High-Yield Production of Nano-Lateral Size Graphene Oxide by High-Power Ultrasonication
Abstract
1. Introduction
- Intensive oxidation of graphite by increasing concentration of oxidizing agents or increasing the timescale/cycles of oxidation process
- Centrifugation of GO in aqueous dispersion and separation of the fractions with smaller dimensions
- Breakdown of GO sheets by high-power ultrasonication
- Selective precipitation of larger GO sheets by protonation with organic solvents or by pH adjustment
- Exfoliation of graphite nanofibers with very small diameter
- Electrical breakdown of graphite by arc-discharge
- Ball milling of graphite in the presence of oxidizing agents
- Electrochemical exfoliation of graphite electrodes
2. Materials and Methods
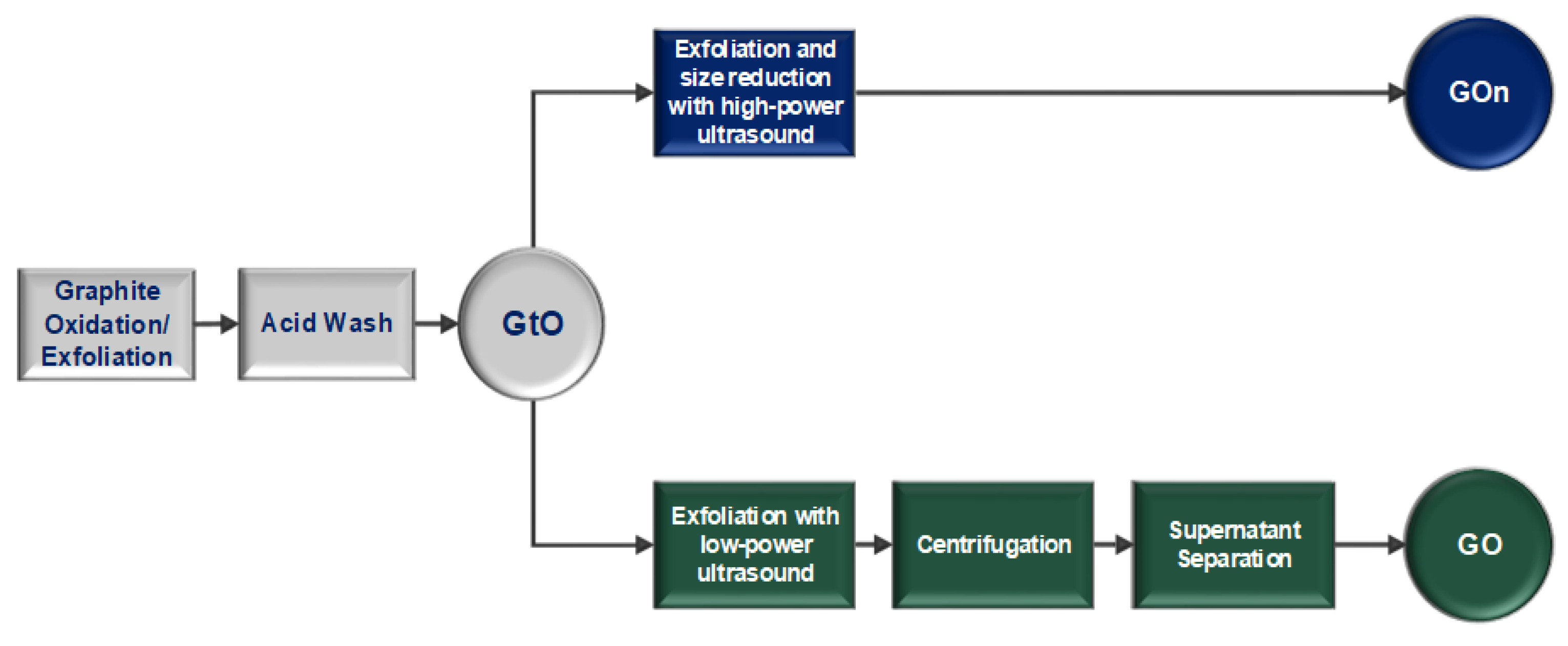
2.1. GO Lateral Dimensions Reduction Based on Centrifugation
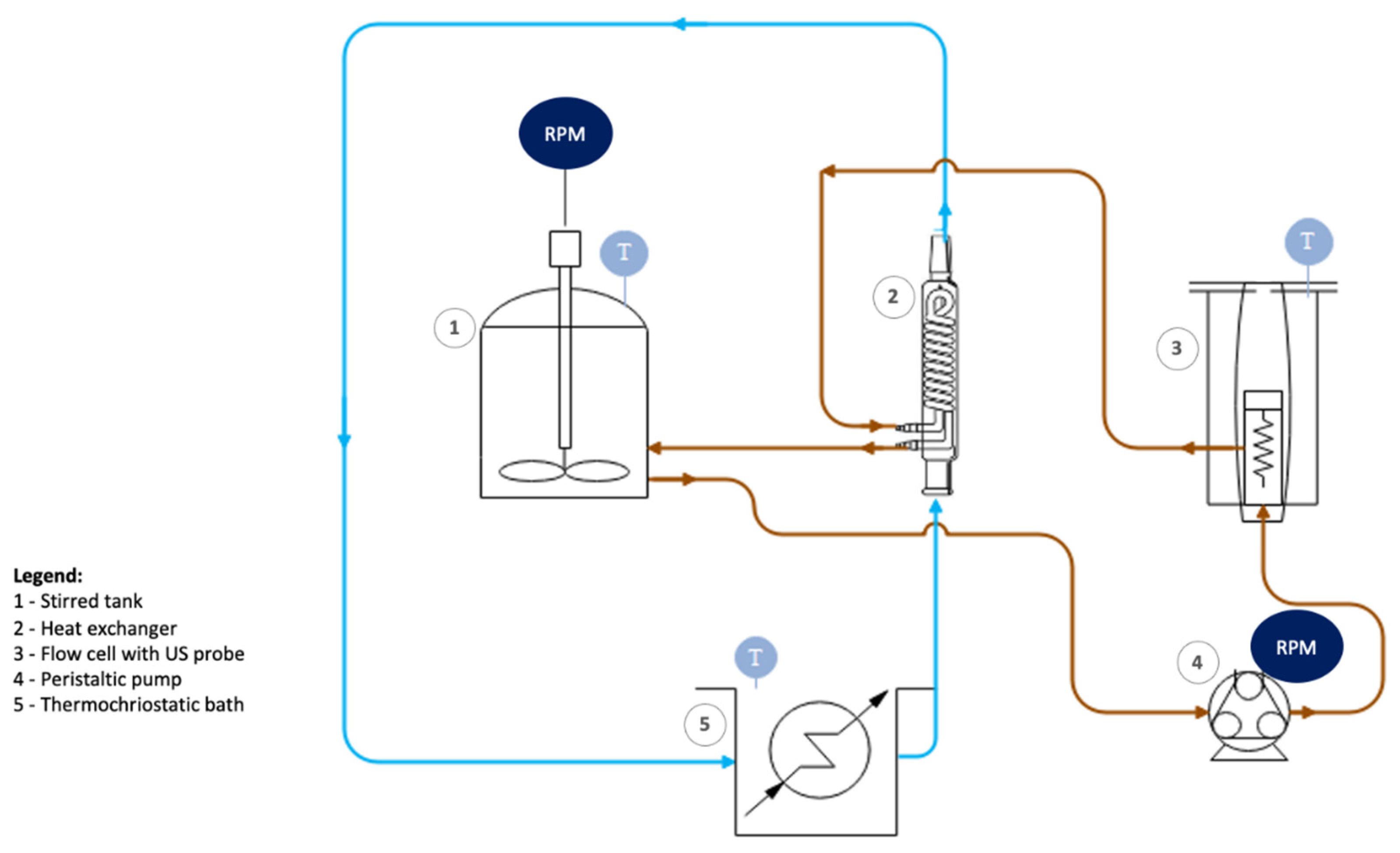
2.2. GO Lateral Dimensions Reduction Based on High-Power Ultrasonication
