Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents
Abstract
:1. Introduction
2. Data and Methods
2.1. Data
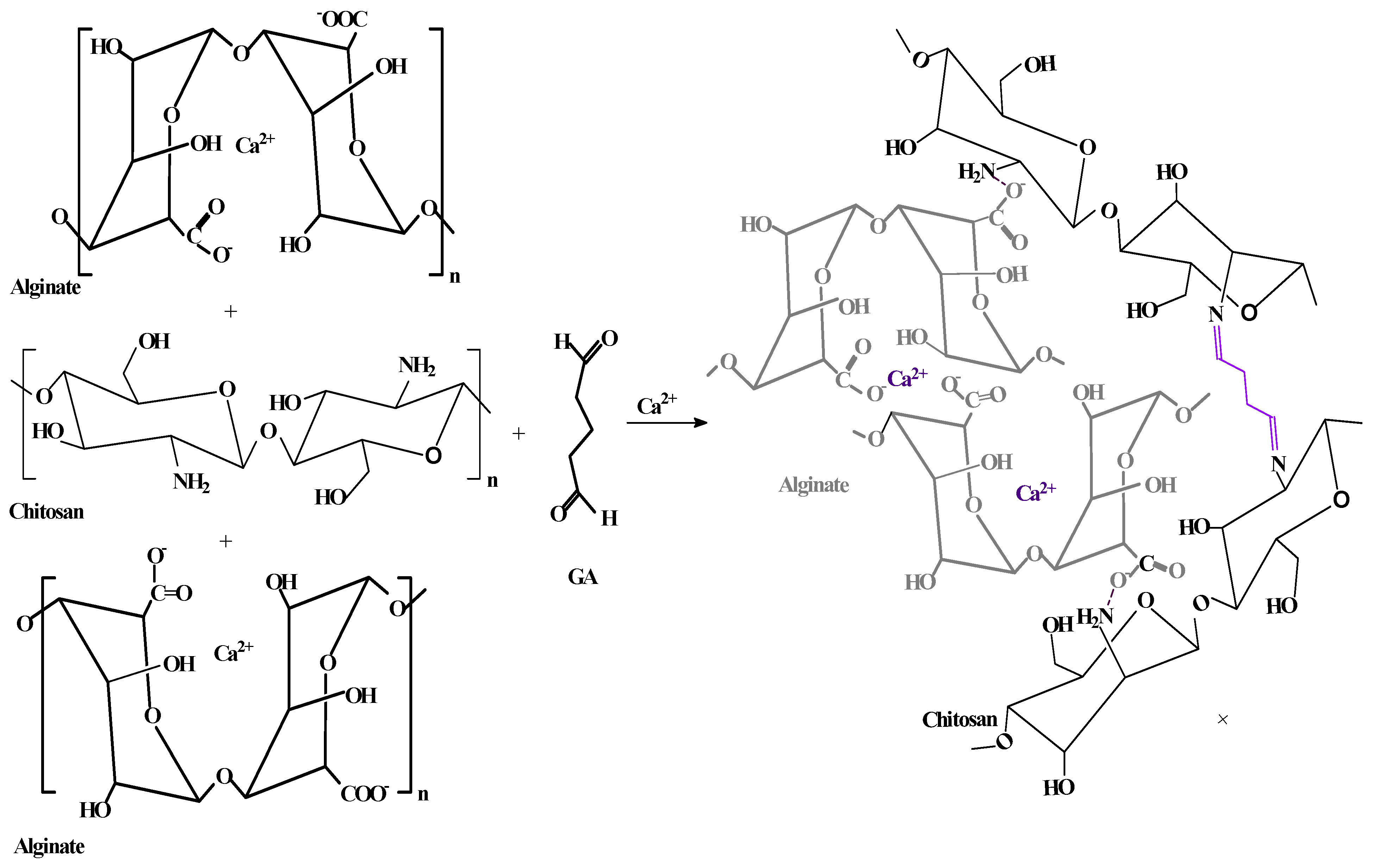
2.2. Synthesis of the Sorbents
2.3. Characterization
2.4. Sorption Tests
2.5. Describing of Sorption Isotherms and Uptake Kinetics
2.6. Treatment of Real Metal-Containing Groundwater
3. Results and Discussion
3.1. Characterization
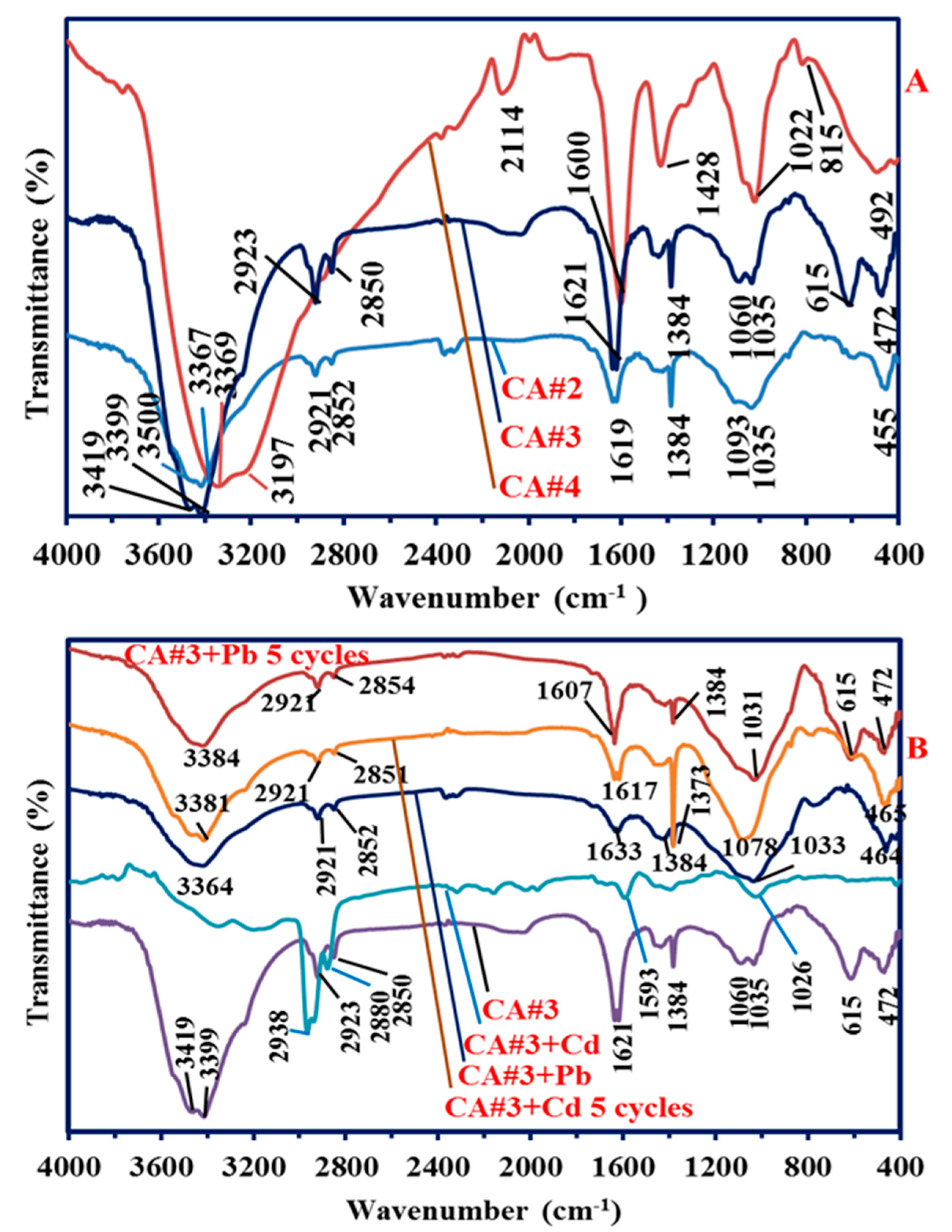
3.1.1. Fourier Transform Infrared Analysis
3.1.2. Thermogravimetric Analysis and Textural Properties (N2 Adsorption)
