Aggregation Induced Emission and Nonlinear Optical Properties of an Intramolecular Charge-Transfer Compound
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Materials
2.2. Experimental Equipment
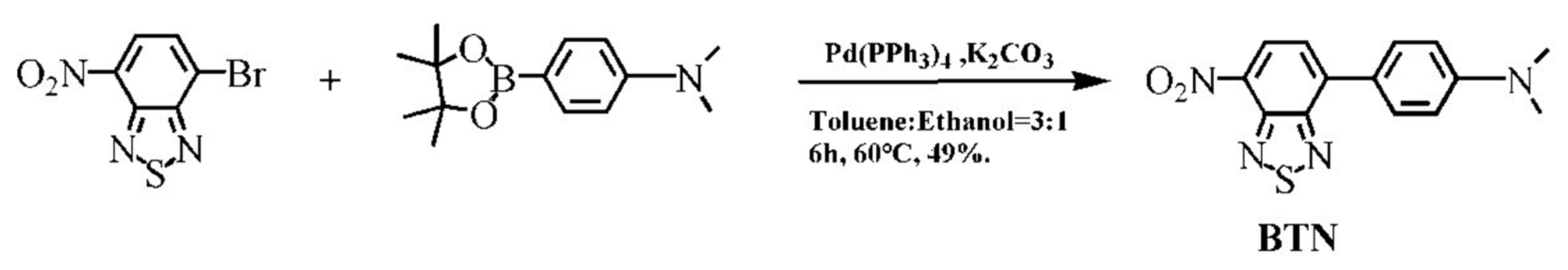
2.3. Synthesis
3. Results and Discussion
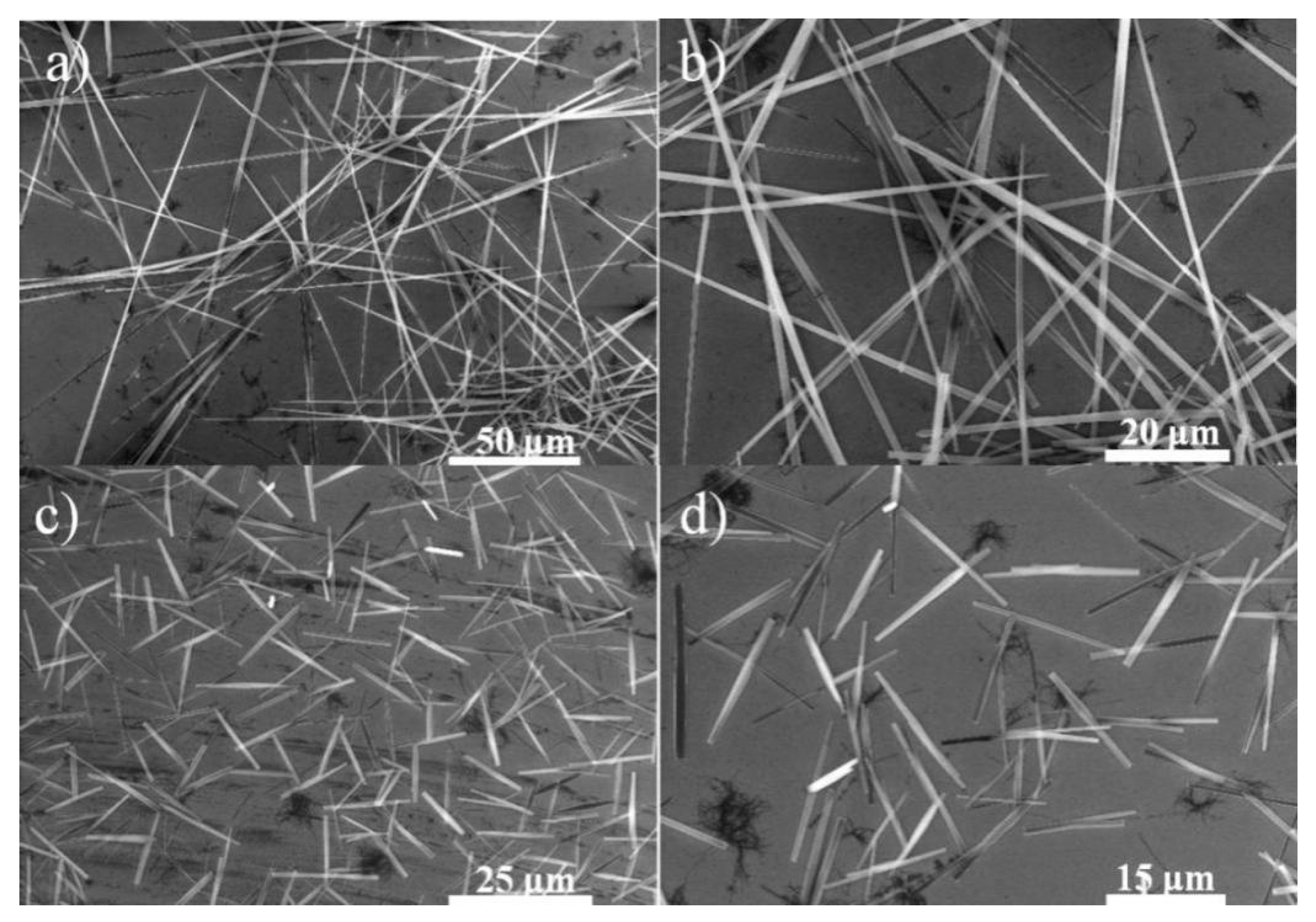
3.1. Self-Assembly
3.2. Crystal Structure
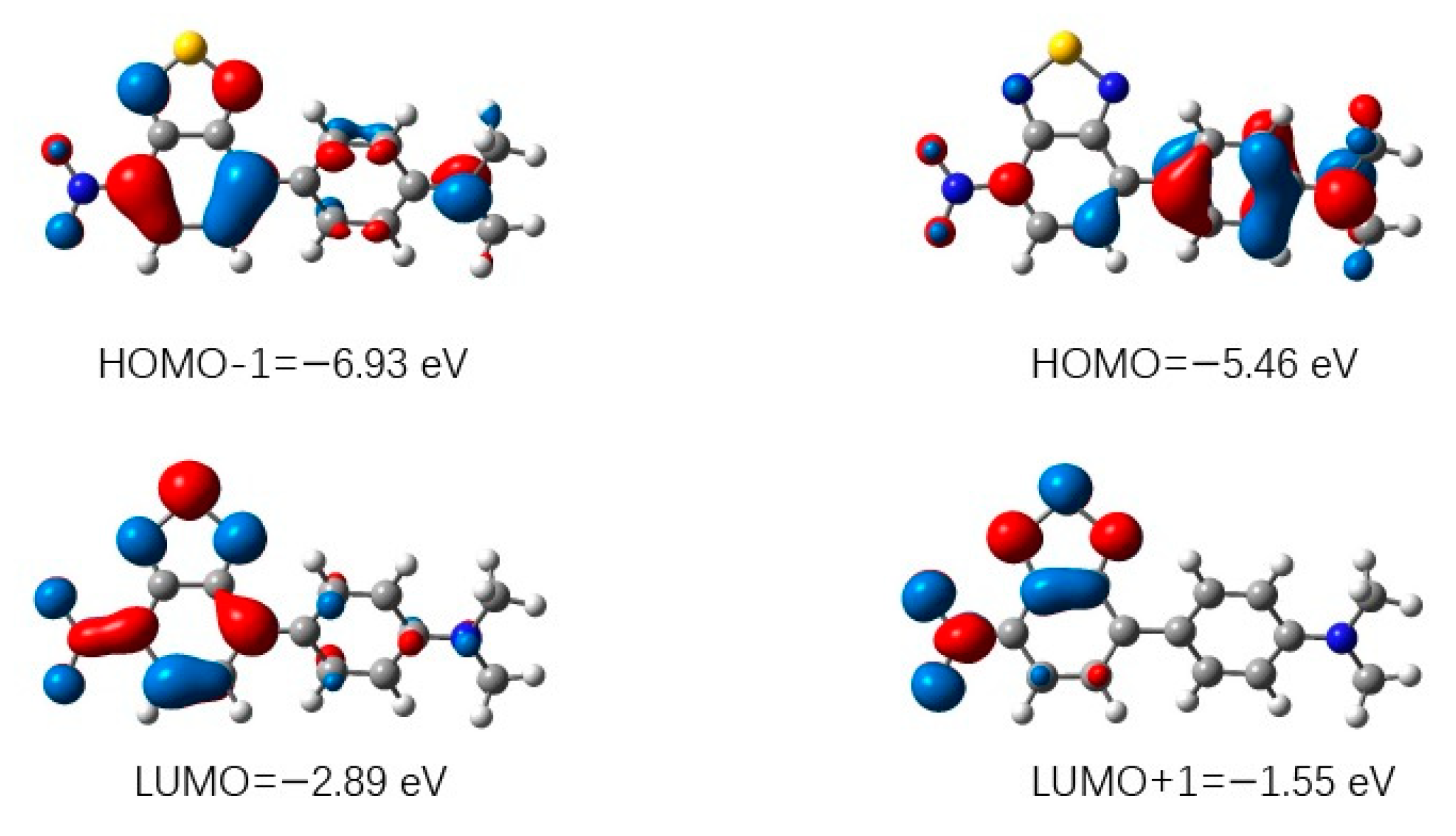
3.3. Theoretical Analysis of Compounds
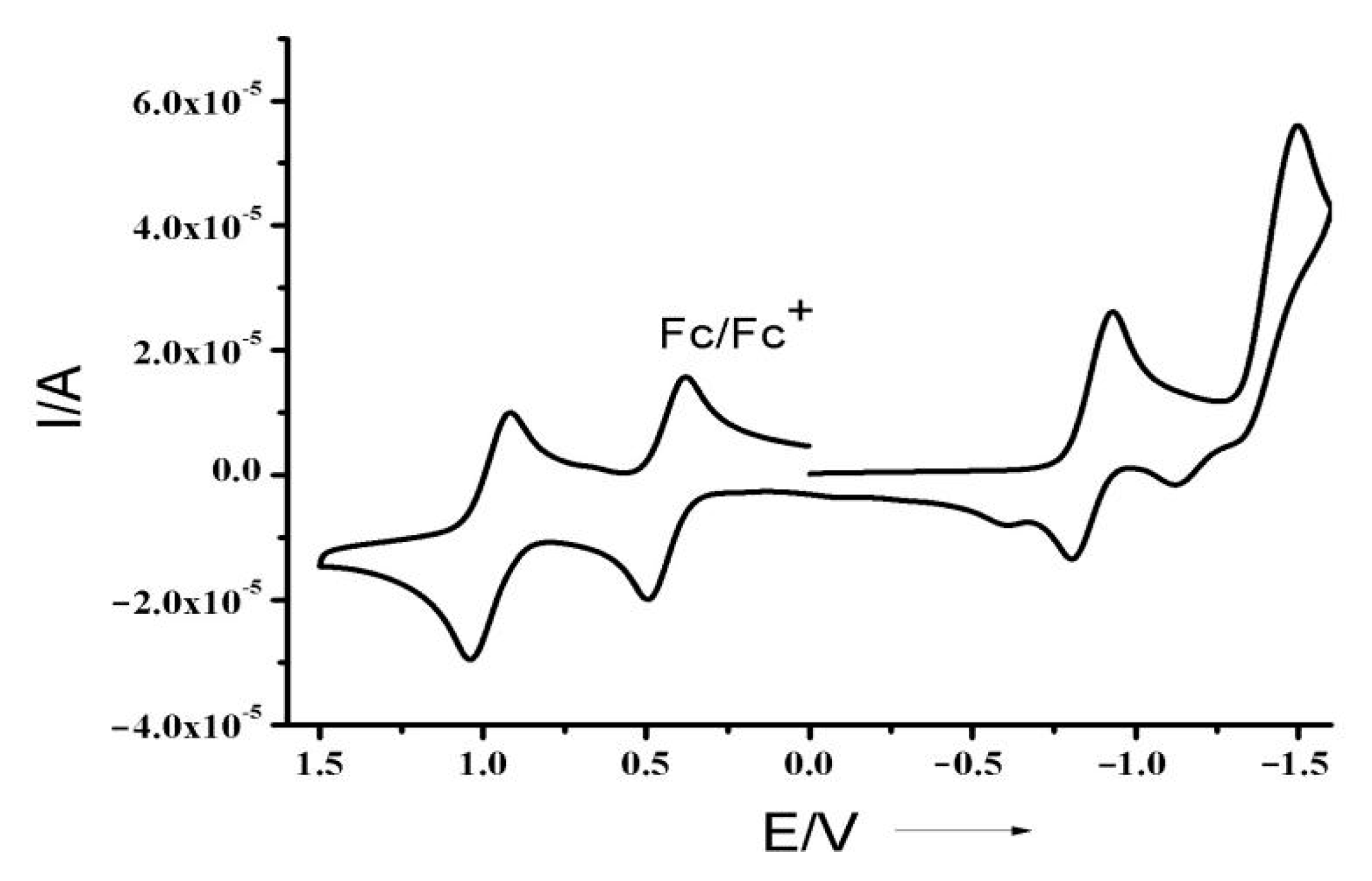
3.4. Electrochemical Properties
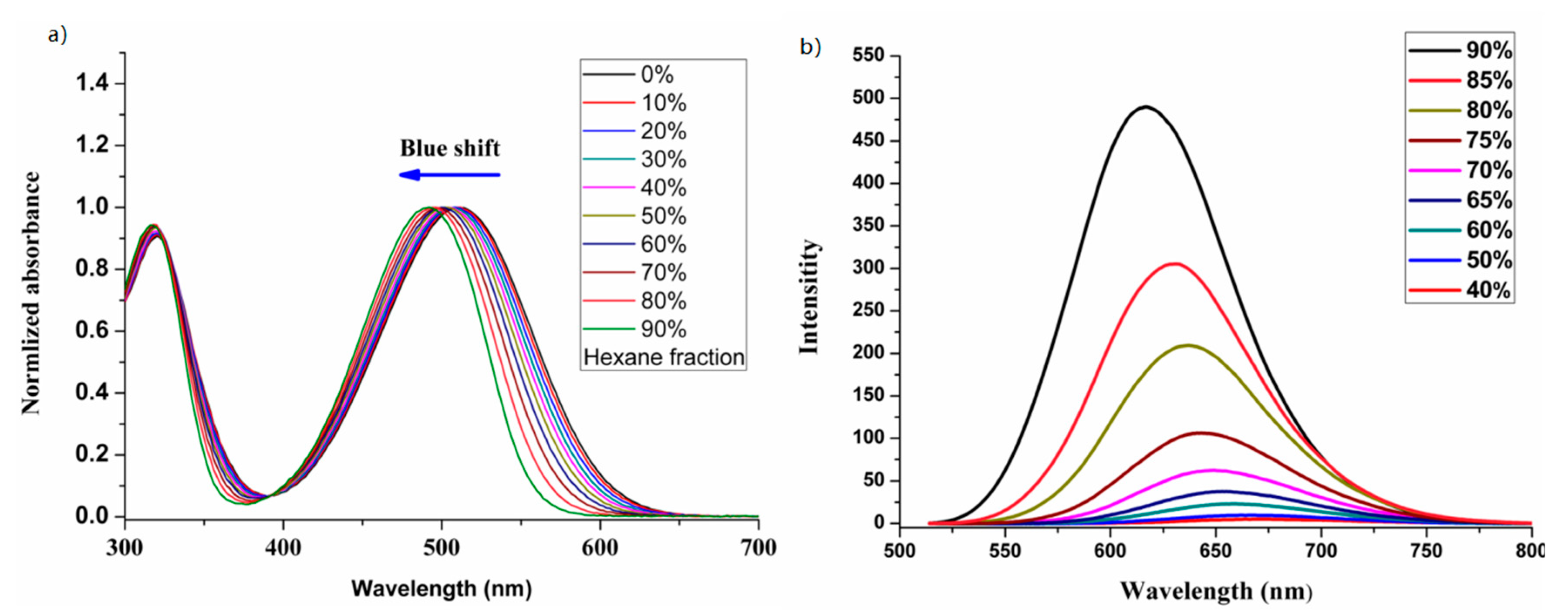
3.5. Linear Optical Properties
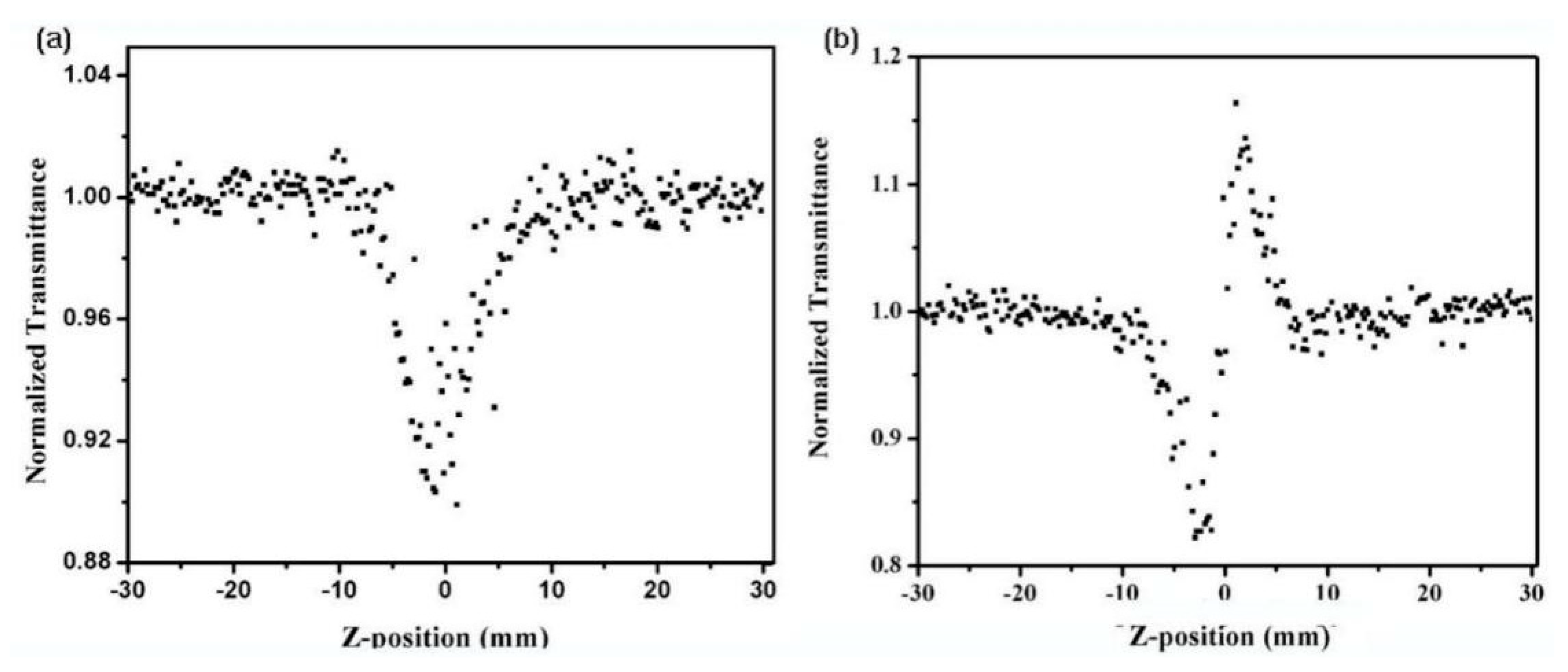
3.6. Nonlinear Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leung, N.L.C.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.Y.; Tang, B.Z. Restriction of Intramolecular Motions: The General Mechanism behind Aggregation-Induced Emission. Chem. Eur. J. 2014, 20, 15349–15353. [Google Scholar] [CrossRef]
- Luo, J.D.; Xie, Z.L.; Lam, J.W.Y.; Cheng, L.; Chen, H.Y.; Qiu, C.F.; Kwok, H.S.; Zhan, X.W.; Liu, Y.Q.; Zhu, D.B.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lam, J.W.Y.; Kwok, R.T.K.; Liu, B.; Tang, B.Z. Aggregation-induced emission: Fundamental understanding and future developments. Mater. Horiz. 2019, 6, 428–433. [Google Scholar] [CrossRef]
