Abstract
Structural instability during cycling is an important factor affecting the electrochemical performance of nickel-rich ternary cathode materials for Li-ion batteries. In this work, enhanced structural stability and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials are achieved by Ga doping. Compared with the pristine electrode, Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 electrode exhibits remarkably improved electrochemical performance and thermal safety. At 0.5C rate, the discharge capacity increases from 169.3 mAh g−1 to 177 mAh g−1, and the capacity retention also rises from 82.8% to 89.8% after 50 cycles. In the charged state of 4.3 V, its exothermic temperature increases from 245.13 °C to more than 271.24 °C, and the total exothermic heat decreases from 561.7 to 225.6 J·g−1. Both AC impedance spectroscopy and in situ XRD analysis confirmed that Ga doping can improve the stability of the electrode/electrolyte interface structure and bulk structure during cycling, which helps to improve the electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode material.
1. Introduction
With the rapid development of portable electronic products and electric vehicles, higher requirements have been put forward on the energy density, safety, cycle life, and cost of lithium-ion batteries (LIBs). The nickel-rich ternary layered material LiNi1−x−yCoxMnyO2 such as LiNi0.8Co0.1Mn0.1O2 (NCM811) and LiNi0.6Co0.2Mn0.2O2 (NCM622) exhibit high capacity and low cost, showing a promising application prospect [1,2]. However, with the increase in nickel content, the cycle performance, thermal stability, and safety gradually decrease [3,4]; this happens due to factors such as surface residual alkali, transition metal dissolution, cation mixing, surface irreversible formation of NiO phases, intergranular cracks, and micro-strains [1,2,3,4,5,6,7,8,9]. Among the nickel-rich LiNi1−x−yCoxMnyO2 materials, NCM622 material can be prepared in the air, has a higher lithium ion diffusion coefficient, and has better structural stability [10,11,12]; therefore, it has become the preferred choice for research and commercial application.
In order to improve the electrochemical performance of NCM622 material, some modification methods have been investigated in recent years. One useful method is to coat the surface of NCM622 material with Al2O3 [13], Co3O4 [14], SiO2 [15], Li3PO4 [16], Mn3(PO4)2 [17], Li1.3Al0.3Ti1.7(PO4)3 [18], LiAlO2 [19], and Li2Si2O5 [20] in order to improve the stability of the electrode/electrolyte interface and, thus, enhance the capacity, Coulomb efficiency, cyclability, and thermal safety performance. Another effective method is to prepare heterogeneous structural materials. For example, the formation of a heterogeneous interface layer of rock salt phase on the primary particles has been reported to significantly improve the cycle stability of NCM622 material at high temperatures [21]. In addition, element doping is also a very important modification method. Schipper et al. [22] concluded that Zr doping can suppress the phase change of NCM622 from the layered structure to the spinel structure. Liu et al. [23] reported that Mo doping for NCM622 can suppress the loss of lattice oxygen, enhance the cation order, broaden the Li+ migration channel, and improve the electrochemical performance under high voltage. Huang et al. [24,25] confirmed that Na and Mg doping can promote Li+ migration and improve the rate performance of NCM622. Mofid et al. [26] replaced Co in NCM622 by both Fe and Al, which reduced the degree of cation mixing and improved the cycle stability remarkably. Because F has strong electronegativity, a more stable crystal structure can be obtained while F ions are doped into the oxygen site of NCM622 material. All of the F-doped samples [27], including those co-doped with Na and Mg electrode materials [28,29], showed a good rate performance and excellent cycle performance. Recently, the doping and coating of dual functional modified materials prepared by using the same source, such as PO43– gradient doping and Li3PO4 coating dual functional materials [30], Zr doping and amorphous Li2ZrO3 coating dual functional materials [31], have also been reported to greatly improve the cycle performance of NCM622 under high temperature and high voltage.
Ga doping should also be an effective method for stabilizing the layered cathode materials, because the ion radius of Ga3+ is close to that of Co3+ and Ni3+ but has a strong polarization, which is beneficial for increasing the covalence of the O-TM layer in layered lithium transition metal oxides [32]. It has also been proved that Ga doping can significantly improve the structural stability and cycling performance of LiNiO2 [33,34], LiCoO2 [35], LiNi0.8Co0.2O2 [36], and Li[Li0.2Mn0.54Co0.13Ni0.13]O2 [37]. However, Ga doping has not been used to improve the structural stability and cycling performance of nickel-rich LiNi1−x−yCoxMnyO2 materials. Although Ga is a dispersed rare metal, its reserves on the earth have reached 1 million tons, which is about 1/7 of those of cobalt, and its price is less than four times that of cobalt [38]; however, since the amount of doped Ga is very small, the increase in cost should be minimal. Therefore, the research on Ga doping to improve the electrochemical performance of nickel-rich LiNi1−x−yCoxMnyO2 materials seems necessary.
In this project, the high-temperature solid-state reaction method was employed to synthesize Ga-doped NCM622 materials. The effect of annealing temperature and Ga content on the structural and electrochemical properties of Ga-doped NCM622 materials was thoroughly investigated. The Ga-doped materials prepared under the optimized synthesis conditions exhibited remarkably improved structural stability and electrochemical performance.
2. Experimental
Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 (x = 0, 0.01, 0.02, 0.03 and 0.05) materials were prepared by the high-temperature solid-state reaction method. Stoichiometric Li2CO3 (AR, Tianjin Fuchen Chemical Reagent Co., Ltd., Tianjin, China), Ni0.6Co0.2Mn0.2(OH)2 (Henan Kelong NewEnergy Co., Ltd., Xinxiang, China), and Ga2O3 (AR, Shanghai Macleans Biochemical Technology Co., Ltd., Shanghai, China) with cation mole ratio of Li:(Ni + Co + Mn):Ga = 1.08:(1 − x):x were mixed, thoroughly grinded, and then transferred into the furnace (TM-0914P, Beijing Ying’an Meicheng Scientific Instrument Co., Ltd., Beijing, China), where they were preheated at 500 °C for 6 h; they were subsequently calcined for 12 h at 800 °C, 850 °C, and 900 °C, respectively, with a heating rate of 5 °C min−1, followed by a cooling down to 500 °C with a rate of 2 °C min−1, and then naturally cooling to room temperature.
The structures and microscopic morphologies of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 materials were analyzed using an X-ray diffractometer (Rigaku, D/Max-2600-PC, Tokyo, Japan) and field emission scanning electron microscopy (FEI, Quanta-400F, Hillsboro, OR, USA), respectively. X-ray photoelectron spectroscopy (XPS) and energy dispersive X-ray (EDX) spectroscopy analysis for the sample with x = 0.02 were carried out in situ using an X-ray photoelectron spectrometer (PHI, 5000 Versaprobe II, Kanagawa, Japan) and a field emission transmission electron microscope (FEI, Tecnai G2 F30, Hillsboro, OR, USA), respectively. The electrochemical properties of the as-prepared materials were tested by galvanostatic charge–discharge test using CR2032 coin cells, in which the cathode electrodes, comprised of 80% active material, 10% Super P, and 10% poly(vinylidene fluoride) (PVdF), were pasted on porous Al foil; a Li metal chip was used as anode; 1 mol L−1 LiPF6/EC + DEC + DMC (volume ratio 1:1:1) was used as the electrolyte; and Celgard 2400 membrane (Charlotte, NC, USA) was used as the separator. The coin cells were assembled in a glove box (Etelux Lab2000, Etelux Inert Gas System (Beijing) Co., Ltd., Beijing, China) filled with argon and then installed on a land battery system (LANHE CT2001A, Wuhan Jinnuo Electronics Co., Ltd., Wuhan, China) to test the charge–discharge performance in the voltage range of 2.8–4.3 V. Electrochemical workstation (IM6eX, Zahner Elektrik GmbH & Co. KG, Kronach, Germany) was used to test the electrochemical impedance spectroscopy of the fully discharged cathode electrodes (2.8 V vs. Li/Li+), in which the frequency range was 100 mHz–100 KHz, and the AC amplitude was 5 mV. The thermal stability of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 materials (typical weight 6.2 mg) in the charged state (4.3 V, vs. Li/Li+) was analyzed with a differential scanning calorimeter (Q2000, TA Instruments, New Castle, DE, USA) under the following test conditions: nitrogen atmosphere; heating rate, 5 °C·min−1; and temperature range, 50–350 °C.