3.1.3. Determination of pHPZC
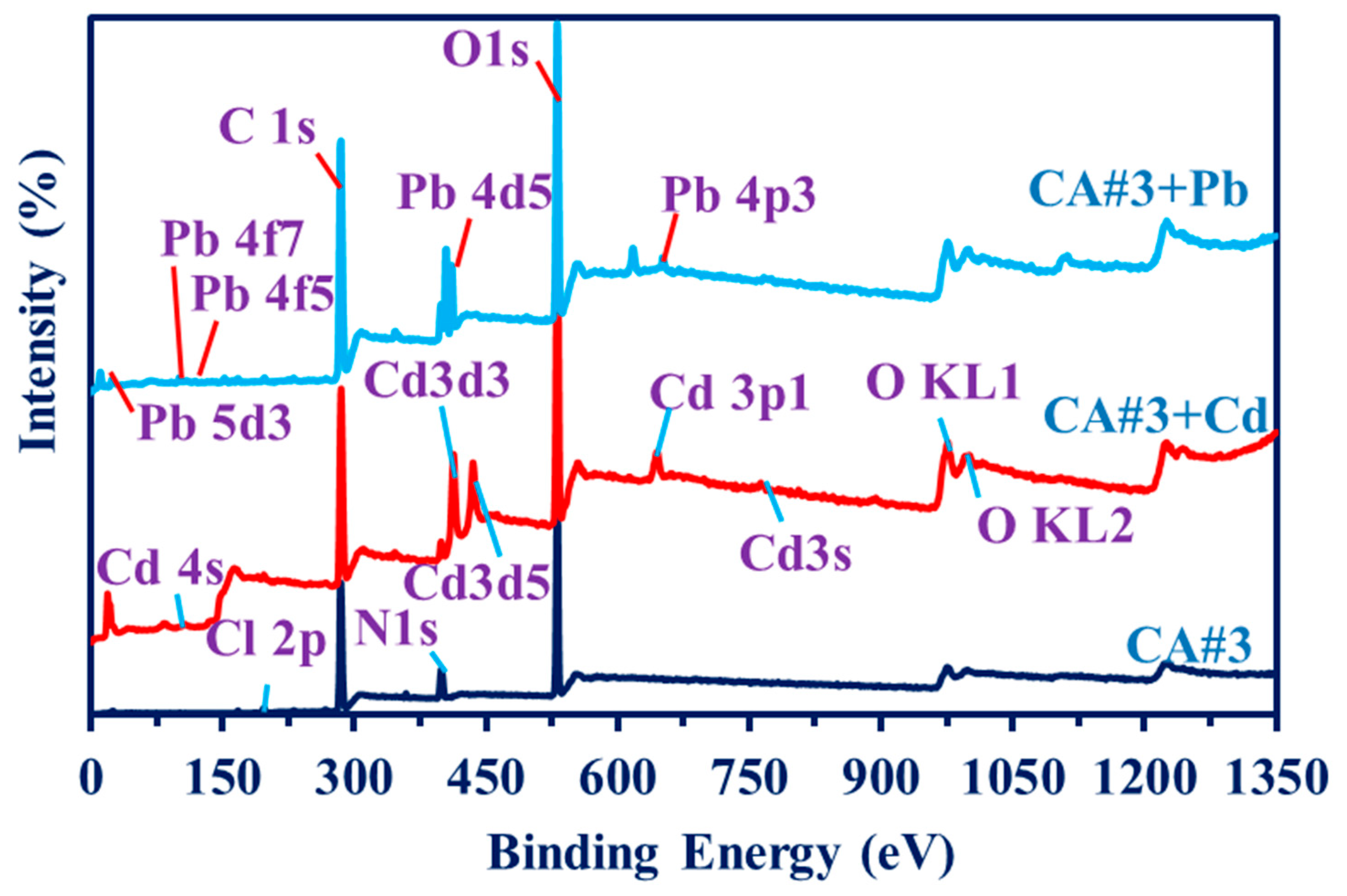
3.1.4. XPS Characterization
3.1.5. Elemental Analysis
3.1.6. SEM-EDX Analysis
3.2. Sorption Properties
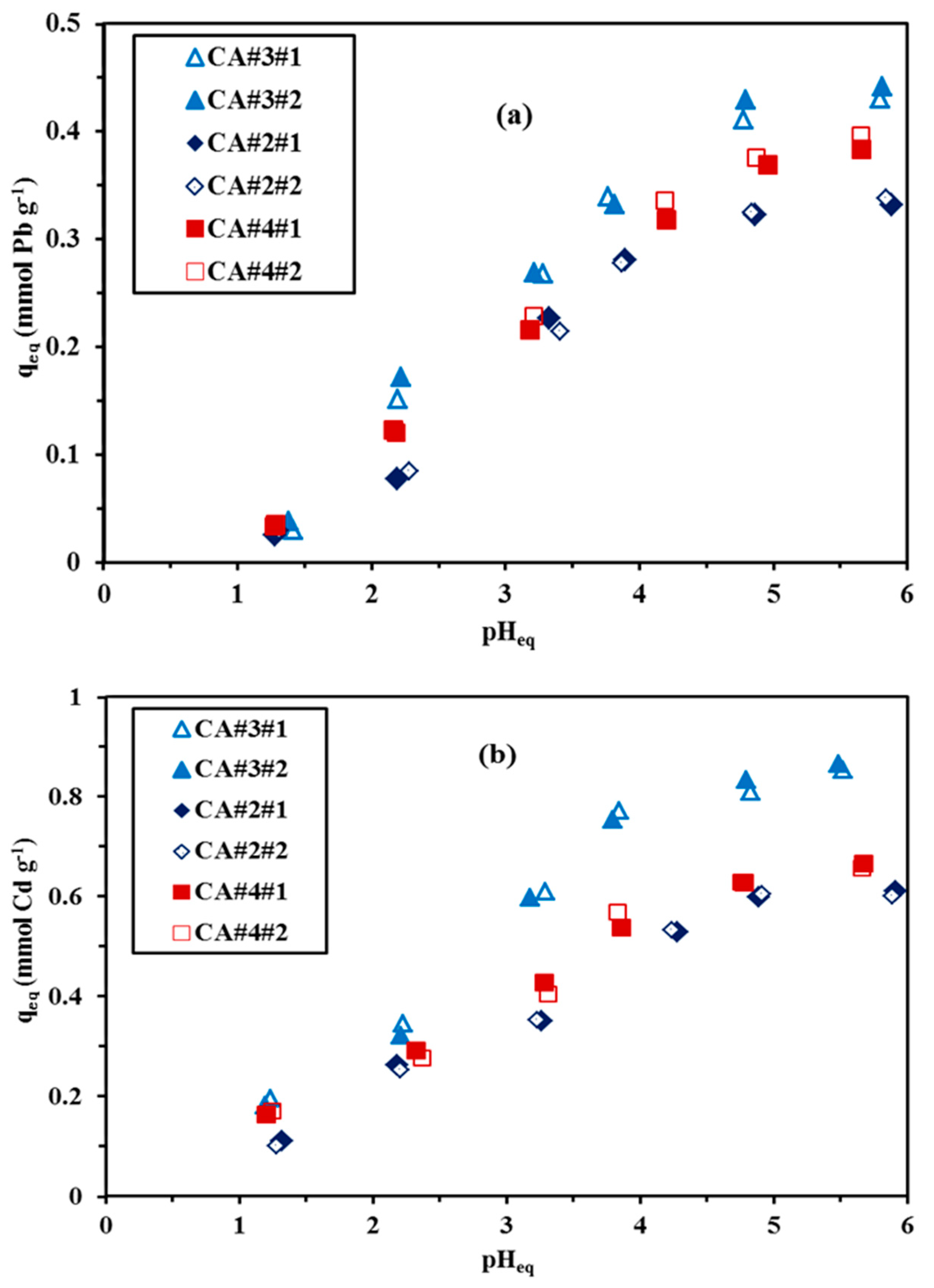
3.2.1. pH Effect
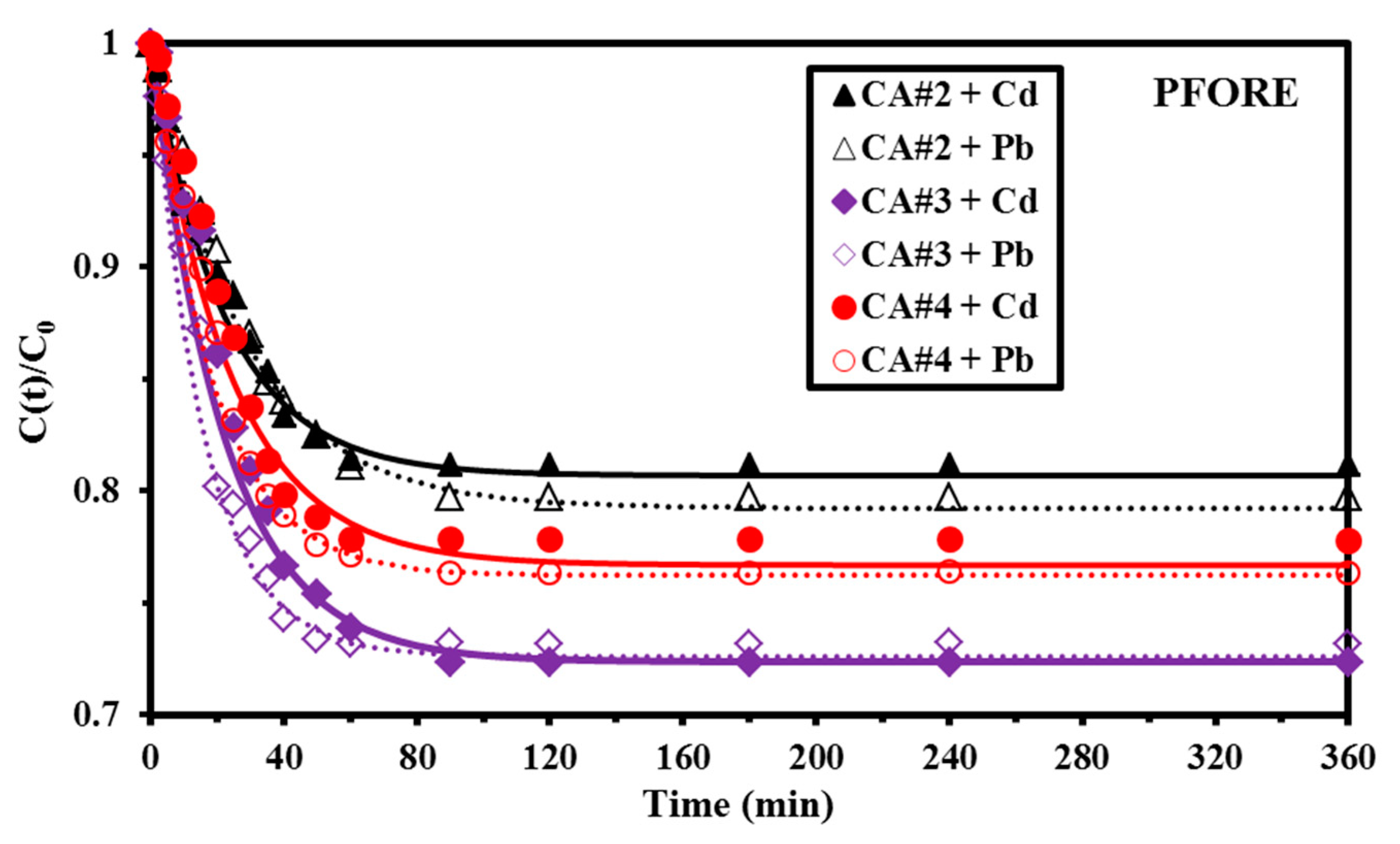
3.2.2. Sorption Kinetics
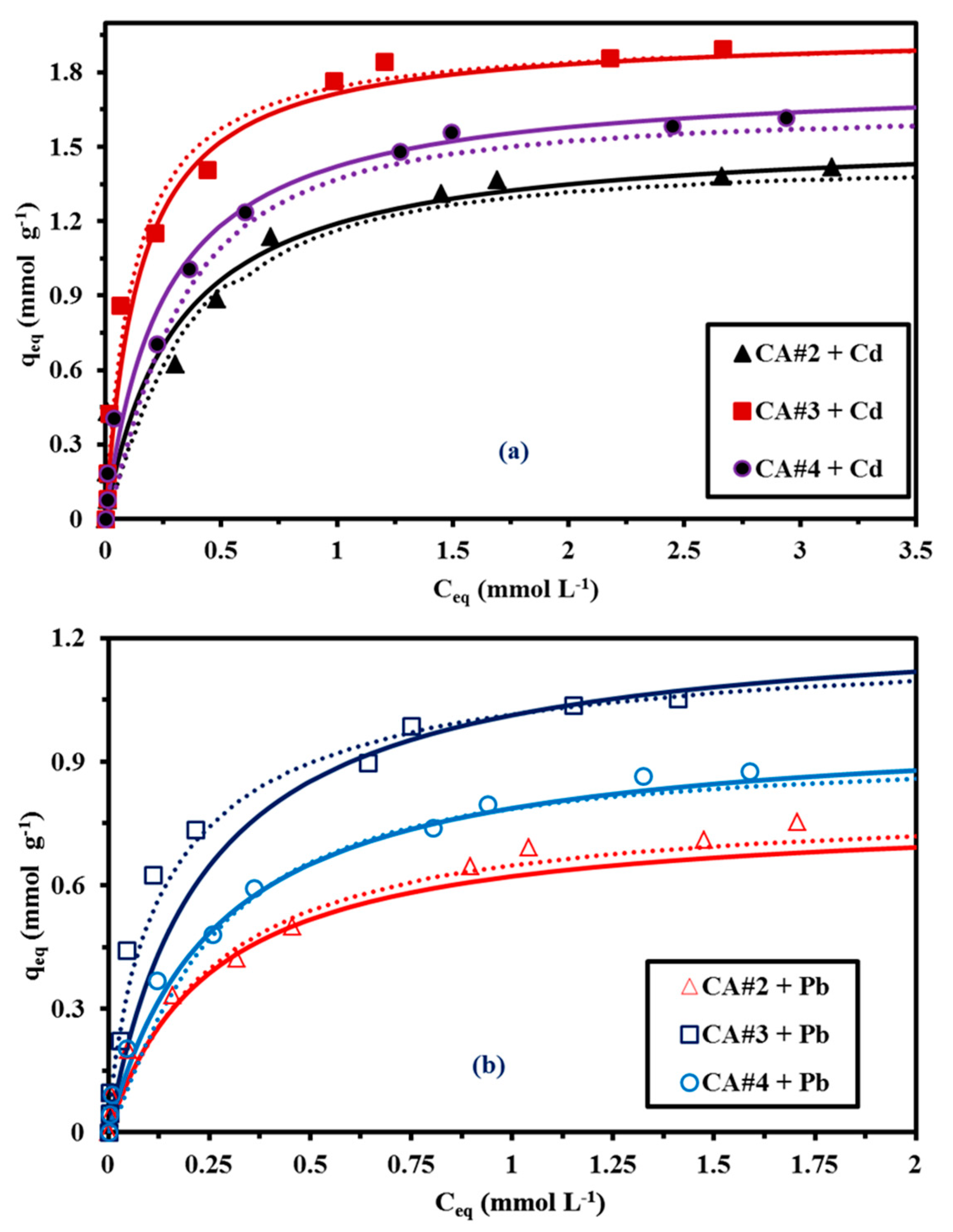
3.2.3. Sorption Isotherms
3.2.4. Expected Binding Scheme
3.2.5. Metal Desorption and Sorbent Recycling
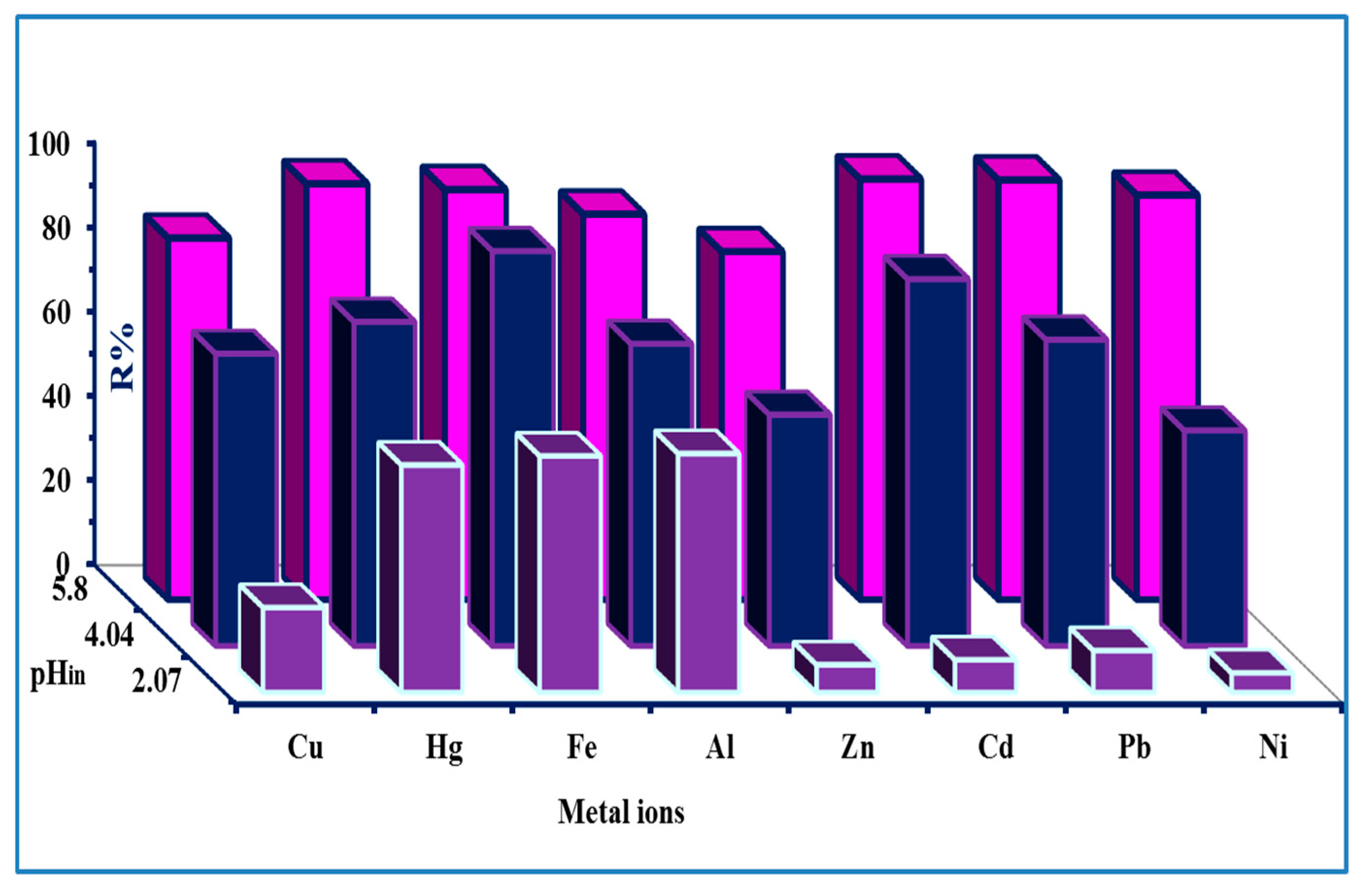
3.2.6. Sorption Characteristic (Multi-Component Solution)
3.3. Treatment of Contaminated Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; p. 541. [Google Scholar]
- Selim, M.T.; Salem, S.S.; Mohamed, A.A.; El-Gamal, M.S.; Awad, M.F.; Fouda, A. Biological treatment of real textile effluent using Aspergillus flavus and Fusarium oxysporium and their consortium along with the evaluation of their phytotoxicity. J. Fungi 2021, 7, 193. [Google Scholar] [CrossRef]
- Fouda, A.; Salem, S.S.; Wassel, A.R.; Hamza, M.F.; Shaheen, T.I. Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon 2020, 6, e04896. [Google Scholar] [CrossRef]
- EU. Council Directive 98/83/EC of 3 November 1998 on the quality of water intented for human consumption. In Document 31998L0083; European Union: Brussels, Belgium, 1998. [Google Scholar]
- USEPA. The Drinking Water Standards and Health Advisories; Office of Water, U.S. Environmental Protection Agency: Washington, DC, USA, 2012; Volume 822-S-12-001, pp. 1–12.
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture. Available online: http://www.fao.org/docrep/003/T0234E/T0234E00.htm (accessed on 20 June 2017).