- Qian, H.; Cousins, M.E.; Horak, E.H.; Wakefield, A.; Liptak, M.D.; Aprahamian, I. Suppression of Kasha’s rule as a mechanism for fluorescent molecular rotors and aggregation-induced emission. Nat. Chem. 2017, 9, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.G.; Zhang, X.Q.; Xu, B.J.; Zhou, X.; Ma, C.P.; Zhang, Y.; Liu, S.W.; Xu, J.R. Recent advances in organic mechanofluorochromic materials. Chem. Soc. Rev. 2012, 41, 3878–3896. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Q.; Chang, N.; Li, C.H.; Mei, J.; Deng, C.M.; Luo, X.L.; Liu, Z.P.; Bo, Z.S.; Dong, Y.Q.; Tang, B.Z. Locking the phenyl rings of tetraphenylethene step by step: Understanding the mechanism of aggregation-induced emission. Chem. Commun. 2012, 48, 10675–10677. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Ju, C.; Yang, G.; Fron, E.; Coutino-Gonzalez, E.; Semin, S.; Fan, C.; Balok, R.S.; Cremers, J.; Tinnemans, P.; et al. Aggregation Induced Enhancement of Linear and Nonlinear Optical Emission from a Hexaphenylene Derivative. Adv. Funct. Mater. 2016, 26, 8968–8977. [Google Scholar] [CrossRef]
- Qi, J.; Sun, C.W.; Zebibula, A.; Zhang, H.Q.; Kwok, R.T.K.; Zhao, X.Y.; Xi, W.; Lam, J.W.Y.; Qian, J.; Tang, B.Z. Real-Time and High-Resolution Bioimaging with Bright Aggregation-Induced Emission Dots in Short-Wave Infrared Region. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef]