3. Results and Discussion
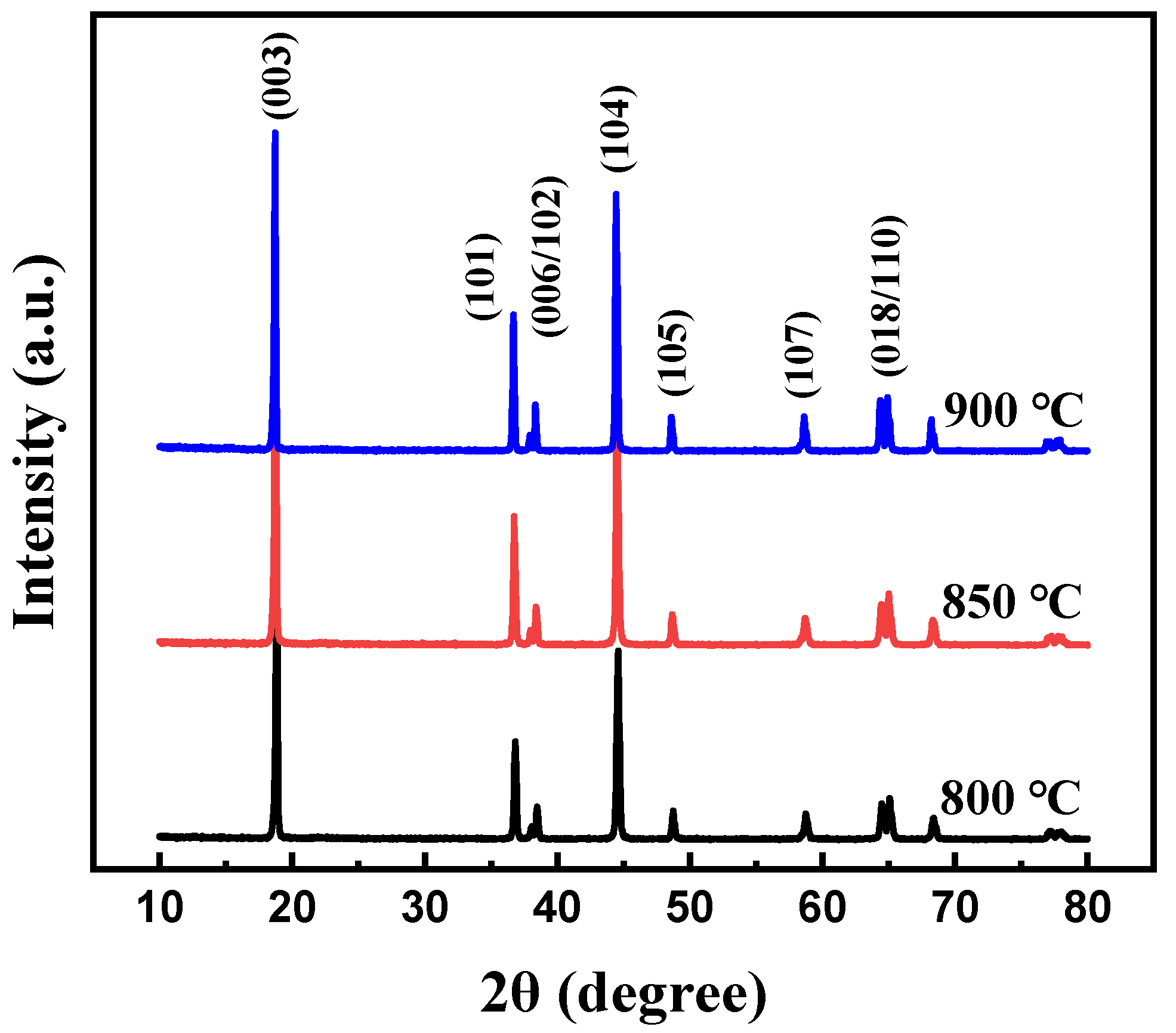
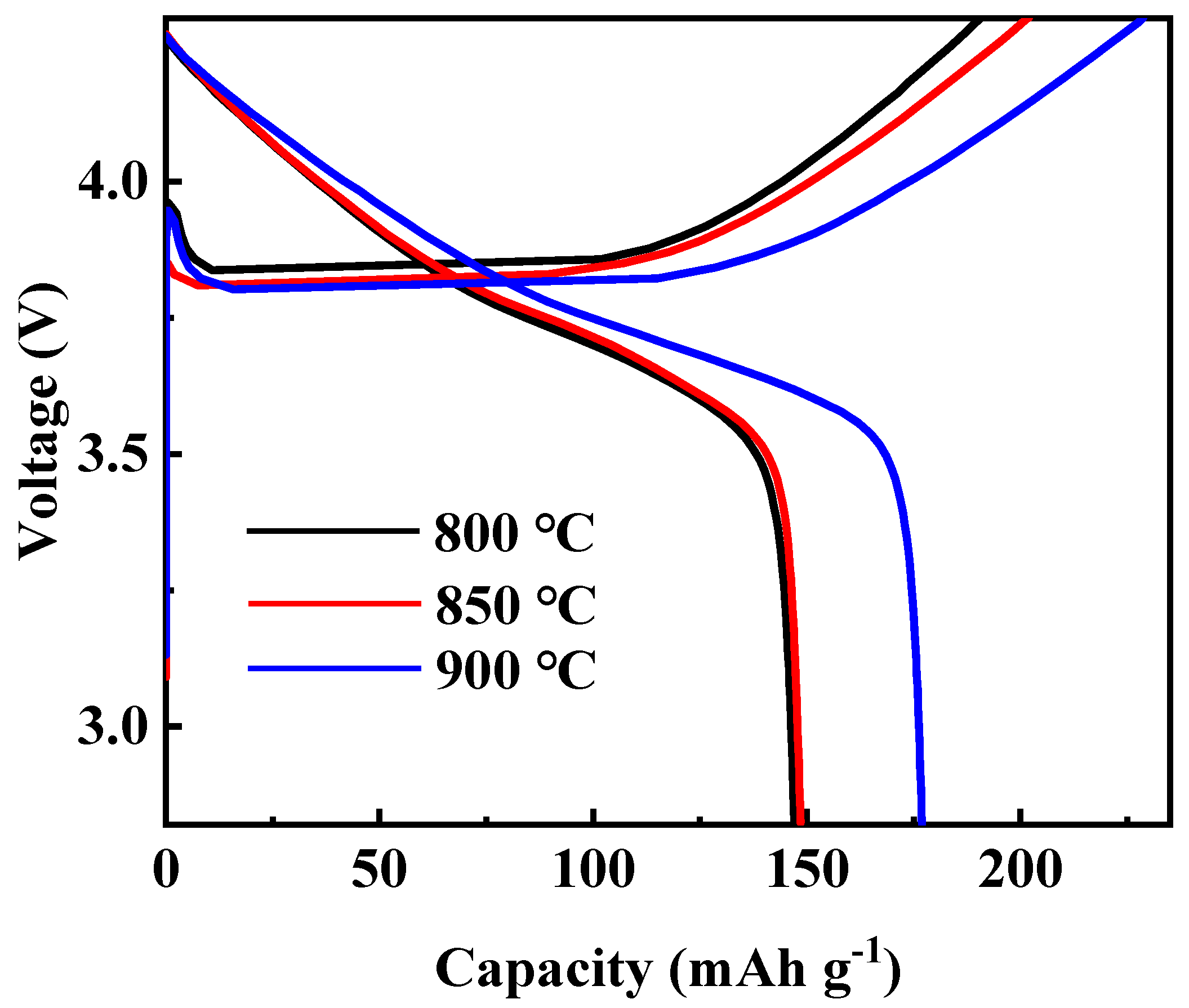
At first, the calcination temperature for the synthesis of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 (x = 0, 0.01, 0.02, 0.03 and 0.05) materials was optimized. Figure 1 shows the XRD patterns of the Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 sample prepared at different calcination temperatures. It is clear that all peaks can be indexed to the layer α-NaFeO2 structure with space group R3-m, and no impurity phase appeared. Low cation disorder for all samples can be confirmed by the lattice parameter ratio of c/a > 4.9 and the ratio of I(003)/I(104) > 1.2. With the increase in calcination temperature, the splitting degree of double peaks (006)/(102) and (108)/(110) rose, and the ratio of c/a gradually increased from 4.9485 for the sample prepared at 800 °C to 4.9548 for the sample prepared at 900 °C, indicating an improved cation ordering. This was further confirmed by the following charge–discharge tests, as shown in Figure 2. Among the three samples, the sample calcinated at 900 °C shows the smallest polarization and the highest discharge capacity. Its discharge capacity at a rate of 0.5 C reached 177.0 mAh g−1. In the following work, samples calcined at 900 °C are used to discuss the influence of Ga doping on the structure and electrochemical performance of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 (x = 0, 0.01, 0.02, 0.03 and 0.05) materials.
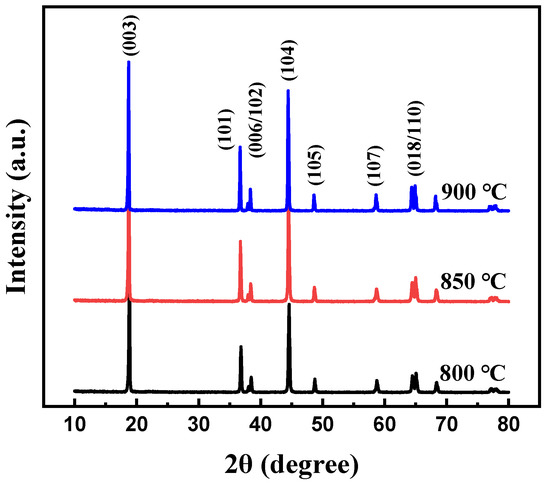
Figure 1.
XRD patterns of Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 calcinated at different temperatures.
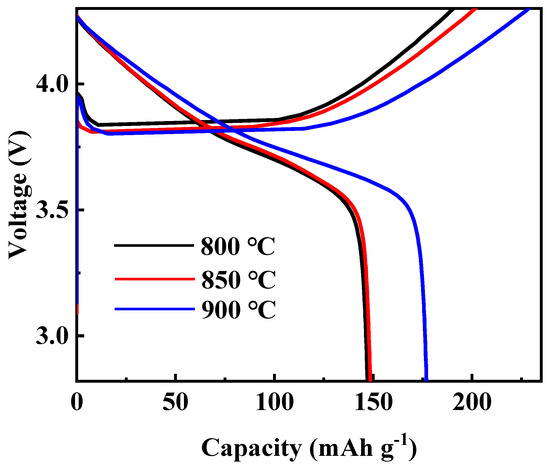
Figure 2.
Initial charge–discharge curves of Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 calcinated at different temperatures.
