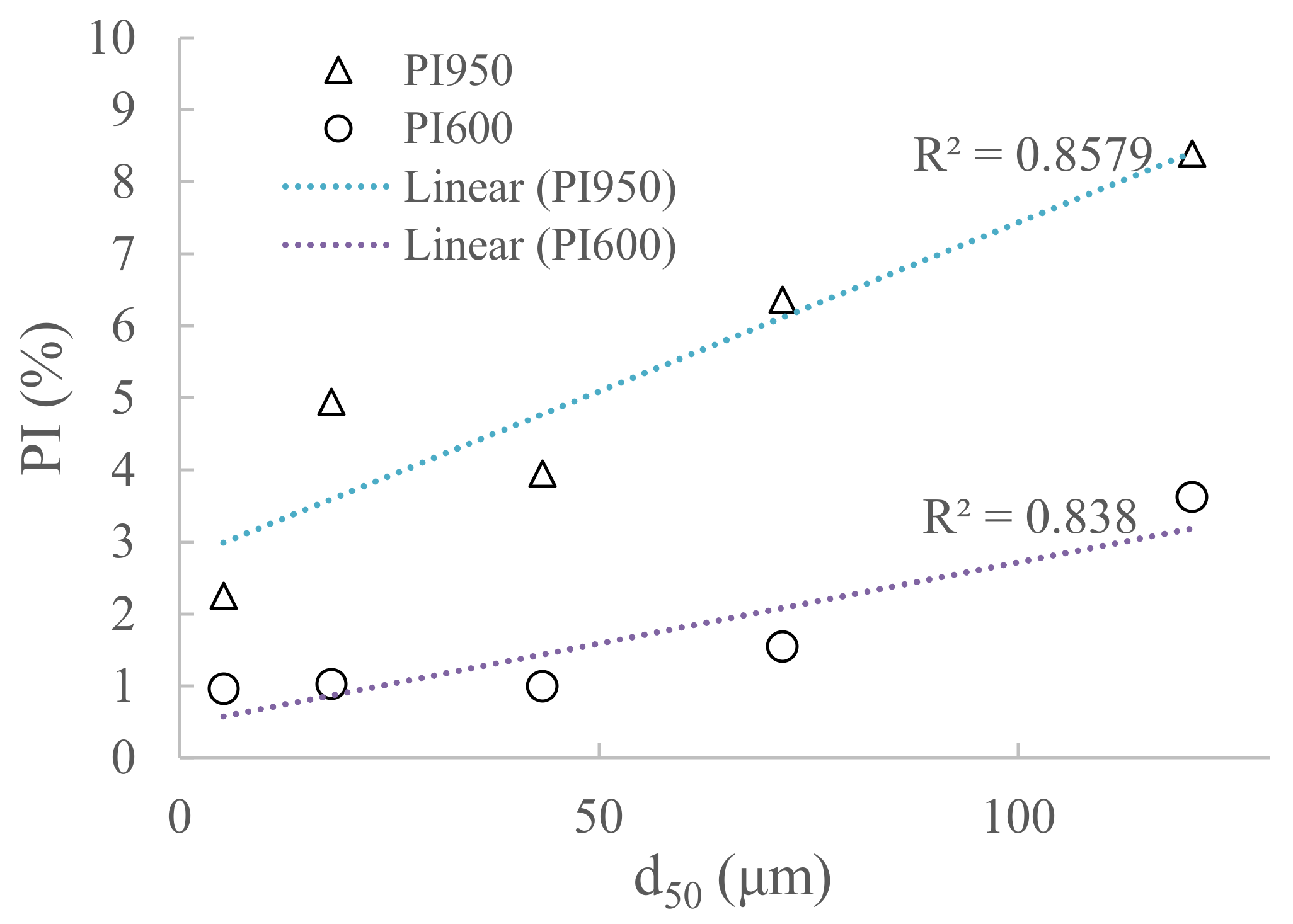

| Parametar | CEM I | WBA1 | WBA2 | WBA3 | WBA4 | WBA5 |
|---|---|---|---|---|---|---|
| P2O5 (mass %) | 0.22 | 2.60 | 1.84 | 1.82 | 1.35 | 4.03 |
| CaO (mass %) | 59.80 | 48.70 | 51.90 | 46.75 | 16.25 | 47.35 |
| MgO (mass %) | 2.01 | 4.79 | 3.75 | 8.26 | 4.30 | 4.71 |
| TiO2 (mass %) | 0.23 | 0.15 | 0.15 | 0.34 | 1.17 | 0.25 |
| SO3 (mass %) | 3.33 | 4.77 | 3.58 | 2.73 | 0.60 | 3.95 |
| Na2Oeq (mass %) | 1.67 | 10.90 | 6.60 | 4.63 | 4.59 | 4.72 |
| Pozzolanic oxides (SiO2+Al2O3+Fe2O3) | 29.97 | 12.29 | 13.03 | 28.81 | 54.68 | 19.70 |
| Free CaO (mass %) | 2.50 | 8.60 | 13.50 | 7.30 | 0.50 | 8.80 |
| Free MgO (mass %) | 0.75 | 4.20 | 3.80 | 3.30 | 0.50 | 4.50 |
| Cl- (mass %) | 0.04 | 0.06 | <0.003 | 0.04 | 0.04 | <0.003 |
| LOI (950 °C) | 3.6 | 13.4 | 13.8 | 3.8 | 8.3 | 12.7 |
| pH | 12.86 | 13.37 | 13.25 | 13.15 | 12.97 | 13.22 |
| Density (g/cm3) | 3.1 | 2.59 | 2.59 | 2.59 | 2.63 | 2.33 |
| Bulk density (kg/m3) | n/a | 0.38 | 0.38 | 0.91 | 0.61 | 0.55 |
| d50 (μm) | 9.6 | 18.2 | 43.3 | 71.9 | 120.7 | 17.8 |
| Technology used | - | Pulverized fuel combustor | Pulverized fuel combustor | Grate combustor | Grate combustor | Bubbling fluidized bed |
| Type of wood used | - | Beech, oak, hornbeam, poplar, cherry | Beech, oak, hornbeam | Beech, oak, abies, picea | Beech, oak, hornbeam | Beech, oak, hornbeam, poplar |
| Average temperature, °C | - | 700–750 | 700–750 | 700–950 | 800 | 850 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carević, I.; Štirmer, N.; Serdar, M.; Ukrainczyk, N. Effect of Wood Biomass Ash Storage on the Properties of Cement Composites. Materials 2021, 14, 1632. https://doi.org/10.3390/ma14071632
Carević I, Štirmer N, Serdar M, Ukrainczyk N. Effect of Wood Biomass Ash Storage on the Properties of Cement Composites. Materials. 2021; 14(7):1632. https://doi.org/10.3390/ma14071632
Chicago/Turabian StyleCarević, Ivana, Nina Štirmer, Marijana Serdar, and Neven Ukrainczyk. 2021. "Effect of Wood Biomass Ash Storage on the Properties of Cement Composites" Materials 14, no. 7: 1632. https://doi.org/10.3390/ma14071632