- Chen, S.H.; Chen, N.; Yan, Y.L.; Liu, T.F.; Yu, Y.W.; Li, Y.J.; Liu, H.B.; Zhao, Y.S.; Li, Y.L. Controlling growth of molecular crystal aggregates for efficient optical waveguides. Chem. Commun. 2012, 48, 9011–9013. [Google Scholar] [CrossRef]
- Chen, S.H.; Qin, Z.H.; Liu, T.F.; Wu, X.Z.; Li, Y.J.; Liu, H.B.; Song, Y.L.; Li, Y.L. Aggregation-induced emission on benzothiadiazole dyads with large third-order optical nonlinearity. Phys. Chem. Chem. Phys. 2013, 15, 12660–12666. [Google Scholar] [CrossRef]
- Ju, C.; Li, X.; Yang, G.; Yuan, C.; Semin, S.; Feng, Y.; Rasing, T.; Xu, J. Polymorph dependent linear and nonlinear optical properties of naphthalenyl functionalized fluorenones. Dyes Pigments 2019, 166, 272–282. [Google Scholar] [CrossRef]
- Xu, J.L.; Liu, X.F.; Lv, J.; Zhu, M.; Huang, C.S.; Zhou, W.D.; Yin, X.D.; Liu, H.B.; Li, Y.L.; Ye, H.P. Morphology transition and aggregation-induced emission of an intramolecular charge-transfer compound. Langmuir 2008, 24, 4231–4237. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wen, L.; Zhou, W.; Lv, J.; Guo, Y.; Zhu, M.; Liu, H.; Li, Y.; Jiang, L. Asymmetric and Symmetric Dipole-Dipole Interactions Drive Distinct Aggregation and Emission Behavior of Intramolecular Charge-Transfer Molecules. J. Phys. Chem. C 2009, 113, 5924–5932. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, H.; Liu, H.; Zhou, C.; Zhao, Y.; Li, Y.; Li, Y. Crystal Hierarchical Supramolecular Architectures from 1-D Precursor Single-Crystal Seeds. J. Phys. Chem. C 2010, 114, 2925–2931. [Google Scholar] [CrossRef]