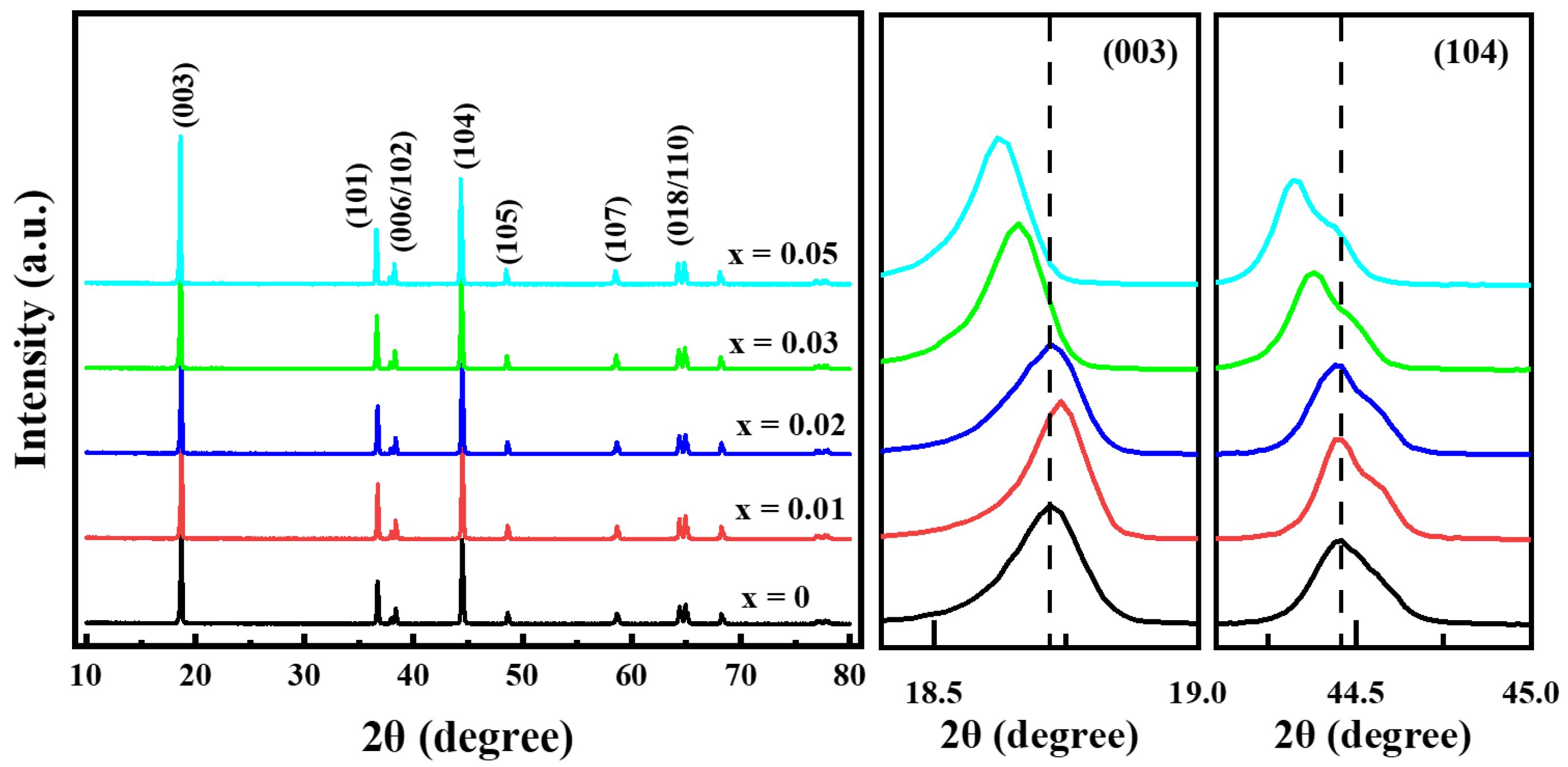
Figure 3 shows the XRD patterns of the prepared Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 (x = 0, 0.01, 0.02, 0.03 and 0.05) materials. All of the samples still exhibited a well-defined layered structure based on a hexagonal α-NaFeO2 structure with low cation mixing between Li+ and Ni2+ in the lithium layer; no impurity phases emerged even for the sample with x = 0.05. As the Ga content increased, the lattice parameters of a and c increased gradually from 2.8695 and 14.2202 Å for x = 0 to 2.8763 and 14.2601 Å for x = 0.05, respectively; this occurred due to the fact that the Ga3+ ionic radius (0.62 Å) is similar to the average ionic radius of Ni2+ (0.69 Å) and Mn4+ (0.53 Å) but is larger than that of Co3+ (0.545 Å) and Ni3+ (0.56 Å). This implies that Ga3+ was doped into the crystal lattice successfully. Although the lattice parameters of a and c improved gradually as the Ga content increased, little change occurred for the lattice parameter ratio of c/a, indicating that small quantities of Ga3+ doping do not generate a negative effect on the two-dimensional layered structure of LiNi0.6Co0.2Mn0.2O2 materials.
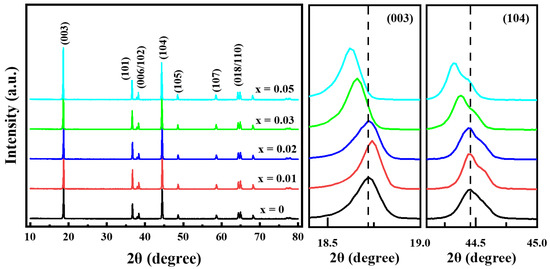
Figure 3.
XRD patterns of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 samples.
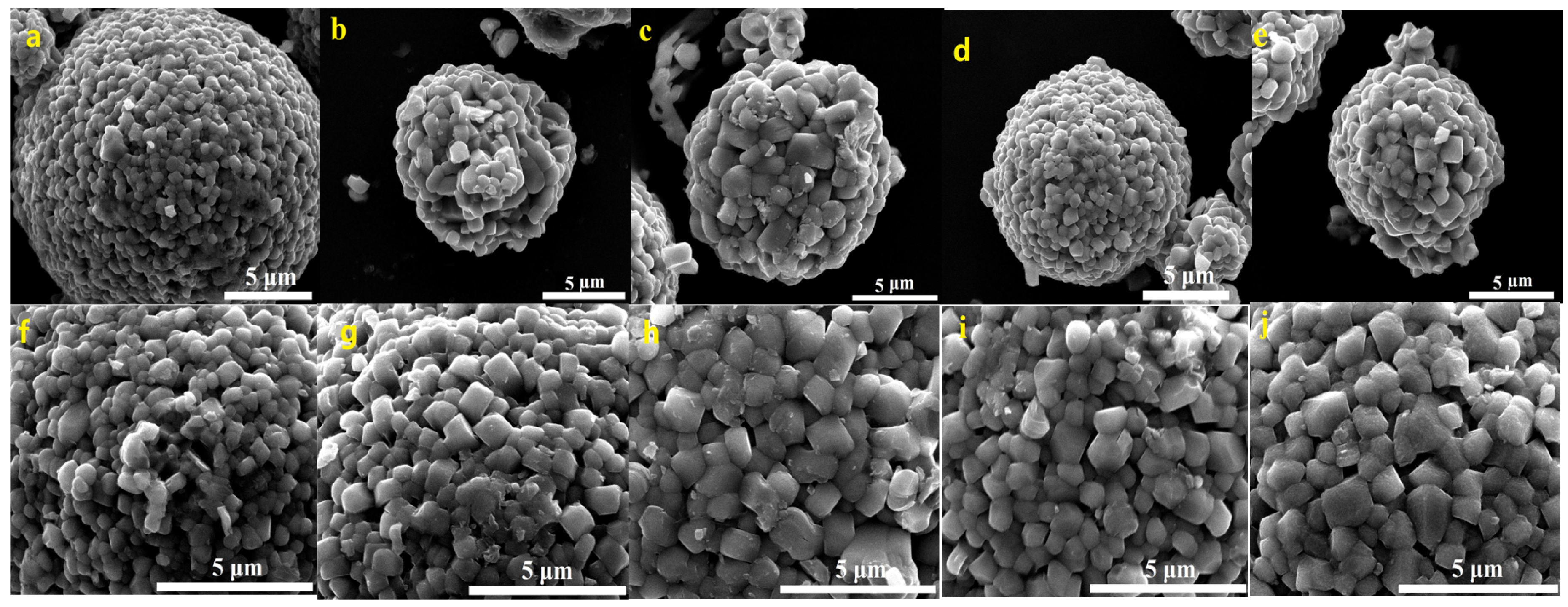
FESEM images of the prepared Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 (x = 0, 0.01, 0.02, 0.03 and 0.05) materials are shown in Figure 4. It can be seen that all five samples present the morphology of dense agglomerated secondary spherical particles with a particle size of ~10 µm. It is noted that the primary particle size increased from ~300 nm for x = 0 µm to ~1 µm for x = 0.02–0.05; the crystal planes and grain boundaries became clearer, indicating that a moderate Ga doping is beneficial for promoting crystal growth.
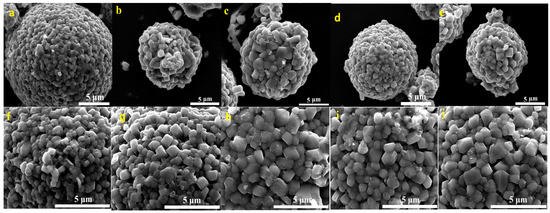
Figure 4.
FESEM images of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2: x = 0 (a,f), x = 0.01 (b,g), x = 0.02 (c,h), x = 0.03 (d,i), x = 0.05 (e,j).
