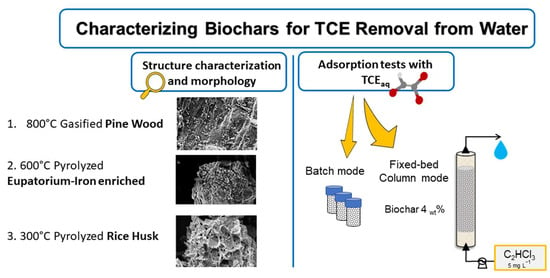
Biochar from Pine Wood, Rice Husks and Iron-Eupatorium Shrubs for Remediation Applications: Surface Characterization and Experimental Tests for Trichloroethylene Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochars Description
2.2. SEM-EDS Acquisition and BET Analysis
2.3. Kinteric and Isotherm Tests
2.4. Column Experiments
2.5. Calculations
2.6. Analytical Methods
3. Results and Discussion
3.1. Physicochemical Properties of Biochars
3.2. Adsorption Test in Batch Reactors
3.3. Column Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciampi, P.; Esposito, C.; Papini, M.P. Hydrogeochemical Model Supporting the Remediation Strategy of a Highly Contaminated Industrial Site. Water 2019, 11, 1371. [Google Scholar] [CrossRef] [Green Version]
- Sale, T.; Newell, C.; Stroo, H.; Hinchee, R.; Johnson, P. Frequently Asked Questions Regarding Management of Chlorinated Solvents in Soils and Groundwater; Environmental Security Technology Certification Program Office (DOD): Arlington, VA, USA, 2008. [Google Scholar]
- Casasso, A.; Tosco, T.; Bianco, C.; Bucci, A.; Sethi, R. How can we make pump and treat systems more energetically sustainable? Water 2020, 12, 67. [Google Scholar] [CrossRef] [Green Version]
- Mackay, D.M.; Cherry, J.A. Groundwater contamination: Pump-and-treat remediation. Environ. Sci. Technol. 1989, 23, 630–636. [Google Scholar] [CrossRef]
- Baric, M.; Majone, M.; Beccari, M.; Papini, M.P. Coupling of polyhydroxybutyrate (PHB) and zero valent iron (ZVI) for enhanced treatment of chlorinated ethanes in permeable reactive barriers (PRBs). Chem. Eng. J. 2012, 195–196, 22–30. [Google Scholar] [CrossRef]
- Ghaeminia, M.; Mokhtarani, N. Remediation of nitrate-contaminated groundwater by PRB-Electrokinetic integrated process. J. Environ. Manag. 2018, 222, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Al-Tabbaa, A. Sustainability: A new imperative in contaminated land remediation. Environ. Sci. Policy 2014, 39, 25–34. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Qin, C.; Wang, H.; Yuan, X.; Xiong, T.; Zhang, J.; Zhang, J. Understanding structure-performance correlation of biochar materials in environmental remediation and elec-trochemical devices. Chem. Eng. J. 2020, 382, 122977. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Gaunt, J.; Cowie, A. Biochar for Environmental Management: Science and Technology; Routledge: London, UK, 2012. [Google Scholar] [CrossRef]
- Wu, P.; Ata-Ul-Karim, S.T.; Singh, B.P.; Wang, H.; Wu, T.; Liu, C.; Fang, G.; Zhou, D.; Wang, Y.; Chen, W. A scientometric review of biochar research in the past 20 years (1998–2018). Biochar 2019, 1, 23–43. [Google Scholar] [CrossRef] [Green Version]
- Sørmo, E.; Silvani, L.; Thune, G.; Gerber, H.; Schmidt, H.P.; Smebye, A.B.; Cornelissen, G. Waste timber pyrolysis in a medium-scale unit: Emission budgets and biochar quality. Sci. Total Environ. 2020, 718, 137335. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ippolito, J.A.; Cui, L.; Novak, J.; Johnson, M.G. Biochar for Mine-land Reclamation. In Biochar from Biomass and Waste; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 75–90. [Google Scholar]