- Pagano, M.; Castagnolo, D.; Bernardini, M.; Fallacara, A.L.; Laurenzana, I.; Deodato, D.; Kessler, U.; Pilger, B.; Stergiou, L.; Strunze, S. The Fight against the Influenza A Virus H1N1: Synthesis, Molecular Modeling, and Biological Evaluation of Benzofurazan Derivatives as Viral RNA Polymerase Inhibitors. ChemMedChem 2014, 9, 129–150. [Google Scholar] [CrossRef]
- Mackenzie, C.R.; Zhang, J. Self-Assembly Molecules. U.S. Patent 07,655,412, 2 February 2010. [Google Scholar]
- Xu, J.; Semin, S.; Rasing, T.; Rowan, A.E. Organized Chromophoric Assemblies for Nonlinear Optical Materials: Towards (Sub)wavelength Scale Architectures. Small 2015, 11, 1113–1129. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef]
- Xu, J.; Semin, S.; Cremers, J.; Wang, L.; Savoini, M.; Fron, E.; Coutino, E.; Chervy, T.; Wang, C.; Li, Y.; et al. Controlling Microsized Polymorphic Architectures with Distinct Linear and Nonlinear Optical Properties. Adv. Opt. Mater. 2015, 3, 948–956. [Google Scholar] [CrossRef]
- Xu, J.; Semin, S.; Niedzialek, D.; Kouwer, P.H.J.; Fron, E.; Coutino, E.; Savoini, M.; Li, Y.; Hofkens, J.; Uji-I, H.; et al. Self-Assembled Organic Microfibers for Nonlinear Optics. Adv. Mater. 2013, 25, 2084–2089. [Google Scholar] [CrossRef]