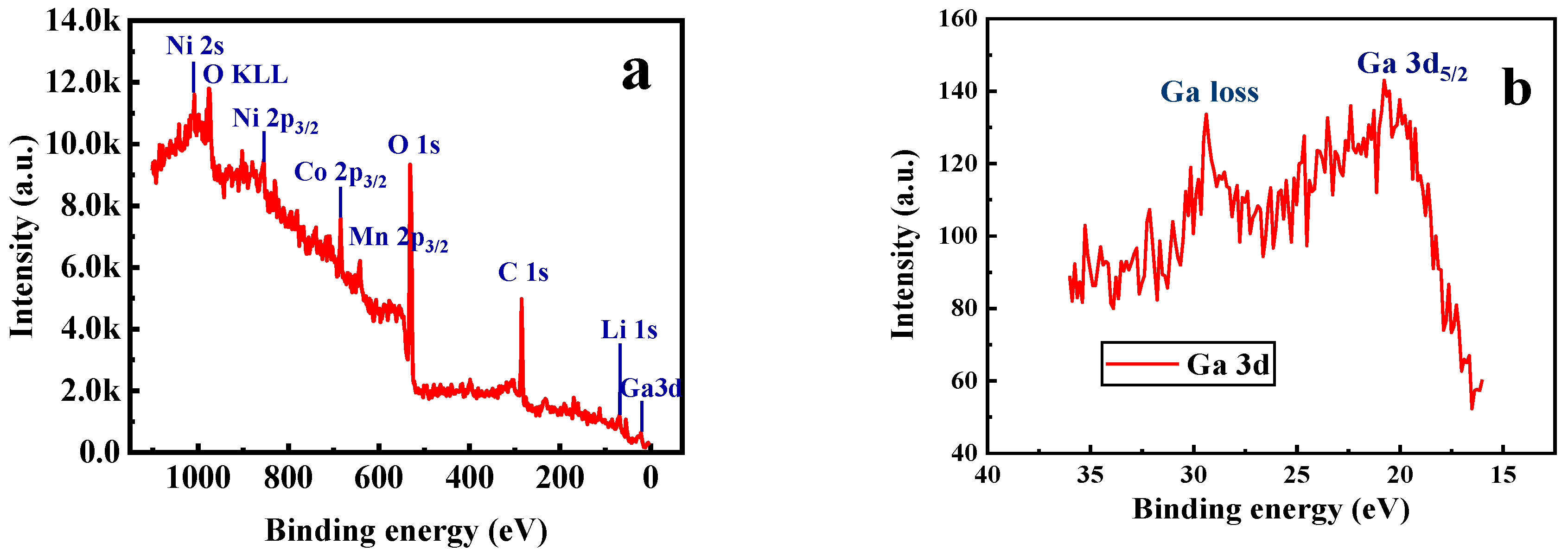
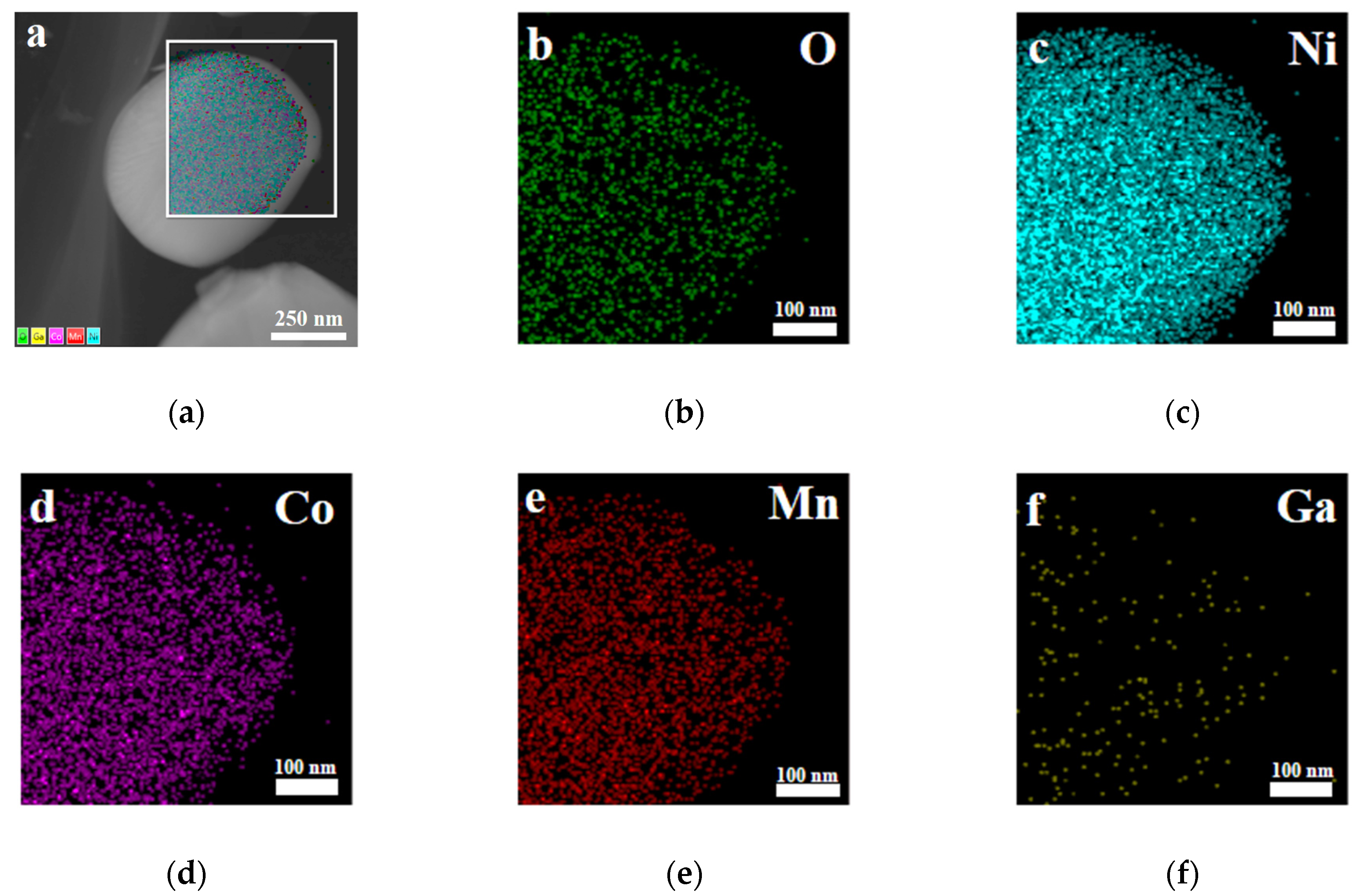
Furthermore, X-ray photoelectron spectroscopy (XPS) and energy dispersive X-ray (EDX) spectroscopy analysis were carried out for the sample with x = 0.02. As shown in Figure 5a, the photoelectron peak of Ga 3d can be observed in the XPS survey spectra. It can be seen from Figure 5b that the binding energy corresponding to the Ga 3d5/2 peak is 20.8 eV, which is consistent well with the reported data for Ga2O3 [39], indicating that the valence state of the doped Ga ions remains +3. Figure 6 presents the EDX mapping of the Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 material. As in the case of Ni, Co, Mn, and O atoms, the doped Ga element is also uniformly distributed, which can conform uniform Ga doping in the material.
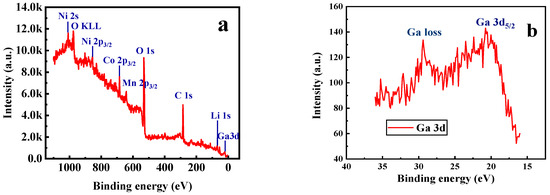
Figure 5.
XPS spectra of Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2. (a) Survey scan; (b) Ga 3d spectra.
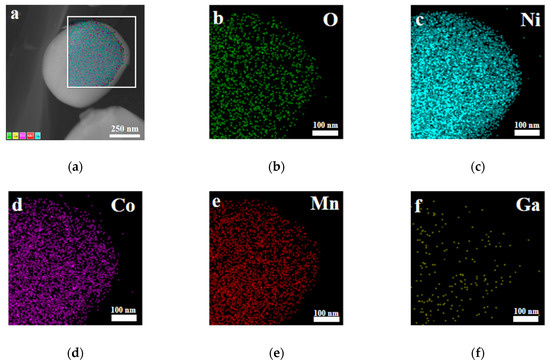
Figure 6.
Energy dispersive X-ray (EDX) spectroscopy mapping of Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2. (a) Layered image; (b) O atoms; (c) Ni atoms; (d) Co atoms; (e) Mn atoms; (f) Ga atoms.
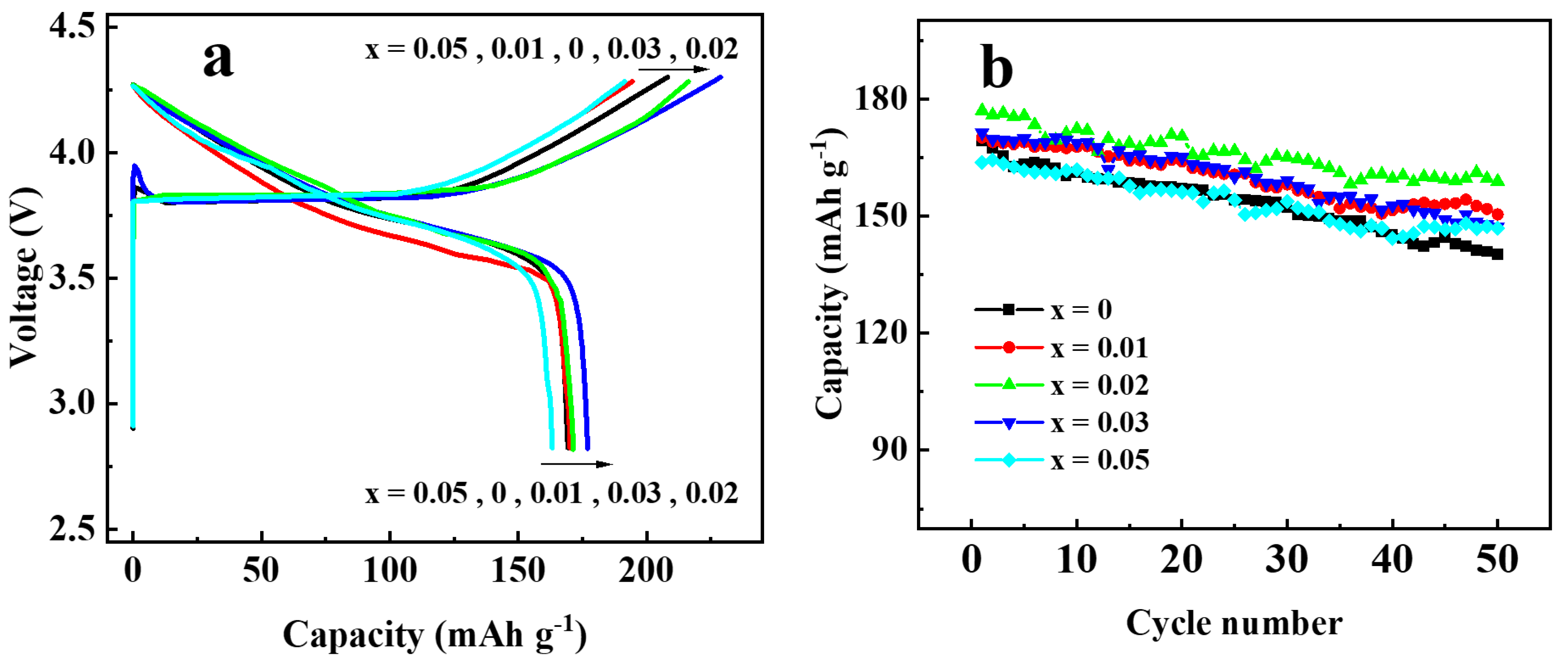
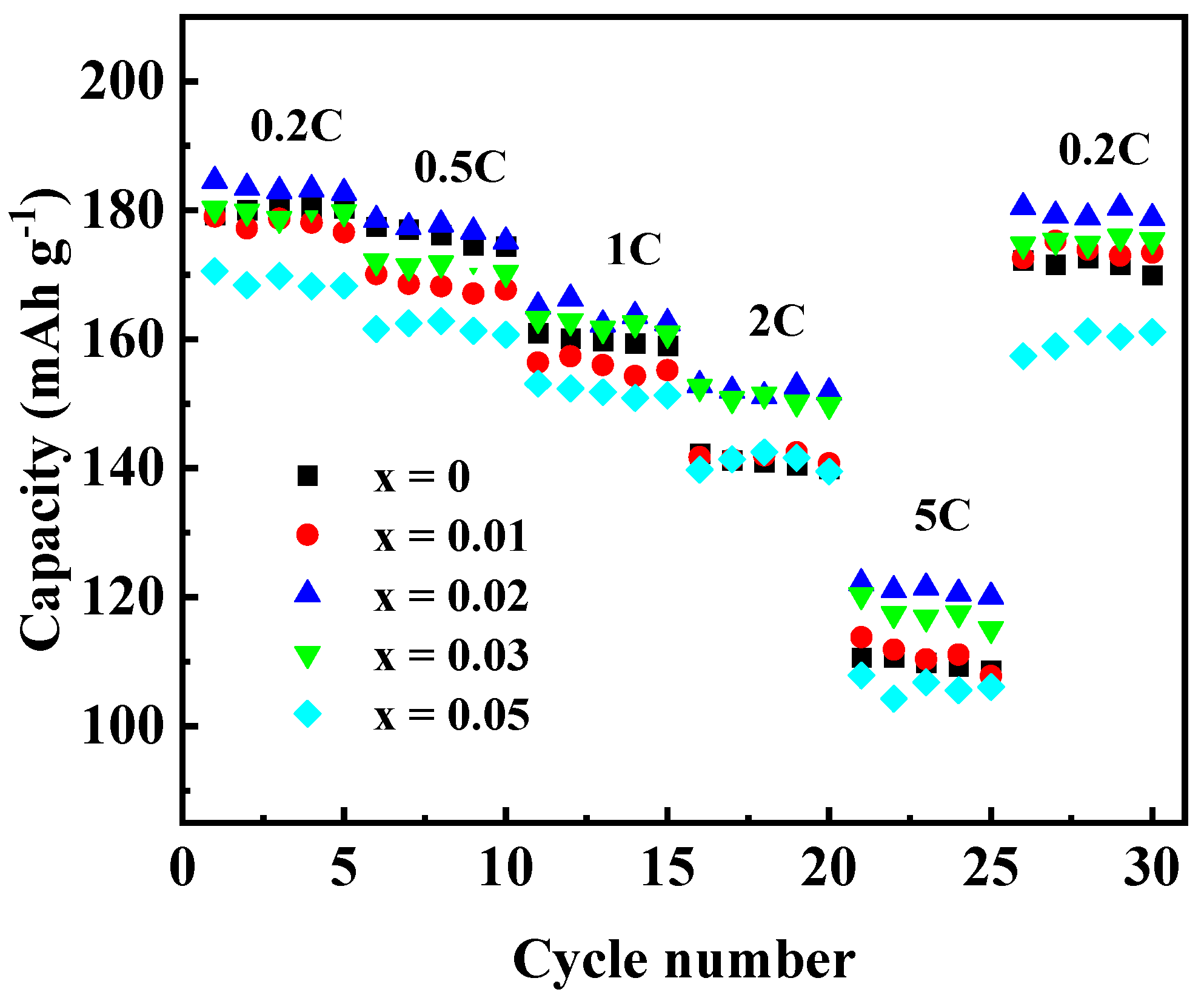
To investigate the effect of Ga doping on the electrochemical performance of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 materials, the as-prepared materials were assembled into 2032 coin cells for the charging and discharging tests. Figure 7 shows the initial charge–discharge curves (Figure 7a) and the cycling performances (Figure 7b) at 0.5 C (1 C = 200 mA g−1) in the voltage between 2.8 and 4.3 V. The discharge capacity of the samples with Ga content x = 0, 0.01, 0.02, 0.03, and 0.05 were found to be 169.3, 170.1, 177.0, 171.3, and 163.2 mAh g−1 in the first cycle, and 140.2, 150.4, 158.9, 147.3 and 146.9 mAh g−1 after 50 cycles, with the capacity retention of 82.8, 88.4, 89.8, 86.0, and 89.7%, respectively. Enhanced discharge capacity for the sample with x = 0.02 may be attributed to the improved cation order. However, when the Ga content was x ≥0.03, the discharge capacity dropped again, which may be due to the excessive doping of Ga3+ without electrochemical activity. In addition, the rate performance of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 was also investigated, as shown in Figure 8. Among the five samples, the one with x = 0.02 still showed the best electrochemical performance, with a discharge capacity that reached 183.4 mAh g−1 at 0.2 C and 121.1 mAh g−1 at 5 C, respectively. It was noted that the capacity from 26 cycles to 30 cycles at 0.2 C, followed by a charge–discharge at 5 C from 21 to 25 cycles, still retained 97.9% of its initial discharge capacity at 0.2 C in the first cycle. The above results reveal that a Ga substitution for 2% of the transition metal elements in the NCM622 material can significantly improve the electrochemical performance.
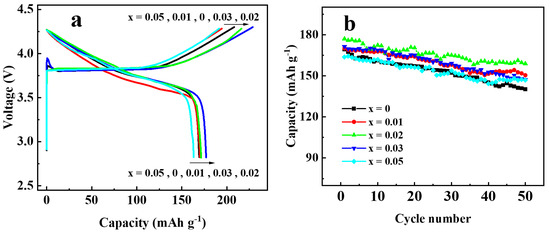
Figure 7.
Charge–discharge performance of Li[Ni0.6Co0.2Mn0.2 ]1−xGaxO2 at 0.5C rate: (a) initial charge–discharge curves; (b) cycling performance.
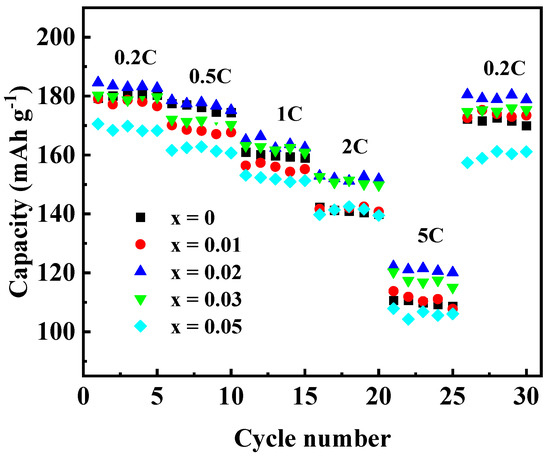
Figure 8.