- Gwenzi, W.; Chaukura, N.; Noubactep, C.; Mukome, F.N. Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision. J. Environ. Manag. 2017, 197, 732–749. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; Jin, F.; McMillan, O.; Al-Tabbaa, A. Qualitative and quantitative characterisation of adsorption mechanisms of lead on four biochars. Sci. Total Environ. 2017, 609, 1401–1410. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; Zhengqian, Y.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Rodríguez-Vila, A.; Asensio, V.; Forján, R.; Covelo, E.F. Chemical fractionation of Cu, Ni, Pb and Zn in a mine soil amended with compost and biochar and vegetated with Brassica juncea L. J. Geochem. Explor. 2015, 158, 74–81. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Siggins, A.; Thorn, C.; Healy, M.G.; Abram, F. Simultaneous adsorption and biodegradation of trichloroethylene occurs in a biochar packed column treating contaminated landfill leachate. J. Hazard. Mater. 2021, 403, 123676. [Google Scholar] [CrossRef]
- Piai, L.; Blokland, M.; van der Wal, A.; Langenhoff, A. Biodegradation and adsorption of micropollutants by biological activated carbon from a drinking water production plant. J. Hazard. Mater. 2000, 388, 122028. [Google Scholar] [CrossRef]
- Dalahmeh, S.S.; Alziq, N.; Ahrens, L. Potential of biochar filters for onsite wastewater treatment: Effects of active and inactive biofilms on adsorption of per- and polyfluoroalkyl substances in laboratory column experiments. Environ. Pollut. 2019, 247, 155–164. [Google Scholar] [CrossRef]
- Daňo, M.; Viglašová, E.; Galamboš, M.; Štamberg, K.; Kujan, J. Surface complexation models of pertechnetate on biochar/montmorillonite composite-batch and dynamic sorption study. Materials 2020, 13, 3108. [Google Scholar] [CrossRef]
- Chaves Fernandes, B.C.; Ferreira Mendes, K.; Dias Júnior, A.F.; da Silva Caldeira, V.P.; da Silva Teófilo, T.M.; Severo Silva, T.; Mendonça, V.; de Freitas Souza, M.; Valadão Silva, D. Impact of pyrolysis temperature on the Properties of Eucaluptus Wood-Derived Biochar. Materials 2020, 13, 5841. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, Y.; Zheng, B.; Cai, X. Competitive removal of Cd(ii) and Pb(ii) by biochars produced from water hyacinths: Performance and mechanism. RSC Adv. 2016, 6, 5223–5232. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Silvani, L.; Vrchotova, B.; Kastanek, P.; Demnerova, K.; Pettiti, I.; Papini, M.P. Characterizing Biochar as Alternative Sorbent for Oil Spill Remediation. Sci. Rep. 2017, 7, srep43912. [Google Scholar] [CrossRef] [PubMed]
- Martinsen, V.; Alling, V.; Nurida, N.; Mulder, J.; Hale, S.; Ritz, C.; Rutherford, D.; Heikens, A.; Breedveld, G.D.; Cornélissen, G. pH effects of the addition of three biochars to acidic Indonesian mineral soils. Soil Sci. Plant Nutr. 2015, 61, 821–834. [Google Scholar] [CrossRef] [Green Version]
- Silvani, L.; Cornelissen, G.; Smebye, A.B.; Zhang, Y.; Okkenhaug, G.; Zimmerman, A.R.; Thune, G.; Sævarsson, H.; Hale, S.E. Can biochar and designer biochar be used to remediate per- and polyfluorinated alkyl substances (PFAS) and lead and antimony contaminated soils? Sci. Total Environ. 2019, 694, 133693. [Google Scholar] [CrossRef] [PubMed]
- Silvani, L.; Di Palma, P.R.; Riccardi, C.; Eek, E.; Hale, S.E.; Viotti, P.; Papini, M.P. Use of biochar as alternative sorbent for the active capping of oil contaminated sediments. J. Environ. Chem. Eng. 2017, 5, 5241–5249. [Google Scholar] [CrossRef]