- Jordan, B.J.; Ofir, Y.; Patra, D.; Caldwell, S.T.; Kennedy, A.; Joubanian, S.; Rabani, G.; Cooke, G.; Rotello, V.M. Controlled Self-Assembly of Organic Nanowires and Platelets Using Dipolar and Hydrogen-Bonding Interactions. Small 2008, 4, 2074–2078. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Tong, B.; Shen, J.; Shi, J.; Han, T.; Chen, L.; Zhi, J.; Lu, P.; Ma, Y.; Dong, Y. Aggregation-Induced Emission Enhancement of Aryl-Substituted Pyrrole Derivatives. J. Phys. Chem. B 2010, 114, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Hilger, A.; Gisselbrecht, J.-P.; Tykwinski, R.R.; Boudon, C.; Schreiber, M.; Martin, R.E.; Luthi, H.P.; Gross, M.; Diederich, F. Electronic Characteristics of Arylated Tetraethynylethenes: A Cooperative Computational and Electrochemical Investigation. J. Am. Chem. Soc. 1997, 119, 2069–2078. [Google Scholar] [CrossRef]
- Fernandez, I.; Bickelhaupt, F.M. The activation strain model and molecular orbital theory: Understanding and designing chemical reactions. Chem. Soc. Rev. 2014, 43, 4953–4967. [Google Scholar] [CrossRef] [PubMed]
- Chong Delano, P. MP2 or B3LYP computed bond distances compared with CCSD(T)cc-pVQZ. Can. J. Chem. 2018, 96, 27. [Google Scholar] [CrossRef]
- Zheng, S.; Phillips, H.; Geva, E.; Dunietz, B.D. Ab Initio Study of the Emissive Charge-Transfer States of Solvated Chromophore-Functionalized Silsesquioxanes. J. Am. Chem. Soc. 2012, 134, 6944–6947. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Zhou, Y.; Semin, S.; Yang, G.; Tinnemans, P.; Duan, Y.; Feng, Y.; Rasing, T.; Xu, J. Solvent dependent linear and nonlinear optical properties of triphenylamine unit incorporated difluoroboron β-diketonate complexes. Dyes Pigments 2019, 162, 776–785. [Google Scholar] [CrossRef]
- Anant, P.; Lucas, N.T.; Jacob, J. A Simple Route toward the Synthesis of Bisbenzothiadiazole Derivatives. Org. Lett. 2008, 10, 5533–5536. [Google Scholar] [CrossRef]
- 3Kato, S.; Matsumoto, T.; Ishi, T.; Thiemann, T.; Shigeiwa, M.; Gorohmaru, H.; Maeda, S.; Yamashita, Y.; Mataka, S. Strongly red-fluorescent novel donor-pi-bridge-acceptor-pi-bridge-donor (D-pi-A-pi-D) type 2,1,3-benzothiadiazoles with enhanced two-photon absorption cross-sections. Chem. Commun. 2004, 2004, 2342–2343. [Google Scholar] [CrossRef]
- Hamanoue, K.; Hirayama, S.; Nakayama, T.; Teranishi, H. Nonradiative relaxation process of the higher excited-states of meso-substituted anthracenes. J. Phys. Chem. Lett. 1980, 84, 2074–2078. [Google Scholar] [CrossRef]
- Fridman, N.; Speiser, S.; Kaftory, M. Chromotropic Behavior of Lophine Nitro-Derivatives. Cryst. Growth Des. 2006, 6, 2281–2288. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, K.; Yao, Z.; Zou, B.; Xu, J.; Bu, X.-H. Multi-Stimuli-Responsive Fluorescence Switching from a Pyridine-Functionalized Tetraphenylethene AIEgen. ACS Appl. Mater. Interfaces 2018, 10, 5819–5827. [Google Scholar] [CrossRef]