Rate capabilities of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2.
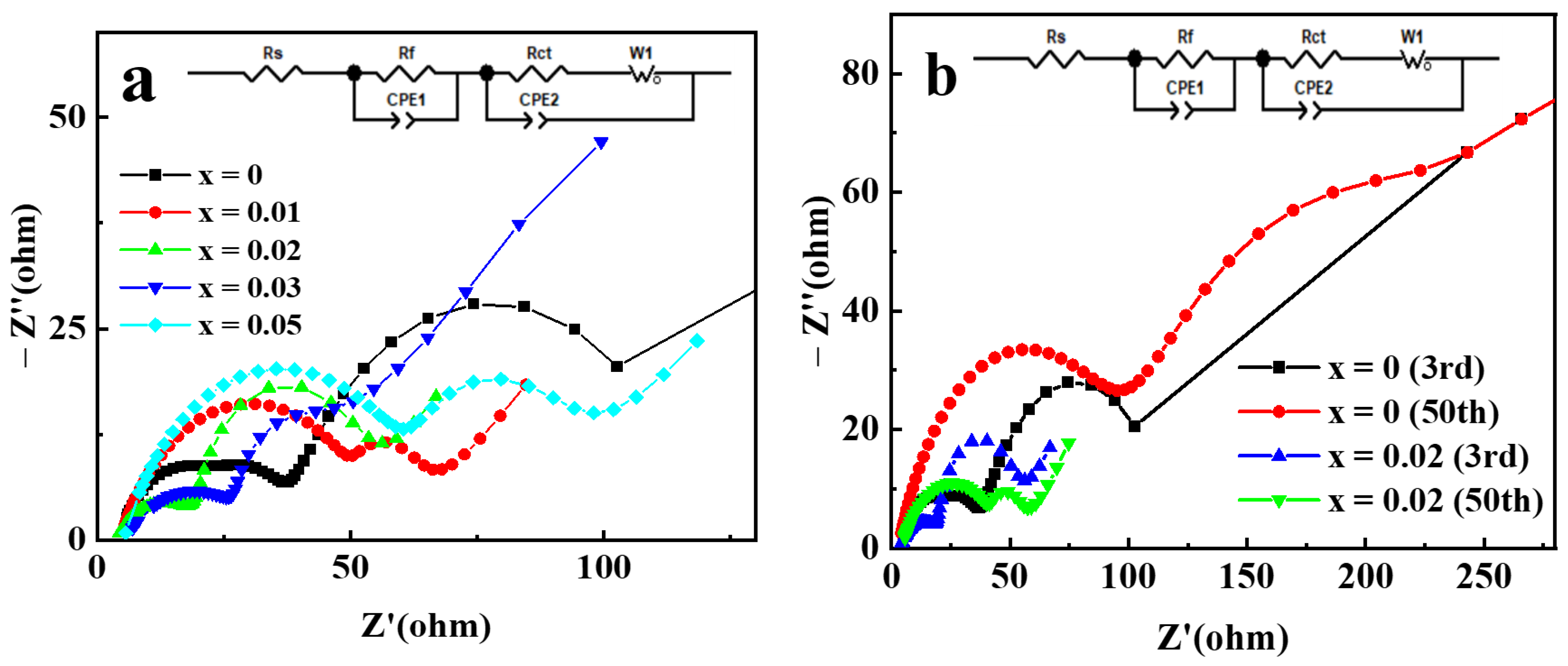
Electrochemical impedance spectroscopy (EIS) analysis was used to further clarify the mechanism for the improvement of the electrochemical performance of NCM622 by Ga doping. The Nyquist plots of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 electrodes are displayed in Figure 9. The Nyquist plots consist of the following: (a) a semicircle in the high frequency range assigned to surface film resistance; (b) another semicircle in the medium frequency range assigned to charge transfer impedance; and (c) a sloped line in the low-frequency range assigned to the impedance of diffusion of lithium ions [40]. The analysis of the plots was performed by fitting the equivalent circuit; the fitting results for Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 electrodes after three cycles are shown in Table 1. Compared with pristine electrodes, the Ga-doped cathode electrodes with x = 0.01, 0.02 and 0.03 exhibited minor decreased surface film resistance (Rf), and remarkably reduced charge transfer resistance (Rct). This indicates that Ga doping can improve the electrochemical activity in the interface of electrode/electrolyte, thereby helping to reduce electrochemical polarization and enhance the capacity and rate performance. However, the Rf value for the sample with Ga content x = 0.05 increased significantly, which may be due to the presence of impurity phases on the surface of the material. Although impurity phases are not observed in Figure 1, the research based on synchrotron XRD analysis confirmed the limited solubility of Ga in the LiNiO2 and the formation of impurity phase Li5GaO4 for the Ga-doped LiNiO2 samples [34]. The Li+ diffusion coefficient can also be calculated by using the method in reference [28] to process the EIS data. The obtained Li+ diffusion coefficients for the samples with Ga content x = 0, 0.01, 0.02, 0.03, and 0.05 are 1.30 × 10−12, 5.80 × 10−11, 8.85 × 10−11, 6.92 × 10−11, and 3.81 × 10−11 cm2 s−1, respectively. The change trend of the Li+ diffusion coefficient is completely consistent with that of the charge transfer impedance, which may be related to the synergy of charge transfer. Compared with the pristine sample, the Ga-doped sample with x = 0.02 exhibited a Li+ diffusion coefficient as high as about 53 times, which is consistent with its best electrochemical performance. EIS analysis of Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 was further conducted after 50 cycles, and the results, compared with those of a pristine electrode, are presented in Figure 9b. The Rf and Rct of the pristine electrode increased from 18.62 and 32.34 Ω in the 3rd cycle to 96.26 and 135.6 Ω in the 50th cycle, respectively, resulting in an increase in the interface impedance by 180.9 Ω. However, the Rf of the Ga-doped sample with x = 0.02 increased by 19.31 Ω, and the Rct decreased by 17.82 Ω due to the improved electrode activation, resulting in an increase in the total interface impedance by only 1.5 Ω after 50 cycles. It can be concluded that Ga doping can effectively suppress the increase in interface impedance of the Ga-doped cathode electrode during cycling, which is beneficial for enhancing capacity retention.