- Juriš, P.; Rataj, D.; Ondrašovič, M.; Sokol, J.; Novák, P. Hygienic and Ecological Requirements on Recycling of Organic Wastes in Agriculture; M. Vaško: Prešov, Slovakia, 2000. [Google Scholar]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Hamza, M.F. Photocatalytic degradation of real textile and tannery effluent using biosynthesized magnesium oxide nanoparticles (MgO-NPs), heavy metal adsorption, phytotoxicity, and antimicrobial activity. J. Environ. Chem. Eng. 2021, 9, 105346. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Djordjevic, A.B.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Dai, S.; Yin, Z.; Lu, H.; Jia, R.; Xu, J.; Song, X.; Li, L.; Shu, Y.; Zhao, X. Toxicological assessment of combined lead and cadmium: Acute and sub-chronic toxicity study in rats. Food Chem. Toxicol. 2014, 65, 260–268. [Google Scholar] [CrossRef]
- Cobbina, S.J.; Chen, Y.; Zhou, Z.; Wu, X.; Zhao, T.; Zhang, Z.; Feng, W.; Wang, W.; Li, Q.; Wu, X.; et al. Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J. Hazard. Mater. 2015, 294, 109–120. [Google Scholar] [CrossRef]
- El-Boshy, M.; Ashshi, A.; Gaith, M.; Qusty, N.; Bokhary, T.; Altaweel, N.; Abdelhady, M. Studies on the protective effect of the artichoke (Cynara scolymus) leaf extract against cadmium toxicity-induced oxidative stress, hepatorenal damage, and immunosuppressive and hematological disorders in rats. Environ. Sci. Pollut. Res. 2017, 24, 12372–12383. [Google Scholar] [CrossRef]
- Abdou, H.M.; Hassan, M.A. Protective role of Omega-3 polyunsaturated fatty acid against lead acetate-induced toxicity in liver and kidney of female rats. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Šmirjákova, S.; Ondrašovičová, O.; Kašková, A.; Lakticova, K. The effect of cadmium and lead pollution on human and animal health. Folia Vet. 2005, 49, 31–32. [Google Scholar]
- Kavak, D. Removal of lead from aqueous solutions by precipitation: Statistical analysis and modeling. Desalin. Water Treat. 2013, 51, 1720–1726. [Google Scholar] [CrossRef]
- Lu, X.; Huang, Z.; Liang, Z.; Li, Z.; Yang, J.; Wang, Y.; Wang, F. Co-precipitation of Cu and Zn in precipitation of struvite. Sci. Total. Environ. 2021, 764, 144269. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.M.; Bailey, P.J.; Tasker, P.A.; Turkington, J.R.; Grant, R.A.; Love, J.B. Solvent extraction: The coordination chemistry behind extractive metallurgy. Chem. Soc. Rev. 2014, 43, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Van der Hoogerstraete, T.; Binnemans, K. Highly efficient separation of rare earths from nickel and cobalt by solvent extraction with the ionic liquid trihexyl(tetradecyl)phosphonium nitrate: A process relevant to the recycling of rare earths from permanent magnets and nickel metal hydride batteries. Green Chem. 2014, 16, 1594–1606. [Google Scholar] [CrossRef] [Green Version]
- Swain, N.; Mishra, S. A review on the recovery and separation of rare earths and transition metals from secondary resources. J. Clean. Prod. 2019, 220, 884–898. [Google Scholar] [CrossRef]
- Wu, S.; Wang, L.; Zhang, P.; El-Shall, H.; Moudgil, B.; Huang, X.; Zhao, L.; Zhang, L.; Feng, Z. Simultaneous recovery of rare earths and uranium from wet process phosphoric acid using solvent extraction with D2EHPA. Hydrometallurgy 2018, 175, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Sharaf, M.; Yoshida, W.; Kubota, F.; Goto, M. Selective extraction of scandium by a long alkyl chain carboxylic acid/organophosphonic ester binary extractant. Solvent Extr. Ion Exch. 2018, 36, 647–657. [Google Scholar] [CrossRef]
- Huang, X.; Dong, J.; Wang, L.; Feng, Z.; Xue, Q.; Meng, X. Selective recovery of rare earth elements from ion-adsorption rare earth element ores by stepwise extraction with HEH(EHP) and HDEHP. Green Chem. 2017, 19, 1345–1352. [Google Scholar] [CrossRef]
- Agarwal, V.; Safarzadeh, M.S. Solvent extraction and separation of cerium(III) and samarium(III) from mixed rare earth solutions using PC88A. Miner. Metall. Process. 2017, 34, 125–131. [Google Scholar] [CrossRef]
- Hamza, M.F.; El Aassy, I.E. Solid phase extraction of uranium removal from underground water, Wadi Naseib, Southwestern Sinai, Egypt. Desalin. Water Treat. 2014, 52, 331–338. [Google Scholar] [CrossRef]
- Hamza, M.F.; Mahfouz, M.G.; Abdel-Rahman, A.A.-H. Adsorption of uranium (VI) ions on hydrazinyl amine and 1, 3, 4-thiadiazol-2 (3 H)-thion chelating resins. J. Dispers. Sci. Technol. 2012, 33, 1544–1551. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J. Environ. Chem. Eng. 2021, 9, 104693. [Google Scholar] [CrossRef]
- Otrembska, P.; Gega, J. Separation of nickel(II) and cadmium(II) ions with ion-exchange and membrane processes. Sep. Sci. Technol. 2016, 51, 2675–2680. [Google Scholar] [CrossRef]
- Lai, Y.C.; Lee, W.J.; Huang, K.L.; Wu, C.M. Metal recovery from spent hydrodesulfurization catalysts using a combined acid-leaching and electrolysis process. J. Hazard. Mater. 2008, 154, 588–594. [Google Scholar] [CrossRef]
- He, C.; Salih, K.A.; Wei, Y.; Mira, H.; Abdel-Rahman, A.A.-H.; Elwakeel, K.Z.; Hamza, M.F.; Guibal, E. Efficient recovery of rare earth elements (Pr (III) and Tm (III)) from mining residues using a new phosphorylated hydrogel (Algal Biomass/PEI). Metals 2021, 11, 294. [Google Scholar] [CrossRef]
- Hamza, M.F.; Ahmed, F.Y.; El-Aassy, I.; Fouda, A.; Guibal, E. Groundwater purification in a polymetallic mining area (SW Sinai, Egypt) using functionalized magnetic chitosan particles. Water Air Soil Pollut. 2018, 229, 1–14. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdel-Rahman, M.A.; Farag, M.M.; Shehal-Deen, A.; Mohamed, A.A.; Alsharif, S.M.; Saied, E.; Moghanim, S.A.; Azab, M.S. Catalytic degradation of wastewater from the textile and tannery industries by green synthesized hematite (α-Fe2O3) and magnesium oxide (MgO) nanoparticles. Curr. Res. Biotechnol. 2021, 3, 29–41. [Google Scholar] [CrossRef]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Giraldo, J.D.; Rivas, B.L.; Elgueta, E.; Mancisidor, A. Metal ion sorption by chitosan-tripolyphosphate beads. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Hamza, M.F. Grafting of quaternary ammonium groups for uranium (VI) recovery: Application on natural acidic leaching liquor. J. Radioanal. Nucl. Chem. 2019, 322, 519–532. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.-C.; Guibal, E. Uranium and europium sorption on amidoxime-functionalized magnetic chitosan micro-particles. Chem. Eng. J. 2018, 344, 124–137. [Google Scholar] [CrossRef]