- Liu, Z.B.; Tian, J.G.; Zang, W.P.; Zhou, W.Y.; Zhang, C.P.; Zhang, G.Y. Influence of nonlinear absorption on Z-scan measurements of nonlinear refraction. Chin. Phys. Lett. 2003, 20, 509–512. [Google Scholar]
- Reichert, M.; Hu, H.; Ferdinandus, M.R.; Seidel, M.; Zhao, P.; Reed, J.M.; Fishman, D.A.; Webster, S.; Hagan, D.J.; Van Stryland, E.W.; et al. Measurement of Nonlinear Refraction Dynamics of CS2. In Proceedings of the IEEE 2014 Conference on Lasers and Electro-Optics, San Jose, CA, USA, 8–13 June 2014. [Google Scholar]
- Wu, X.; Xiao, J.; Sun, R.; Jia, J.; Yang, J.; Ao, G.; Shi, G.; Wang, Y.; Zhang, X.; Song, Y. Twistacene contained molecule for optical nonlinearity: Excited-state based negative refraction and optical limiting. Opt. Laser Technol. 2018, 102, 93–99. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Y.L.; Xu, Y.; Fun, H.; Fang, G.Y.; Wang, Y.X.; Xin, X.Q. Studies on two interesting microporous polymeric clusters {[Et4N](2)[MS4Cu4(CN)(4)]}(n) (M = Mo or W) with three-dimensional open frameworks: Synthesis, structural characterization, strong optical non-linearities and large optical limiting properties. Dalton Trans. 2000, 2823–2829. [Google Scholar] [CrossRef]


| Formula | C14H12N4O2S |
|---|---|
| CCDC number | 1977053 |
| Mr | 300.34 |
| crystal size (mm3) | 0.40 × 0.20 × 0.07 |