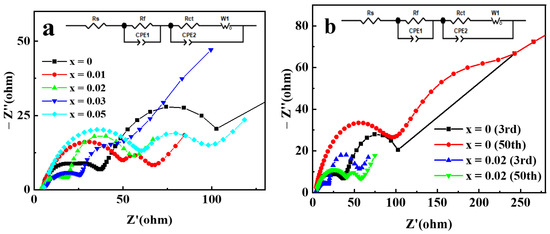
Figure 9.
The Nyquist plots of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 electrodes: (a) after 3 cycles; (b) comparison after 50 cycles with 3 cycles for x = 0 and 0.02 electrodes.

Table 1.
Electrochemical impedance spectroscopy (EIS) fitting results of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 electrodes after 3 cycles.
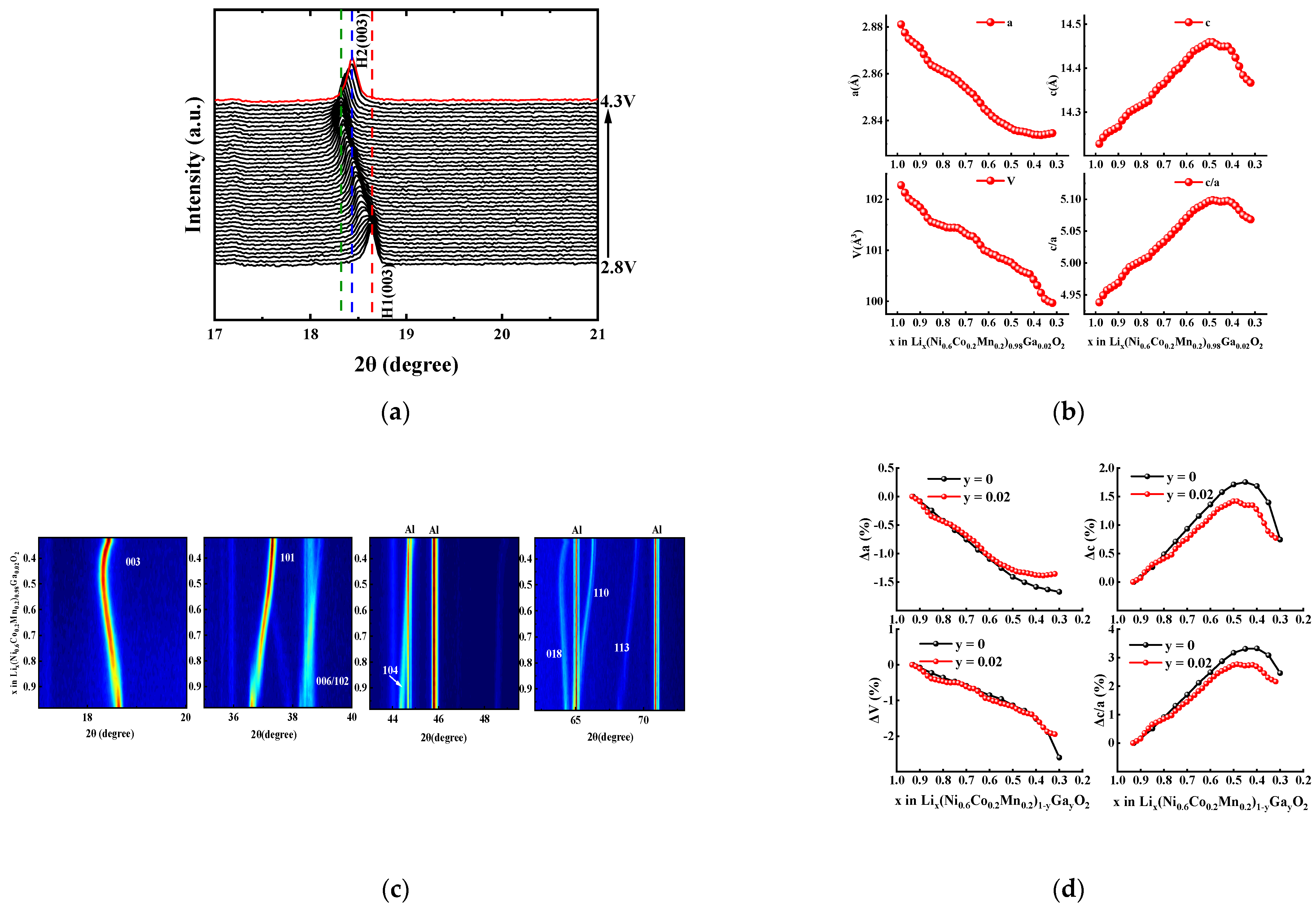
For LiNi1−x−yCoxMnyO2 materials, the increase in interface impedance during cycling is mainly ascribed to the electrode/electrolyte interface side reaction (e.g., electrolyte decomposition, formation of SEI (solid electrolyte interface) film, and transition metal dissolution) and structural instability (e.g., cation mixing, NiO phase formation, microcracks, and microstrains caused by phase transition). The interface side reactions are often coupled with structural instability. For example, new interface side reactions occur on the generated microcracks, which can accelerate capacity fading during cycling [1,2]. Therefore, improving structural stability is very important in order to enhance the cycle life of LiNi1−x−yCoxMnyO2 materials. In this work, in situ XRD analysis was performed on the Ga-doped sample with x = 0.02 during the charge–discharge process, the results of which are shown in Figure 10. As shown in Figure 10a,b, the hexagonal phase structure is maintained all over the charging process—i.e., without obvious diffraction peaks ascribed to the monoclinic phase or the two-hexagonal phase—which is similar to that of LiNi0.98Ga0.02O2 material [33]. This should be attributed to the stabilizing effect of Ga doping on the layered structure.
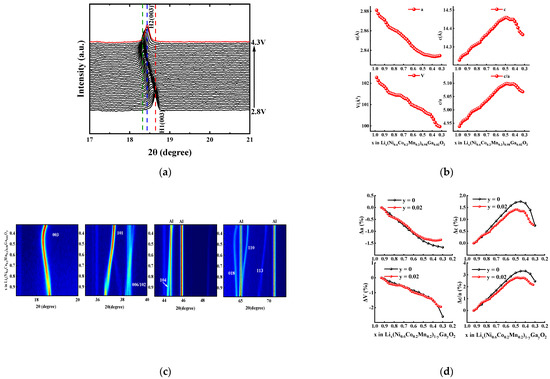
Figure 10.
In situ XRD analysis for Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 electrode during charging process from 2.8 V to 4.3 V: (a) (003) diffraction peaks; (b) contour plots; (c) changes in lattice parameters; (d) relative change of lattice parameters (the data for sample with y = 0 from reference [5]).
It can be seen from the refined lattice parameters (Figure 10c) that the c value, which represents the interslab distance of the Li layers, increased initially with the deintercalation of Li+ ions due to the increase in Coulomb repulsion. However, when most of the Li+ ions were removed (corresponding to Li content x = 0.47 in LixMO2), a contraction of interslab distance occurred with a decrease in c value, which also led to a sharp contraction of c/a and V. For Ni-rich ternary materials, the severe change in the crystal lattice along the c-direction during the charging–discharging process presumably causes the instability of the lattice structure [5,34]. As shown in Figure 10d, the relative changes in lattice parameters of Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 are compared with the reported values of undoped NCM622 material [5] during the delithiation process. It can be seen that the rapid contraction occurred for NCM622 material, while the Li content y in LixMO2 was less than 0.45, and the lattice parameters c, c/a, and unit cell volume V shrunk by 1, 0.82, and 1.33%, respectively, until the Li content y value reached 0.3 (4.32 V vs. Li/Li+) [5]. However, for the Ga-doped sample Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2, the shrinkage of lattice parameters c, c/a, and V in almost the same delithiation range decreased to 0.57, 0.6, and 0.72%, respectively. In addition, the total unit cell volume of Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 decreased by 1.94% in the entire charging process (4.3 V vs. Li/Li+), which is smaller than the 2.6% of NCM622 material [5]. The above results confirm that Ga doping helps to increase the structural stability, which in turn can decrease the intergranular cracks and mechanical strain occurring in cycling, thus improving the cycle life of NCM622 material.