- Hamza, M.F.; Abdel-Rahman, A.A.-H. Extraction studies of some hazardous metal ions using magnetic peptide resins. J. Dispers. Sci. Technol. 2015, 36, 411–422. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Shokri, E. Synthesis and characterization of magnetic amidoximated chitosan-g poly (polyacrylonitrile)/laponite RD nanocomposites with enhanced adsorption capacity for Cu2+. Turk. J. Chem. 2017, 41, 135–152. [Google Scholar] [CrossRef]
- Hamza, M.F.; Aly, M.M.; Abdel-Rahman, A.A.-H.; Ramadan, S.; Raslan, H.; Wang, S.; Vincent, T.; Guibal, E. Functionalization of magnetic chitosan particles for the sorption of U (VI), Cu (II) and Zn (II)—hydrazide derivative of glycine-grafted chitosan. Materials 2017, 10, 539. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Alginate and algal-based beads for the sorption of metal cations: Cu(II) and Pb(II). Int. J. Mol. Sci. 2016, 17, 1453. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Hamza, M.F.; Vincent, T.; Faur, C.; Guibal, E. Praseodymium sorption on Laminaria digitata algal beads and foams. J. Colloid Interface Sci. 2017, 504, 780–789. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Algal foams applied in fixed-bed process for lead(II) removal using recirculation or one-pass modes. Mar. Drugs 2017, 15, 315. [Google Scholar] [CrossRef] [Green Version]
- Hamza, M.F.; Fouda, A.; Elwakeel, K.Z.; Wei, Y.; Guibal, E.; Hamad, N.A. Phosphorylation of Guar Gum/Magnetite/Chitosan Nanocomposites for Uranium (VI) Sorption and Antibacterial Applications. Molecules 2021, 26, 1920. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon 2009, 37, 1215–1221. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Proc. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Uber die adsorption in lasungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Radwan, T.M.; Blackburn, G.A.; Whyatt, J.D.; Atkinson, P.M. Dramatic loss of agricultural land due to urban expansion threatens food security in the Nile Delta, Egypt. Remote Sens. 2019, 11, 332. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, D.; Abotalib, A.Z.; El-Bastaweesy, M.; El-Said, M.A.; Melegy, A.; Garamoon, H. Geo-environmental impacts of hydrogeological setting and anthropogenic activities on water quality in the Quaternary aquifer southeast of the Nile Delta, Egypt. J. Afr. Earth Sci. 2020, 172, 103947. [Google Scholar] [CrossRef]
- Negm, A.M.; Sakr, S.; Abd-Elaty, I.; Abd-Elhamid, H.F. An overview of groundwater resources in Nile Delta aquifer. Groundw. Nile Delta 2018, 73, 3–44. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Chichester, UK, 2006; pp. 1–23. [Google Scholar] [CrossRef]
- Lu, S.; Chen, L.; Hamza, M.F.; He, C.; Wang, X.; Wei, Y.; Guibal, E. Amidoxime functionalization of a poly(acrylonitrile)/silica composite for the sorption of Ga(III)—Application to the treatment of Bayer liquor. Chem. Eng. J. 2019, 368, 459–473. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Guibal, E. Quaternization of algal/PEI beads (a new sorbent): Characterization and application to scandium recovery from aqueous solutions. Chem. Eng. J. 2020, 383, 123210. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elem. Res. 2020, 195, 707–724. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.-H.; El-Aassy, I.E.E.; Fadia, Y.A.; Hamza, M.F. Studies on the uptake of rare earth elements on polyacrylamidoxime resins from natural concentrate leachate solutions. J. Dispers. Sci. Technol. 2010, 31, 1128–1135. [Google Scholar] [CrossRef]
- Wei, Y.; Rakhatkyzy, M.; Salih, K.A.; Wang, K.; Hamza, M.F.; Guibal, E. Controlled bi-functionalization of silica microbeads through grafting of amidoxime/methacrylic acid for Sr (II) enhanced sorption. Chem. Eng. J. 2020, 402, 125220. [Google Scholar] [CrossRef]
- Hernandez-Paredes, J.; Glossman-Mitnik, D.; Esparza-Ponce, H.E.; Alvarez-Ramos, M.E.; Duarte-Moller, A. Band structure, optical properties and infrared spectrum of glycine-sodium nitrate crystal. J. Mol. Struct. 2008, 875, 295–301. [Google Scholar] [CrossRef]
- Hamza, M.F.; Salih, K.A.; Adel, A.-H.; Zayed, Y.E.; Wei, Y.; Liang, J.; Guibal, E. Sulfonic-functionalized algal/PEI beads for scandium, cerium and holmium sorption from aqueous solutions (synthetic and industrial samples). Chem. Eng. J. 2021, 403, 126399. [Google Scholar] [CrossRef]
- Iditoiu, C.; Segal, E.; Chambree, D. Kinetics of non-isothermal behaviour of synthetic cationites with low acidity. J. Therm. Anal. Calorim. 1999, 56, 407–417. [Google Scholar] [CrossRef]
- Chambree, D.; Iditoiu, C.; Segal, E.; Cesaro, A. The study of non-isothermal degradation of acrylic ion-exchange resins. J. Therm. Anal. Calorim. 2005, 82, 803–811. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Gleason, K.K. Particle surface design using an all-dry encapsulation method. Adv. Mater. 2006, 18, 1972–1977. [Google Scholar] [CrossRef]
- Teng, C.-C.; Ma, C.-C.M.; Lu, C.-H.; Yang, S.-Y.; Lee, S.-H.; Hsiao, M.-C.; Yen, M.-Y.; Chiou, K.-C.; Lee, T.-M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Hernan, L.; Morales, J.; Santos, J.; Espinos, J.P.; Gonzalez-Elipe, A.R. Preparation and characterization of diamine intercalation compounds of misfit layer sulfides. J. Mater. Chem. 1998, 8, 2281–2286. [Google Scholar] [CrossRef]
- Liu, C.K.; Bai, R.B.; Ly, Q.S. Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms. Water Res. 2008, 42, 1511–1522. [Google Scholar] [CrossRef]
- Deng, S.B.; Bai, R.B.; Chen, J.P. Aminated polyacrylonitrile fibers for lead and copper removal. Langmuir 2003, 19, 5058–5064. [Google Scholar] [CrossRef]
- Dong, T.; Yang, L.; Pan, F.; Xing, H.; Wang, L.; Yu, J.; Qu, H.; Rong, M.; Liu, H. Effect of immobilized amine density on cadmium(II) adsorption capacities for ethanediamine-modified magnetic poly-(glycidyl methacrylate) microspheres. J. Magn. Magn. Mater. 2017, 427, 289–295. [Google Scholar] [CrossRef]
- Lv, L.; Zhang, J.; Yuan, S.; Huang, L.; Tang, S.; Liang, B.; Pehkonen, S.O. Enhanced adsorption of Cu(II) ions on chitosan microspheres functionalized with polyethylenimine-conjugated poly(glycidyl methacrylate) brushes. RSC Adv. 2016, 6, 78136–78150. [Google Scholar] [CrossRef]