- Silvani, L.; Hjartardottir, S.; Bielská, L.; Škulcová, L.; Cornelissen, G.; Nizzetto, L.; Hale, S.E. Can polyethylene passive samplers predict polychlorinated biphenyls (PCBs) uptake by earthworms and turnips in a biochar amended soil? Sci. Total Environ. 2019, 662, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Premarathna, K.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Li, L.; Quinlivan, P.A.; Knappe, D.R. Effects of activated carbon surface chemistry and pore structure on the adsorption of organic contaminants from aqueous solution. Carbon 2002, 40, 2085–2100. [Google Scholar] [CrossRef]
- Rosales, E.; Meijide, J.; Pazos, M.; Sanromán, M.A. Challenges and recent advances in biochar as low-cost biosorbent: From batch assays to continuous-flow systems. Bioresour. Technol. 2017, 246, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ahmad, M.; Al-Wabel, M.I.; Vithanage, M.; Rajapaksha, A.U.; Kim, H.S.; Lee, S.S.; Ok, Y.S. Adsorptive Removal of Trichloroethylene in Water by Crop Residue Biochars Pyrolyzed at Contrasting Temperatures: Continuous Fixed-Bed Experiments. J. Chem. 2015, 2015, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Lee, S.S.; Oh, S.-E.; Mohan, D.; Moon, D.H.; Lee, Y.H.; Ok, Y.S. Modeling adsorption kinetics of trichloroethylene onto biochars derived from soybean stover and peanut shell wastes. Environ. Sci. Pollut. Res. 2013, 20, 8364–8373. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.; Hayes, M.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Gao, J.; Dai, J.; Han, J.; Wang, Y.; Xie, J.; Yan, Y. Selective adsorption behavior of Pb(II) by mesoporous silica SBA-15-supported Pb(II)-imprinted polymer based on surface molecularly imprinting technique. J. Hazard. Mater. 2011, 186, 197–205. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Alhaija, M.A.; Al-Zuhair, S. Evaluation of an activated carbon packed bed for the adsorption of phenols from petroleum refinery wastewater. Environ. Sci. Pollut. Res. 2017, 24, 7511–7520. [Google Scholar] [CrossRef]
- Ai, J.; Lu, C.; Berg, F.W.V.D.; Yin, W.; Strobel, B.W.; Hansen, H.C.B. Biochar catalyzed dechlorination – Which biochar properties matter? J. Hazard. Mater. 2021, 406, 124724. [Google Scholar] [CrossRef]
- Kameyama, K.; Miyamoto, T.; Iwata, Y. The Preliminary Study of Water-Retention Related Properties of Biochar Produced from Various Feedstock at Different Pyrolysis Temperatures. Materials 2019, 12, 1732. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Lee, S.S.; Rajapaksha, A.U.; Vithanage, M.; Zhang, M.; Cho, J.S.; Lee, S.-E.; Ok, Y.S. Trichloroethylene adsorption by pine needle biochars produced at various pyrolysis temperatures. Bioresour. Technol. 2013, 143, 615–622. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.S.; Khim, J. Peat moss-derived biochars as effective sorbents for VOCs’ removal in groundwater. Environ. Geochem. Health 2017, 41, 1637–1646. [Google Scholar] [CrossRef]
- Siggins, A.; Abram, F.; Healy, M.G. Pyrolysed waste materials show potential for remediation of trichloroethylene-contaminated water. J. Hazard. Mater. 2020, 390, 121909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sohi, S.P.; Liu, S.; Guan, J.; Zhou, J.; Chen, J. Adsorption and reductive degradation of Cr(VI) and TCE by a simply synthesized zero valent iron magnetic biochar. J. Environ. Manag. 2019, 235, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Tang, J.; Cui, M.; Gao, B.; Shen, B. Biochar/iron (BC/Fe) composites for soil and groundwater remediation: Synthesis, applications, and mechanisms. Chemosphere 2020, 246, 125609. [Google Scholar] [CrossRef]