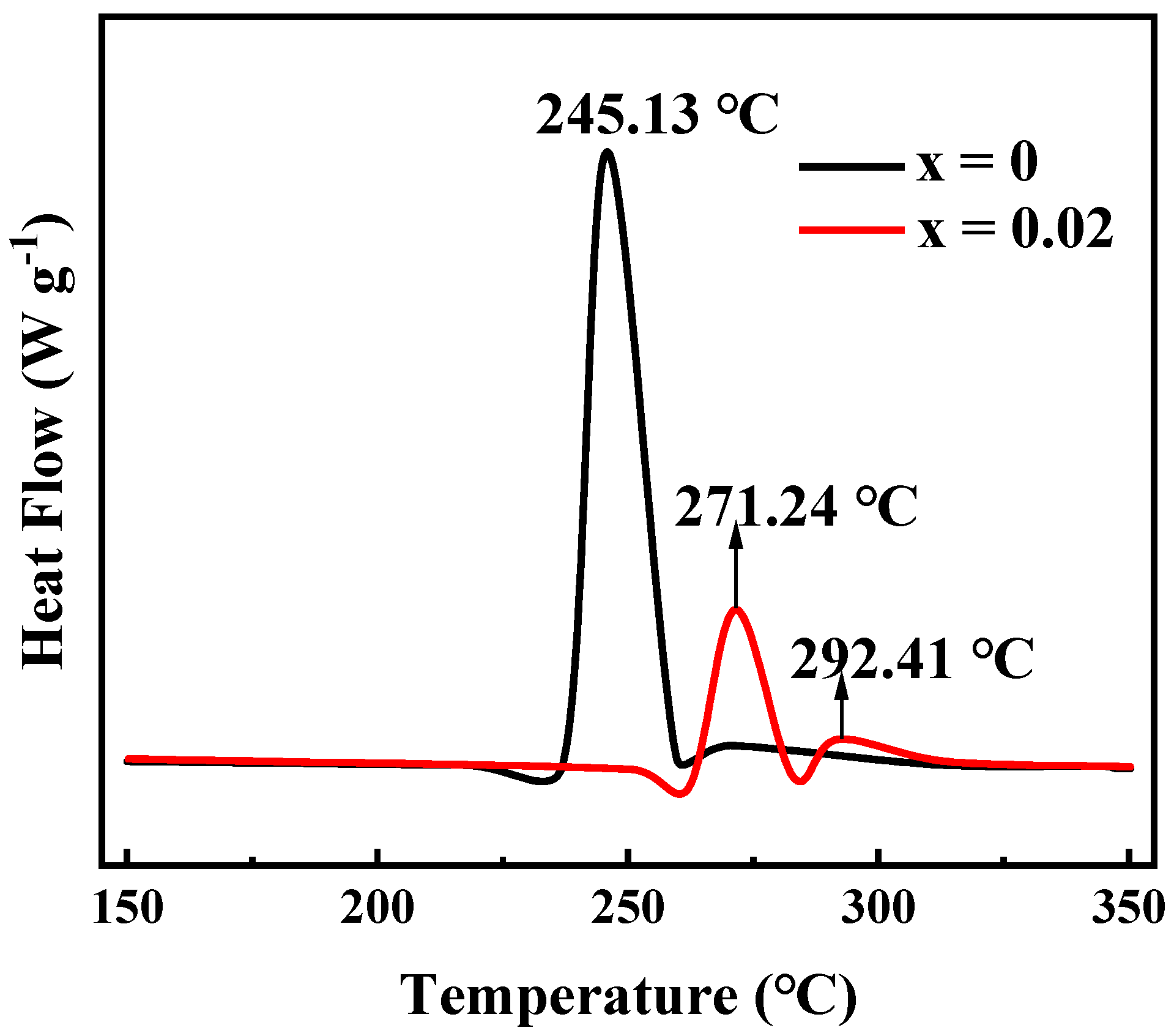
Nickel-rich ternary cathode materials in a deeply delithiated state always suffer from lattice oxygen loss and phase transition at 150–300 °C, accompanied by higher heat generation. Therefore, the thermal stability of nickel-rich cathode materials is a critical aspect for ensuring the safety of lithium rechargeable batteries. Considering this aspect, the thermal stability of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 (x = 0, 0.02) materials was tested after being charged to 4.3 V, the differential scanning calorimeter (DSC) profiles of which are shown in Figure 11. The exothermic peak of LiNi0.6Co0.2Mn0.2O2 appeared at 245.13 °C, and the total generated heat was 561.7 J·g−1. However, for the Ga-doped sample Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2, in addition to an exothermic peak attributed to the phase transition at 271.24 °C, a small exothermic peak also appeared at 292.41 °C, which may be related to the side reaction between the cathode and electrolyte [41]. It is clear that not only the exothermic temperature increases, but the total exothermic heat also decreases to 225.6 J·g−1. This indicates that Ga doping is beneficial for improving the thermal stability of NCM622 material. We believe that this is ascribed to the enhanced structural stabilization by Ga doping.
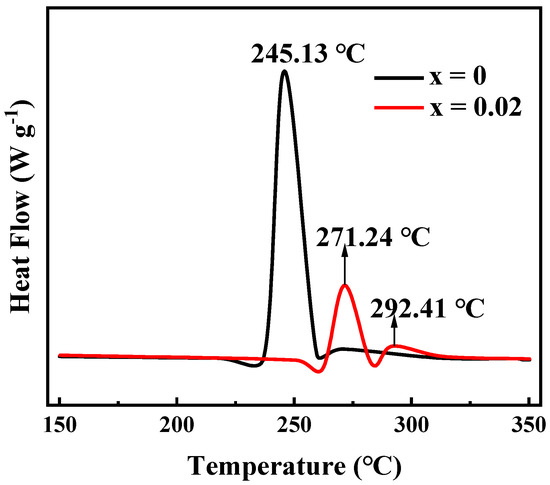
Figure 11.
Differential scanning calorimeter (DSC) curves of Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 (x = 0, 0.02) charged to 4.3 V.
Table 2 lists the performance of some doped LiNi0.6Co0.2Mn0.2O2 materials reported in recent years. These materials were selected because they have the same charge cut-off voltage, which is convenient for performance comparison. It can be seen that the Ga-doped sample synthesized in this work exhibits the highest discharge capacity and rate performance. While comparing the cycle performance, the capacity retention of some samples seemed to be somewhat higher. However, since these were obtained under the circumstances of high rate (1 C) and low initial capacity, the comparison was not feasible. In addition, the Ga-doped sample showed significantly improved thermal stability, while other materials rarely reflected this aspect. On the whole, the obtained Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 in this work exhibits excellent electrochemical and thermal safety, and is a very promising cathode material.

Table 2.
Comparison of electrochemical performance of doped LiNi0.6Co0.2Mn0.2O2 materials from different research.
4. Conclusions
Ga-doped nickel-rich ternary layered Li[Ni0.6Co0.2Mn0.2]1−xGaxO2 (x = 0, 0.01, 0.02, 0.03, 0.05) materials can be successfully prepared at 900 °C using the high-temperature solid-state reaction method. When the Ga content is 0.02, it is not only beneficial for promoting grain growth, but it can also reduce the charge transfer resistance and improve the interface structure and bulk structure stability, thereby significantly improving the electrochemical performance and thermal safety of NCM622 material. Compared with the pristine electrode, the Li(Ni0.6Co0.2Mn0.2)0.98Ga0.02O2 electrode exhibits remarkably improved electrochemical performance and thermal safety. At 0.5 C rate, the discharge capacity increases from 169.3 to 177 mAh g−1, and the capacity retention after 50 cycles also rises from 82.8% to 89.8%. In the charged state of 4.3 V, its exothermic temperature increases from 245.13 °C to more than 271.24 °C, and the total exothermic heat decreases from 561.7 to 225.6 J·g−1. Compared with some doped NCM622 materials reported in recent literature, the obtained Li[Ni0.6Co0.2Mn0.2]0.98Ga0.02O2 in this work exhibits excellent electrochemical and thermal safety and is a very promising cathode material. Enhancing the structural stability of NCM622 material in the charge–discharge process by Ga doping improves cycle stability and thermal safety, which can provide a new idea for improving the performance of long-life high-safety nickel-rich ternary materials for lithium-ion batteries.