- Zheng, L.; Peng, D.; Meng, P. Promotion effects of nitrogenous and oxygenic functional groups on cadmium (II) removal by carboxylated corn stalk. J. Cleaner Prod. 2018, 201, 609–623. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, X.B.; Luo, G.Q.; Xu, Y.Q.; Li, X.; Yao, H. Study on the effects of carrier and modifier on mercury adsorption behavior over halides modified sorbents using temperature programmed desorption method. Fuel Process. Technol. 2018, 178, 293–300. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Benettayeb, A.; Wang, X.; Guibal, E. Efficient removal of uranium, cadmium and mercury from aqueous solutions using grafted hydrazide-micro-magnetite chitosan derivative. J. Mater. Sci. 2020, 55, 4193–4212. [Google Scholar] [CrossRef]
- Hamza, M.F.; Wei, Y.; Mira, H.; Adel, A.-H.; Guibal, E. Synthesis and adsorption characteristics of grafted hydrazinyl amine magnetite-chitosan for Ni (II) and Pb (II) recovery. Chem. Eng. J. 2019, 362, 310–324. [Google Scholar] [CrossRef] [Green Version]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Alexandratos, S.D.; Zhu, X. The effect of hydrogen bonding in enhancing the ionic affinities of immobilized monoprotic phosphate ligands. Materials 2017, 10, 968. [Google Scholar] [CrossRef] [Green Version]
- Marcus, Y. Ion. Properties; Marcel Dekker: New York, NY, USA, 1997; p. 259. [Google Scholar]
- Falyouna, O.; Eljamal, O.; Maamoun, I.; Tahara, A.; Sugihara, Y. Magnetic zeolite synthesis for efficient removal of cesium in a lab-scale continuous treatment system. J. Colloid Interface Sci. 2020, 571, 66–79. [Google Scholar] [CrossRef]
- Pearson, R.G. Acids and bases. Science 1966, 151, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Alexandratos, S.D. Affinity of polymer-supported reagents for lanthanides as a function of donor atom polarizability. Ind. Eng. Chem. Res. 2009, 48, 6173–6187. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, X.; Yao, X.; Ji, H. Thiourea modified hyper-crosslinked polystyrene resin for heavy metal ions removal from aqueous solutions. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Krishnani, K.K.; Meng, X.G.; Christodoulatos, C.; Boddu, V.M. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. J. Hazard. Mater. 2008, 153, 1222–1234. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Zhou, L.P.; Castillo-Araiza, C.O.; De Herdt, E. Cadmium(II), lead(II), and copper(II) biosorption on Baker’s yeast (Saccharomyces cerevesiae). J. Environ. Eng. 2016, 142. [Google Scholar] [CrossRef]
- Mata, Y.N.; Blazquez, M.L.; Ballester, A.; Gonzalez, F.; Munoz, J.A. Biosorption of cadmium, lead and copper with calcium alginate xerogels and immobilized Fucus vesiculosus. J. Hazard. Mater. 2009, 163, 555–562. [Google Scholar] [CrossRef]
- Trakulsujaritchok, T.; Noiphom, N.; Tangtreamjitmun, N.; Saeeng, R. Adsorptive features of poly(glycidyl methacrylate-co-hydroxyethyl methacrylate): Effect of porogen formulation on heavy metal ion adsorption. J. Mater. Sci. 2011, 46, 5350–5362. [Google Scholar] [CrossRef]
- Ferrah, N.; Abderrahim, O.; Amine Didi, M.; Villemein, D. Sorption efficiency of a new sorbent towards cadmium(II): Methylphosphonic acid grafted polystyrene resins. J. Chem. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Demirbas, A.; Pehlivan, E.; Gode, F.; Altun, T.; Arslan, G. Adsorption of Cu(II), Zn(II), Ni(II), Pb(II), and Cd(II) from aqueous solution on amberlite IR-120 synthetic resin. J. Colloid Interface Sci. 2005, 282, 20–25. [Google Scholar] [CrossRef]
- Al Hamouz, O.C.S.; Ali, S.A. Removal of zinc and cadmium ions using a cross-linked polyaminophosphonate. J. Macromol. Sci. A 2013, 50, 375–384. [Google Scholar] [CrossRef]
- Rao, K.S.; Chaudhury, G.R.; Mishra, B.K. Kinetics and equilibrium studies for the removal of cadmium ions from aqueous solutions using Duolite ES 467 resin. Int. J. Miner. Process. 2010, 97, 68–73. [Google Scholar] [CrossRef]
- Wang, F.; Wang, L.J.; Li, J.S.; Sun, X.Y.; Han, W.Q. Adsorption behavior and mechanism of cadmium on strong-acid cation exchange resin. Trans. Nonferrous Met. Soc. China 2009, 19, 740–744. [Google Scholar] [CrossRef]
- Liu, M.Q.; Tao, Z.A.; Wang, H.C.; Zhao, F.; Sun, Q. Preparation and characterization of a series of porous anion-exchanger chelating fibers and their adsorption behavior with respect to removal of cadmium(II). RSC Adv. 2016, 6, 115222–115237. [Google Scholar] [CrossRef]
- Tighadouini, S.; Radi, S.; Garcia, Y. Selective chemical adsorption of Cd (II) on silica covalently decorated with a β-ketoenol-thiophene-furan receptor. Mol. Syst. Des. Eng. 2020, 5, 1037–1047. [Google Scholar] [CrossRef]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U., Jr. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Ahmed, S.A. Removal of lead and sodium ions from aqueous media using natural wastes for desalination and water purification. Desalin. Water Treat. 2016, 57, 8911–8926. [Google Scholar] [CrossRef]
- Bao, S.Y.; Li, K.; Ning, P.; Peng, J.H.; Jin, X.; Tang, L.H. Highly effective removal of mercury and lead ions from wastewater by mercaptoamine-functionalised silica-coated magnetic nano-adsorbents: Behaviours and mechanisms. Appl. Surf. Sci. 2017, 393, 457–466. [Google Scholar] [CrossRef]
- Deniz, S.; Tasci, N.; Yetimoglu, E.K.; Kahraman, M.V. New thiamine functionalized silica microparticles as a sorbent for the removal of lead, mercury and cadmium ions from aqueous media. J. Serb. Chem. Soc. 2017, 82, 215–226. [Google Scholar] [CrossRef]
- Othman, M.K.; Al-Qadri, F.A.; Al-Yusufy, F.A. Synthesis, physical studies and uptake behavior of: Copper(II) and lead(II) by Schiff base chelating resins. Spectrochim. Acta A 2011, 78, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Abo-Farha, S.A.; Abdel-Aal, A.Y.; Ashour, I.A.; Garamon, S.E. Removal of some heavy metal cations by synthetic resin purolite C100. J. Hazard. Mater. 2009, 169, 190–194. [Google Scholar] [CrossRef]
- Arar, O. Removal of lead(II) from water by di (2-ethylhexyl) phosphate containing ion exchange resin. Desalin. Water Treat. 2014, 52, 3197–3205. [Google Scholar] [CrossRef]
- Hayeeye, F.; Yu, Q.J.; Sattar, M.; Chinpa, W.; Sirichote, O. Adsorption of Pb2+ ions from aqueous solutions by gelatin/activated carbon composite bead form. Adsorpt. Sci. Technol. 2018, 36, 355–371. [Google Scholar] [CrossRef]