- Lawrinenko, M.; Wang, Z.; Horton, R.; Mendivelso-Perez, D.; Smith, E.A.; Webster, T.E.; Laird, D.A.; Van Leeuwen, J.H. Macroporous Carbon Supported Zerovalent Iron for Remediation of Trichloroethylene. ACS Sustain. Chem. Eng. 2016, 5, 1586–1593. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Dou, X.; Mohan, D.; Sung, J.-K.; Yang, J.E.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]






| Material | Specific Surface Area (m2 g−1) | Total Pores Volume (cm3 g−1) | Micro-Pores Volume (cm3 g−1) |
|---|---|---|---|
| PWBC 1 | 343 ± 2 | 0.383 | 0.136 |
| RHBC | 81.0 ± 0.7 | 0.101 | 0.028 |
| EuFeBC | 97 ± 1 | 0.075 | 0.039 |
| Commercial AC 1 | 712 ± 2 | 0.736 | 0.281 |
| Material | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| qe(cal) (mg g−1) | K1 (min−1) | R2 | qe(cal.) (mg g−1) | K2 (g (mg min−1)) | R2 | |
| PWBC | 0.16 | 0.029 | 0.83 | 34.36 | 0.0053 | 0.99 |
| RHBC | 1.68 | 0.0072 | 0.89 | 19.42 | 0.0041 | 0.99 |
| EuFeBC | 2.28 | 0.0067 | 0.92 | 24.44 | 0.0019 | 0.99 |
| Material | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|
| n | KF (L g−1) | R2 | qmax (mg g−1) | KL (L mg−1)) | R2 | |
| PWBC | 0.50 ± 1.6 × 10−2 | 9.44 ± 0.60 | 0.98 | 109.41 ± 5.62 | 3.8 × 10−2 ± 4.8 × 10−3 | 0.96 |
| RHBC | 0.43 ± 1.4 × 10−2 | 2.83 ± 0.14 | 0.97 | 21.00 ± 0.91 | 5.8 × 10−2 ± 6.3 × 10−3 | 0.93 |
| EuFeBC | 0.37 ± 1.8 × 10−2 | 5.21 ± 0.38 | 0.96 | 30.35 ± 2.49 | 8.2 × 10−2 ± 2.2 × 10−2 | 0.85 |
| Material Type | Peak Temperature (°C) | Surface Area (m2 g−1) | qmax (mg g−1) | Range of Ce (mg L−1) | Reference |
|---|---|---|---|---|---|
| Pine Wood BC | 850 | 343 ± 2 | 109.41 ± 5.62 | 10–120 | Kastanek et al., 2017 [32]; this work |
| Rice husks BC | 300 | 81.0 ± 0.7 | 21.00 ± 0.91 | ||
| Eupatorium shrubs BC + Fe(OOH) | 600 | 97 ± 1 | 30.35 ± 2.49 | ||
| Norit AC Darco® | - | 712 ± 2 | 260.33 ± 22.32 | 10–200 | |
| Herbal Pomace BC | 600 | 374 | 562.157 | 200 | Siggins et al., 2020 [49] |
| Oak BC | 650–720 | 216 | 22.093 | ||
| Spruce BC | 600 | 39 | 46.754 | ||
| GAC Darco® | - | 633 | 90.680 | ||
| Soybean Stover BC | 300 | 5.61 | 12.48 | 2–20 | Ahmad et al., 2012 [53] |
| Soybean Stover BC | 700 | 420.3 | 31.74 | ||
| Peatnut Shell BC | 300 | 3.14 | 12.12 | ||
| Peatnut Shell BC | 700 | 448.2 | 32.02 | ||
| AC Darco® G-60 | - | 1110 | 50.01 | ||
| Pine Needle BC | 300 | 4.09 | 205.417 | 5–50 | Ahmad et al., 2013 [47] |
| Pine Needle | 500 | 13.06 | 320.251 | ||
| Pine Needle | 700 | 390.52 | 211.189 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, M.M.; Silvani, L.; Amanat, N.; Petrangeli Papini, M. Biochar from Pine Wood, Rice Husks and Iron-Eupatorium Shrubs for Remediation Applications: Surface Characterization and Experimental Tests for Trichloroethylene Removal. Materials 2021, 14, 1776. https://doi.org/10.3390/ma14071776
Rossi MM, Silvani L, Amanat N, Petrangeli Papini M. Biochar from Pine Wood, Rice Husks and Iron-Eupatorium Shrubs for Remediation Applications: Surface Characterization and Experimental Tests for Trichloroethylene Removal. Materials. 2021; 14(7):1776. https://doi.org/10.3390/ma14071776
Chicago/Turabian StyleRossi, Marta M., Ludovica Silvani, Neda Amanat, and Marco Petrangeli Papini. 2021. "Biochar from Pine Wood, Rice Husks and Iron-Eupatorium Shrubs for Remediation Applications: Surface Characterization and Experimental Tests for Trichloroethylene Removal" Materials 14, no. 7: 1776. https://doi.org/10.3390/ma14071776