Author Contributions
Conceptualization, J.L., L.W. and X.H.; methodology, J.L., L.W., Y.K.; investigation, Z.L., M.Z., Z.D. and J.Y.; data curation, M.Z., Z.D. and J.Y.; validation, Z.L., J.L., L.W. and Y.K.; writing—original draft preparation, Z.L., J.L.; writing—review and editing, X.H.; funding acquisition, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges under Beijing Municipality (IDHT20180508), the Ministry of Science and Technology of China (No. 2019YFE0100200), the Tsinghua-Foshan Scientific Research Program (No. 2019THFS0132), and the Tsinghua University Initiative Scientific Research Program (No. 2019Z02UTY06). The authors also thank Joint Work Plan for Research Projects under the Clean Vehicles Consortium at the U.S. and China—Clean Energy Research Center (CERC-CVC2.0, 2016–2020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Liu, W.; Oh, P.; Liu, X.; Lee, M.-J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. 2015, 54, 4440–4457. [Google Scholar] [CrossRef]
- Manthiram, A.; Knight, J.C.; Myung, S.-T.; Oh, S.-M.; Sun, Y.-K. Nickel-rich and lithium-rich layered oxide cathodes: Progress and perspectives. Adv. Energy Mater. 2016, 6, 1501010. [Google Scholar] [CrossRef]
- Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Source 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Ma, L.; Nie, M.; Xia, J.; Dahn, J.R. A systematic study on the reactivity of different grades of charged Li[NixMnyCoz]O2 with electrolyte at elevated temperatures using accelerating rate calorimetry. J. Power Source 2016, 327, 145–150. [Google Scholar] [CrossRef]
- Biasi, L.; Kondrakov, A.O.; Geßwein, H.; Brezesinski, T.; Hartmann, P.; Janek, J. Between Scylla and Charybdis: Balancing among structural stability and energy density of layered NCM cathode materials for advanced lithium-ion batteries. J. Phys. Chem. C 2017, 121, 26163–26171. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Xie, Q.; You, Y.; Chi, M.; Manthiram, A. Long-term cyclability of NCM-811 at high voltages in lithium-ion batteries: An in-depth diagnostic study. Chem. Mater. 2020, 32, 7796–7804. [Google Scholar] [CrossRef]
- Lee, W.; Muhammad, S.; Kim, T.; Kim, H.; Lee, E.; Jeong, M.; Son, S.; Ryou, J.-H.; Yoon, W.-S. New insight into Ni-rich layered structure for next-generation Li rechargeable batteries. Adv. Energy Mater. 2018, 8, 1701788. [Google Scholar] [CrossRef]
- Min, K.; Kim, K.; Jung, C.; Seo, S.-W.; Song, Y.Y.; Lee, H.S.; Shin, J.; Cho, E. A comparative study of structural changes in lithium nickel cobalt manganese oxide as a function of Ni content during delithiation process. J. Power Source 2016, 15, 111–119. [Google Scholar] [CrossRef]
- Kim, N.Y.; Yim, T.; Song, J.H.; Yu, J.-S.; Lee, Z. Microstructural study on degradation mechanism of layered LiNi0.6Co0.2Mn0.2O2 cathode materials by analytical transmission electron microscopy. J. Power Source 2016, 307, 641–648. [Google Scholar] [CrossRef]
- Saavedra-Arias, J.J.; Rao, C.V.; Shojan, J.; Manivannan, A.; Torres, L.; Ishikawa, Y.; Katiyar, R.S. A combined first-principles computational/experimental study on LiNi0.66Co0.17Mn0.17O2 as a potential layered cathode material. J. Power Source 2012, 211, 12–18. [Google Scholar]
- Kim, Y. First-principles investigation of the structural characteristics of LiMO2 cathode materials for lithium secondary batteries. J. Mol. Struct. 2015, 1099, 317–322. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, J.; Cui, S.; Song, X.; Su, Y.; Deng, W.; Wu, Z.; Wang, X.; Wang, W.; Rao, M.; et al. Kinetics tuning of Li-ion diffusion in layered Li(NixMnyCoz)O2. J. Am. Chem. Soc. 2015, 137, 8364–8367. [Google Scholar] [CrossRef]
- Chen, Y.P.; Zhang, Y.; Wang, F.; Wang, Z.; Zhang, Q. Improve the structure and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode material by nano-Al2O3 ultrasonic coating. J. Alloys Compd. 2014, 611, 135–141. [Google Scholar] [CrossRef]
- Tao, F.; Yan, X.-X.; Liu, J.-J.; Zhang, H.-L.; Chen, L. Effects of PVP-assisted Co3O4 coating on the electrochemical and storage properties of LiNi0.6Co0.2Mn0.2O2 at high cut-off voltage. Electrochim. Acta 2016, 210, 548–556. [Google Scholar] [CrossRef]
- Cho, W.; Kim, S.-M.; Song, J.-H.; Yim, T.; Woo, S.-G.; Lee, K.-W.; Kim, J.-S.; Kim, Y.-J. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Source 2015, 282, 45–50. [Google Scholar] [CrossRef]
- Jo, C.-H.; Cho, D.-H.; Noh, H.-J.; Yashiro, H.-J.; Sun, H.; Myung, Y.-K.; Taek, S. An effective method to reduce residual lithium compounds on Ni-rich Li[Ni0.6Co0.2Mn0.2]O2 active material using a phosphoric acid derived Li3PO4 nanolayer. Nano Res. 2015, 8, 1464–1479. [Google Scholar] [CrossRef]
- Cho, W.; Kim, S.-M.; Lee, K.-W.; Song, J.H.; Jo, Y.N.; Yim, T.; Kim, H.; Kim, J.-S.; Kim, Y.-J. Investigation of new manganese orthophosphate Mn3(PO4)2 coating for nickel-rich LiNi0.6Co0.2Mn0.2O2 cathode and improvement of its thermal properties. Electrochim. Acta 2016, 198, 77–83. [Google Scholar] [CrossRef]
- Choi, J.-W.; Lee, J.-W. Improved electrochemical properties of Li(Ni0.6Mn0.2Co0.2)O2 by surface coating with Li1.3Al0.3Ti1.7(PO4)3. J. Power Source 2016, 307, 63–68. [Google Scholar] [CrossRef]
- Liu, W.; Li, X.; Xiong, D.; Hao, Y.; Li, J.; Kou, H.; Yan, B.; Li, D.; Lu, S.; Koo, A.; et al. Significantly improving cycling performance of cathodes in lithium ion batteries: The effect of Al2O3 and LiAlO2 coatings on LiNi0.6Co0.2Mn0.2O2. Nano Energy 2018, 44, 111–120. [Google Scholar] [CrossRef]
- Liu, S.; Wu, H.; Huang, L.; Xiang, M.; Liu, H.; Zhang, Y. Synthesis of Li2Si2O5-coated LiNi0.6Co0.2Mn0.2O2 cathode materials with enhanced high—voltage electrochemical properties for lithiumion batteries. J. Alloys Compd. 2016, 674, 447–454. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.G.; Jeong, H.Y.; Nam, H.; Cho, J. A new coating method for alleviating surface degradation of LiNi0.6Co0.2Mn0.2O2 cathode material: Nanoscale surface treatment of primary particles. Nano Lett. 2015, 15, 2111–2119. [Google Scholar] [CrossRef]
- Schipper, F.; Dixit, M.; Kovacheva, D.; Talianker, M.; Haik, O.; Grinblat, J.; Erickson, E.M.; Ghanty, C.; Major, D.T.; Markovsky, B.; et al. Stabilizing nickel-rich layered cathode materials by a high-charge cation oping strategy: Zirconium-doped LiNi0.6Co0.2Mn0.2O2. J. Mater. Chem. A 2016, 4, 16073–16084. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Z.K.; Wu, F.; Mu, D.; Wang, L.; Wu, B. The effects of molybdenum doping on LiNi0.6Co0.2Mn0.2O2 cathode material. Solid State Ion. 2019, 337, 107–114. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Jing, Q.; Guo, H.; Li, X.; Yang, Z. Investigation on the effect of Na doping on structure and Li-ion kinetics of layered LiNi0.6Co0.2Mn0.2O2 cathode material. Electrochim. Acta 2016, 192, 120–126. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Zheng, X.; Guo, H. Effect of Mg doping on the structural and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials. Electrochim. Acta 2015, 182, 795–802. [Google Scholar] [CrossRef]
- Mofid, W.E.; Ivanov, S.; Konkin, A.; Bund, A. A high performance layered transition metal oxide cathode material obtained by simultaneous aluminum and iron cationic substitution. J. Power Source 2014, 268, 414–422. [Google Scholar] [CrossRef]
- Yue, P.; Wang, Z.; Li, X.; Xiong, X.; Wang, J.; Wu, X.; Guo, H. The enhanced electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials by low temperature fluorine substitution. Electrochim. Acta 2013, 95, 112–118. [Google Scholar] [CrossRef]
- Xiang, W.; Zhu, C.Q.; Zhang, J.; Shi, H.; Liang, Y.T.; Yu, M.H.; Zhu, X.M.; He, F.R.; Lv, G.P.; Guo, X.D. Synergistic coupling effect of sodium and fluorine co-substitution on enhancing rate capability and cycling performance of Ni-rich cathode for lithium ion battery. J. Alloys Compd. 2019, 786, 56–64. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, G.; Luo, L.; Chen, F.; Xie, T.; Dai, S.; Yuan, M. Enhanced cycling stability of Mg-F co-modified LiNi0.6Co0.2Mn0.2–yMgyO2–zFz for lithium-ion batteries. Trans. Nonferrous Met. Soc. China 2018, 28, 1397–1403. [Google Scholar] [CrossRef]
- Ran, Q.; Zhao, H.; Wang, Q.; Shu, X.; Hu, Y.; Hao, S.; Wang, M.; Liu, J.; Zhang, M.; Li, H. Dual functions of gradient phosphate polyanion doping on improving the electrochemical performance of Ni-rich LiNi0.6Co0.2Mn0.2O2 cathode at high cut-off voltage and high temperature. Electrochim. Acta 2019, 299, 971–978. [Google Scholar] [CrossRef]
- Zhan, X.W.; Gao, S.; Cheng, Y.T. Influence of annealing atmosphere on Li2ZrO3-coated LiNi0.6Co0.2Mn0.2O2 and its high-voltage cycling performance. Electrochim. Acta 2019, 300, 36–44. [Google Scholar] [CrossRef]
- Wen, Y. Introduction to Ion Polarization; Anhui Education Press: Hefei, China, 1985. [Google Scholar]
- Nishida, Y.; Nakane, K.; Satoh, T. Synthesis and properties of gallium-doped as the cathode material for lithium secondary batteries. J. Power Source 1997, 68, 561–564. [Google Scholar] [CrossRef]
- Kitsche, D.; Schweidler, S.; Mazilkin, A.; Geßwein, H.; Fauth, F.; Suard, E.; Hartmann, P.; Brezesinski, T.; Janek, J.; Bianchini, M. The effect of gallium doping on the structure and electrochemical performance of LiNiO2 in lithium-ion batteries. Mater. Adv. 2020, 1, 639–647. [Google Scholar] [CrossRef]
- Kim, J.-J.; Ryu, K.H.; Sakaue, K.; Terauchi, H.; Yo, C.-H. Structural characterization for the chemically Li+ ion extracted LiyCoO2, LiyCo0.95Ga0.05O2, and LiyCo0.9Ga0.1O2 compounds. J. Phys. Chem. Solids 2002, 63, 2037–2045. [Google Scholar] [CrossRef]
- Han, C.J.; Eom, W.S.; Lee, S.M.; Cho, W.I.; Jang, H. Study of the electrochemical properties of Ga-doped LiNi0.8Co0.2O2 synthesized by a sol–gel method. J. Power Source 2005, 144, 214–219. [Google Scholar] [CrossRef]
- Yu, T.; Li, J.; Xu, G.; Li, J.; Ding, F.; Kang, F. Improved cycle performance of Li[Li0.2Mn0.54Co0.13Ni0.13]O2 by Ga doping for lithium ion battery cathode material. Solid State Ion. 2017, 301, 64–71. [Google Scholar] [CrossRef]
- Jaskula, B.W. Gallium. USGS: 2017 Minerals Yearbook. April 2020. Available online: https://pubs.er.usgs.gov/publication/pp1802H (accessed on 23 February 2021).
- Carli, R.; Bianchi, C.L. XPS analysis of gallium oxides. Appl. Surf. Sci. 1994, 74, 99–102. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical impedance spectroscopy of metal oxide electrodes for energy applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Pang, P.; Wang, Z.; Tan, X.; Deng, Y.; Nan, J.; Xing, Z.; Li, H. LiCoO2@LiNi0.45Al0.05Mn0.5O2 as high-voltage lithium-ion battery cathode materials with improved cycling performance and thermal stability. Electrochim. Acta 2019, 327, 135018–135026. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).