- Bhatt, R.R.; Shah, B.A. Sorption studies of heavy metal ions by salicylic acid-formaldehyde-catechol terpolymeric resin: Isotherm, kinetic and thermodynamics. Arabian J. Chem. 2015, 8, 414–426. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, Y.; Huang, J.; Yuan, S. Azido chelating fiber: Synthesis, characterization and adsorption performances towards Hg2+ and Pb2+ from water. Polym. Adv. Technol. 2017, 28, 1418–1427. [Google Scholar] [CrossRef]
- Nicola, R.; Costişor, O.; Ciopec, M.; Negrea, A.; Lazău, R.; Ianăşi, C.; Picioruş, E.-M.; Len, A.; Almásy, L.; Szerb, E.I.; et al. Silica-coated magnetic nanocomposites for pb2+ removal from aqueous solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef] [Green Version]

| C0: (mmol M L−1) | C0 (mmol Cd L−1) | C0 (mmol Pb L−1) | |||||
|---|---|---|---|---|---|---|---|
| 0.902 | 0.4997 | ||||||
| Model | Parameter | CA#2 | CA#3 | CA#4 | CA#2 | CA#3 | CA#4 |
| Exp. | qeq (mmol g−1) | 0.564 | 0.828 | 0.664 | 0.338 | 0.445 | 0.393 |
| PFORE | qeq,1 (mmol g−1) | 0.554 | 0.842 | 0.641 | 0.319 | 0.452 | 0.386 |
| k1 × 102 (min−1) | 4.449 | 4.56 | 4.19 | 3.592 | 5.38 | 6.353 | |
| R2 | 0.985 | 0.974 | 0.973 | 0.946 | 0.987 | 0.963 | |
| EV × 104 | 5.4 | 4.6 | 5.7 | 0.233 | 0.896 | 0.177 | |
| AIC | −109.55 | −85.88 | −92.79 | −98.84 | −69.16 | −72.67 | |
| PSORE | qeq,2 (mmol g−1) | 0.605 | 0.88 | 0.696 | 0.312 | 0.473 | 0.379 |
| k2 × 102 (L mmol−1 min−1) | 9.31 | 11.26 | 13.52 | 22.7 | 32.43 | 46.26 | |
| R2 | 0.973 | 0.883 | 0.879 | 0.928 | 0.889 | 0.835 | |
| EV × 104 | 5.39 | 9.8 | 12.6 | 3.635 | 3.75 | 5.16 | |
| AIC | −110.79 | −78.74 | −68.72 | −86.45 | −73.32 | −58.15 | |
| Model | Parameter | Cd(II) | Pb(II) | ||||
|---|---|---|---|---|---|---|---|
| CA#2 | CA#3 | CA#4 | CA#2 | CA#3 | CA#4 | ||
| Exp. | qm (mmol g−1) | 1.42 | 1.894 | 1.616 | 0.754 | 1.054 | 0.877 |
| Langmuir | qm,L (mmol g−1) | 1.505 | 1.926 | 1.717 | 0.799 | 1.248 | 0.994 |
| bL (L mmol−1) | 3.264 | 4.835 | 3.998 | 3.929 | 4.303 | 3.798 | |
| R2 | 0.96 | 0.913 | 0.983 | 0.901 | 0.913 | 0.935 | |
| AIC | −0.495 | −11.01 | −23.48 | −10.96 | −35.61 | −35.14 | |
| Freundlich | kF | 1.097 | 1.664 | 1.222 | 0.639 | 0.994 | 0.777 |
| nF | 3.955 | 4.529 | 3.982 | 2.454 | 4.675 | 3.079 | |
| R2 | 0.781 | 0.734 | 0.906 | 0.982 | 0.899 | 0.971 | |
| AIC | −0.285 | −12.01 | −26.32 | −15.85 | −26.44 | −39.84 | |
| Sips | qm,S(mmol g−1) | 1.407 | 1.948 | 1.744 | 0.777 | 1.118 | 0.908 |
| bS (L mmol−1) | 4.485 | 4.434 | 4.994 | 4.347 | 4.989 | 5.988 | |
| nS | 0.755 | 0.999 | 0.754 | 0.939 | 0.994 | 0.795 | |
| R2 | 0.916 | 0.896 | 0.919 | 0.958 | 0.939 | 0.971 | |
| AIC | −3.36 | −15.42 | −24.9 | −7.88 | −26.36 | −29.67 | |
| M | Sorbent | pH | Equilibrium Time (min) | qm (mmol g−1) | Ref. |
|---|---|---|---|---|---|
| Cd (II) | Rice husk biomatrix | 6 | 90 | 0.149 | [79] |
| Carboxylated grafted corn stalk | 5.8 | 60 | 0.42 | [68] | |
| EDTA Saccharomyces cerevisiae | 5 | 60 | 0.29 | [80] | |
| alginate beads | 6 | 480 | 0.28 | [81] | |
| Alginate beads with Fucus vesiculosus | 6 | 480 | 0.58 | [81] | |
| HEMA-PGMA functionalized DETA | 5 | 50 | 0.32 | [82] | |
| PS-Methylphosphonic sorbent | 5 | 180 | 0.34 | [83] | |
| Amberlite IR−120 | 4–8 | 300 | 0.9 | [84] | |
| Polyaminophosphonate crosslinked polymer | 4 | 240 | 0.48 | [85] | |
| Duolite ES 467 | 4.8 | 90 | 0.15 | [86] | |
| Cationic resin (001 × 7) | 4–5 | 120 | 3.16 | [87] | |
| Amine chelating fibers on porous anion exchange resin | 3 | 60 | 1.12 | [88] | |
| HAHZ-MG-CH sorbent | 5 | 60 | 2.67 | [70] | |
| Thiophene-furan-β-ketoenol grafted on silica | 6.0 | 30 | 0.75 | [89] | |
| CA#2 | 5 | 50 | 1.42 | This work | |
| CA#3 | 5 | 50 | 1.89 | Thiswork | |
| CA#4 | 5 | 50 | 1.616 | This work | |
| Pb (II) | Rice husk biomatrix | 6 | 90 | 0.28 | [79] |
| Magnetic oak bark biochar | 5.0 | 60 | 0.146 | [90] | |
| Sugarcane bagasse | 5.0 | 60 | 0.005 | [91] | |
| Beet pulp | 5.0 | 60 | 0.009 | [91] | |
| Mercaptoamine on silica-coated magnetic nanosorbent | 6–7 | 120 | 1.41 | [92] | |
| Thiamine on silica microparticles | 5.0 | 120 | 0.19 | [93] | |
| Schiff base resin | 10 | 120 | 0.50 | [94] | |
| Purolite C100 sorbent | 5–6 | 1440 | 0.046 | [95] | |
| Di (2-ethylhexyl) phosphate functionalized resin | 4.0 | 80 | 0.172 | [96] | |
| Gelatin/activated on carbon beads | 5.0 | 60 | 1.79 | [97] | |
| Formaldehyde/salicylic acid/catechol sorbent | 6.0 | 240 | 0.931 | [98] | |
| Tripolyphosphate functionalized Chitosan | 5.0 | 1080 | 1.21 | [34] | |
| Azido chelating fiber | 6.0 | 1440 | 1.50 | [99] | |
| HA-MG-CH | 5.0 | 60 | 2.51 | [35] | |
| Magnetic iron oxide-silica shell nanocomposites | 6 | 90 | 0.0719 | [100] | |
| CA#2 | 5.0 | 50 | 0.75 | This work | |
| CA#3 | 5.0 | 50 | 1.05 | This work | |
| CA#4 | 5.0 | 50 | 0.87 | This work |
| Cycle | Cd(II) | Pb(II) | ||||||
|---|---|---|---|---|---|---|---|---|
| Sorption (%) | Desorption (%) | Sorption (%) | Desorption (%) | |||||
| Average | SD | Average | SD | Average | SD | Average | SD | |
| #1 | 92.43 | 3.85 | 99.98 | 0.15 | 89.69 | 0.53 | 99.23 | 0.87 |
| #2 | 91.09 | 3.07 | 100.40 | 0.5 | 89.03 | 0.75 | 99.79 | 0.83 |
| #3 | 90.71 | 3.58 | 99.92 | 0.36 | 87.28 | 0.41 | 99.24 | 0.46 |
| #4 | 90.05 | 4.38 | 99.85 | 0.1 | 85.59 | 0.42 | 99.49 | 0.55 |
| #5 | 89.07 | 4.52 | 99.85 | 0.52 | 84.56 | 0.65 | 99.22 | 0.26 |
| Loss @ Run #5 (%) | 3.36 | 0.1 | 5.1 | 0.01 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, M.F.; Hamad, N.A.; Hamad, D.M.; Khalafalla, M.S.; Abdel-Rahman, A.A.-H.; Zeid, I.F.; Wei, Y.; Hessien, M.M.; Fouda, A.; Salem, W.M. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials 2021, 14, 2189. https://doi.org/10.3390/ma14092189
Hamza MF, Hamad NA, Hamad DM, Khalafalla MS, Abdel-Rahman AA-H, Zeid IF, Wei Y, Hessien MM, Fouda A, Salem WM. Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents. Materials. 2021; 14(9):2189. https://doi.org/10.3390/ma14092189
Chicago/Turabian StyleHamza, Mohammed F., Nora A. Hamad, Doaa M. Hamad, Mahmoud S. Khalafalla, Adel A.-H. Abdel-Rahman, Ibrahim F. Zeid, Yuezhou Wei, Mahmoud M. Hessien, Amr Fouda, and Waheed M. Salem. 2021. "Synthesis of Eco-Friendly Biopolymer, Alginate-Chitosan Composite to Adsorb the Heavy Metals, Cd(II) and Pb(II) from Contaminated Effluents" Materials 14, no. 9: 2189. https://doi.org/10.3390/ma14092189