| crystal system | Monoclinic |
| space group | P21/n |
| a (Å) | 13.053(3) |
| b (Å) | 6.9678(14) |
| c (Å) | 14.799(3) |
| α(deg) | 90 |
| β (deg) | 106.85(3) |
| γ (deg) | 90 |
| V (Å3) | 1288.2(4) |
| Z | 4 |
| F(000) | 624 |
| calcd (mg m−3) | 1.549 |
| (mm−1) | 0.262 |
| range (deg) | 1.83 to 27.52 |
| R1 [I > 2σ(I)] | 0.0481 |
| wR2 [I > 2σ(I)] | 0.1163 |
| goodness of fit | 1.150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Luo, R.; Li, X.; He, M.; Fu, S.; Xu, J. Aggregation Induced Emission and Nonlinear Optical Properties of an Intramolecular Charge-Transfer Compound. Materials 2021, 14, 1909. https://doi.org/10.3390/ma14081909
Chen S, Luo R, Li X, He M, Fu S, Xu J. Aggregation Induced Emission and Nonlinear Optical Properties of an Intramolecular Charge-Transfer Compound. Materials. 2021; 14(8):1909. https://doi.org/10.3390/ma14081909
Chicago/Turabian StyleChen, Songhua, Rui Luo, Xinyue Li, Meiyun He, Shanshan Fu, and Jialiang Xu. 2021. "Aggregation Induced Emission and Nonlinear Optical Properties of an Intramolecular Charge-Transfer Compound" Materials 14, no. 8: 1909. https://doi.org/10.3390/ma14081909
APA StyleChen, S., Luo, R., Li, X., He, M., Fu, S., & Xu, J. (2021). Aggregation Induced Emission and Nonlinear Optical Properties of an Intramolecular Charge-Transfer Compound. Materials, 14(8), 1909. https://doi.org/10.3390/ma14081909