2.3. Transmission Electron Microscopy (TEM)
2.4. Zeta Potential Measurements
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
2.6. X-ray Photoelectron Spectroscopy (XPS)
2.7. Thermogravimetric Analysis (TGA)
2.8. X-ray Diffraction Analysis (XRD)
2.9. Raman Spectroscopy
3. Results and Discussion
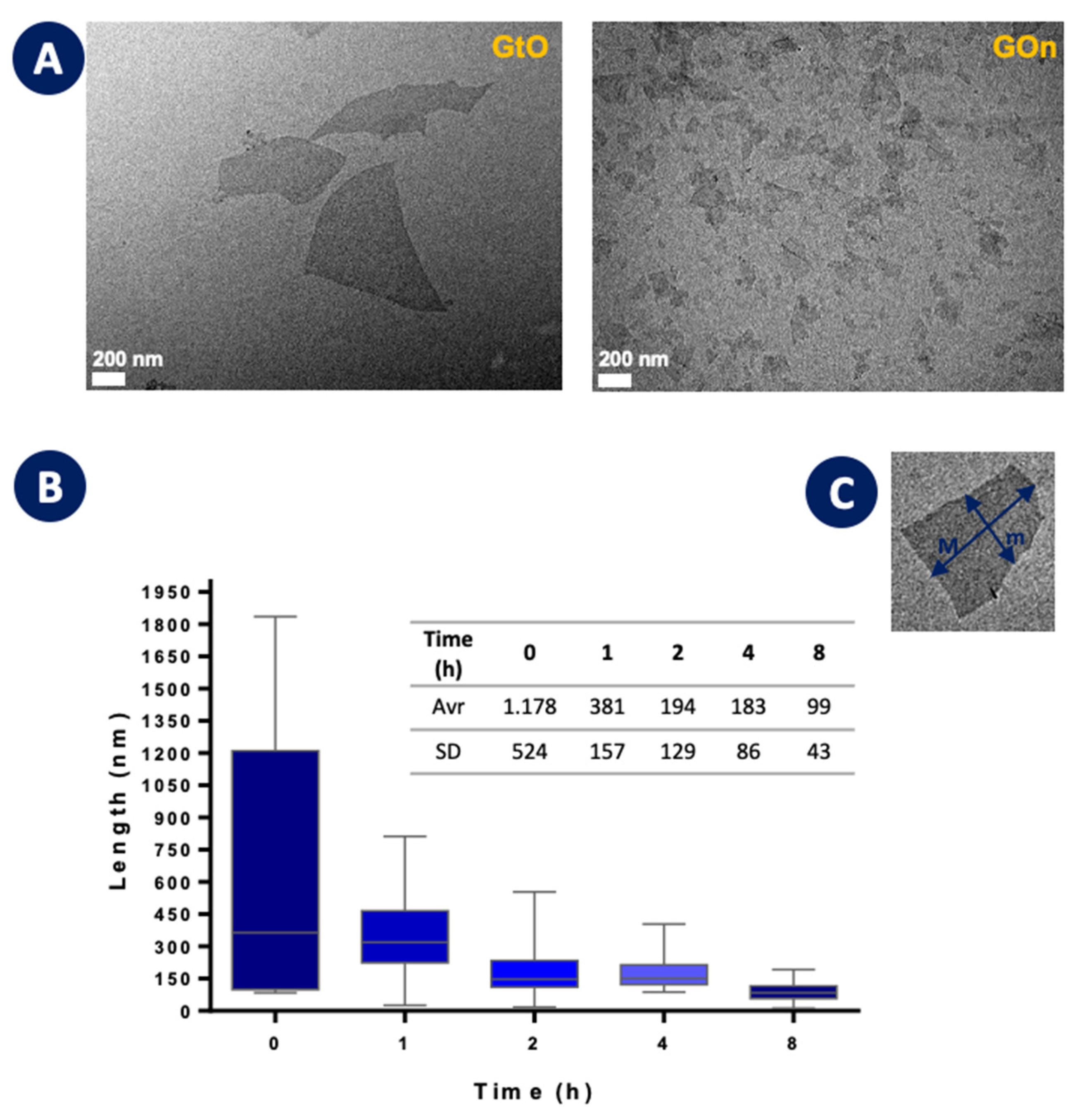
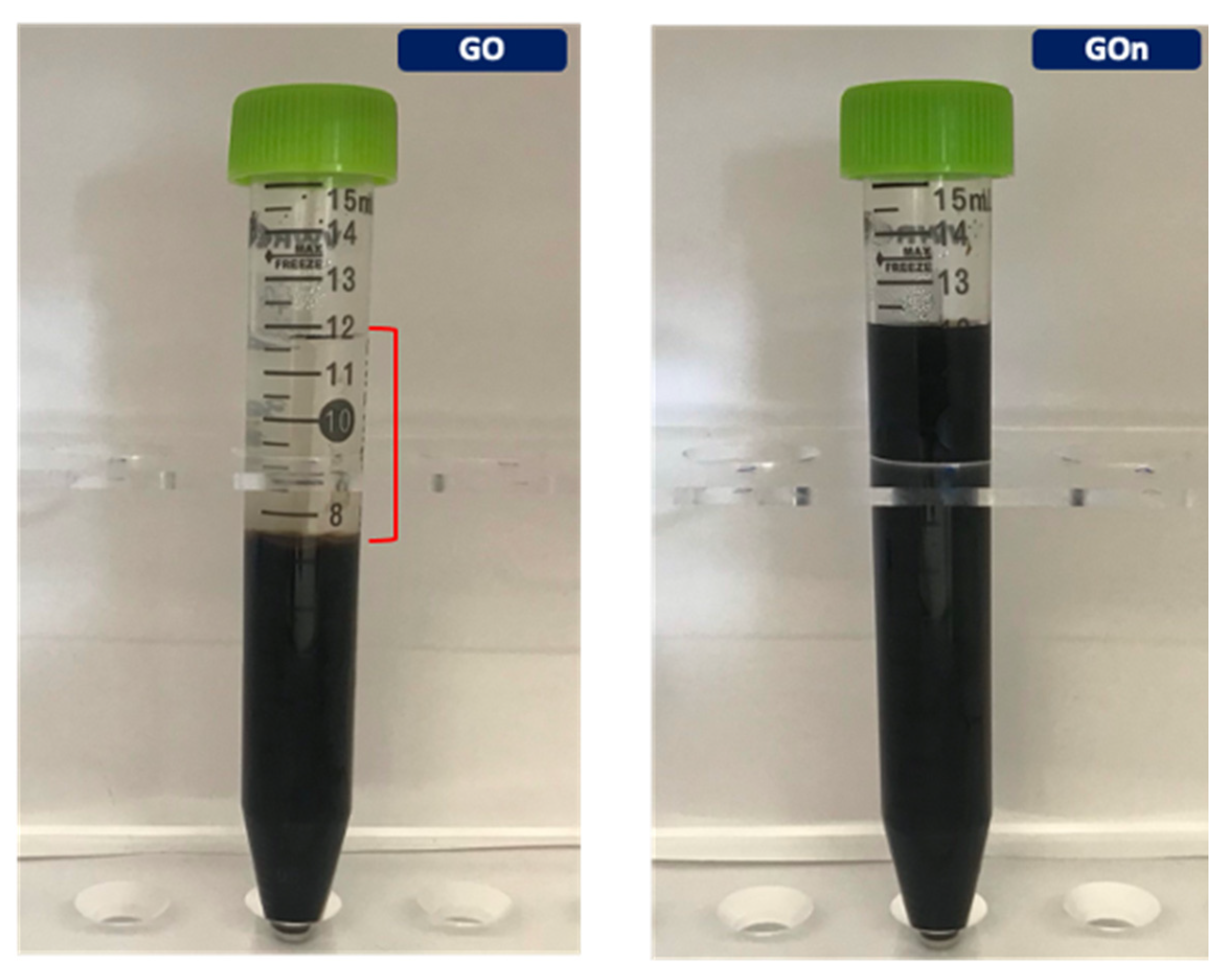
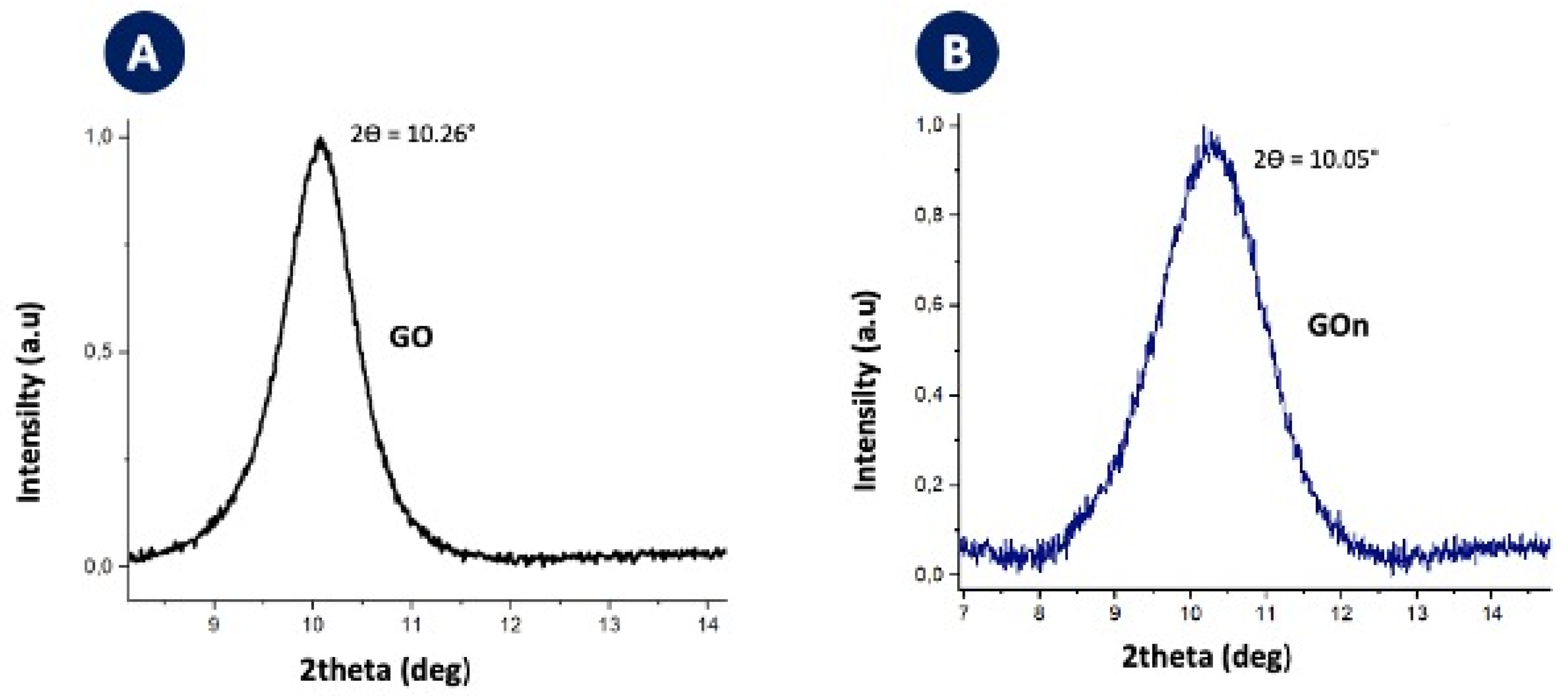
3.1. Morphological Features and Dispersion Stability
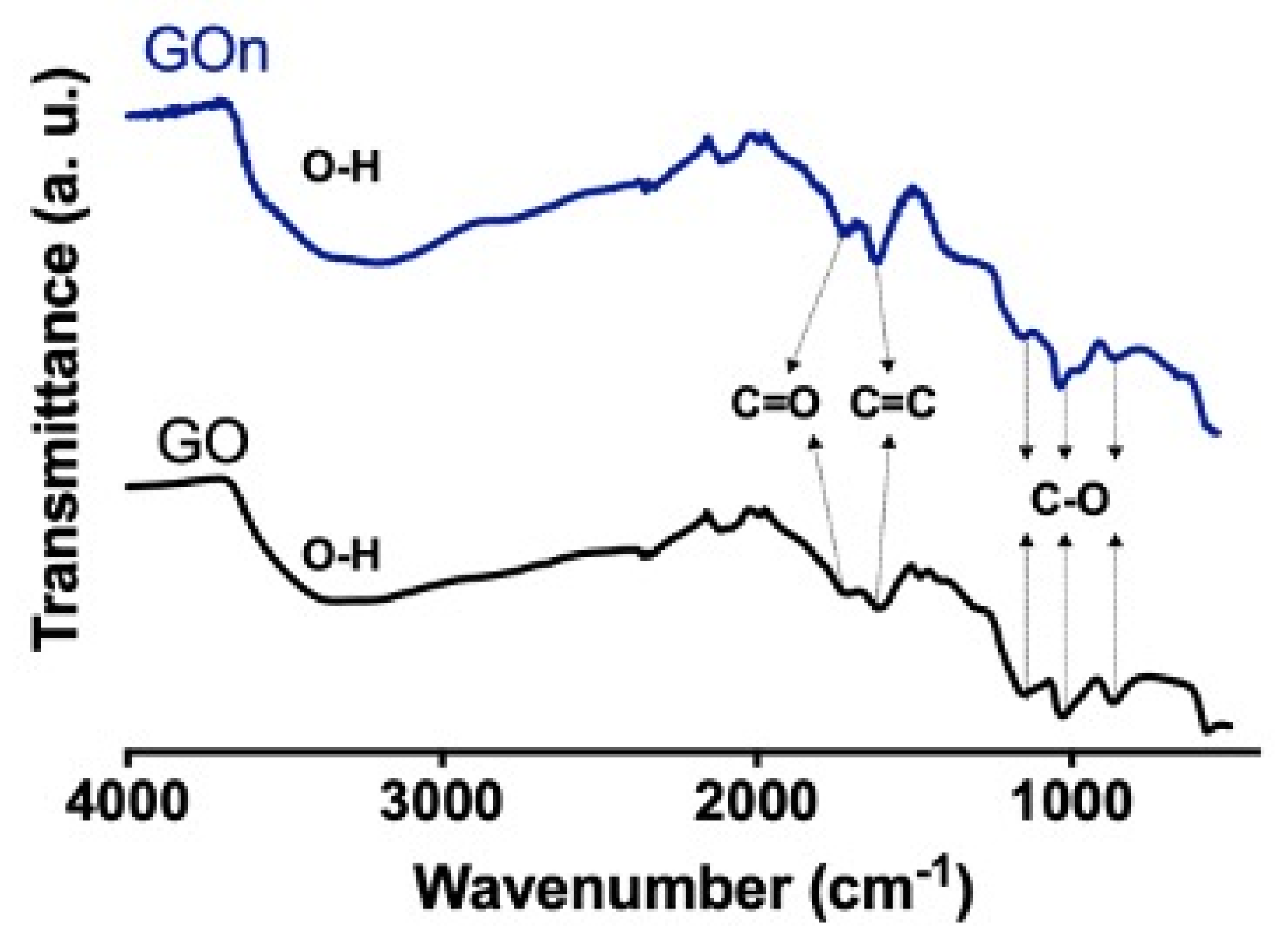
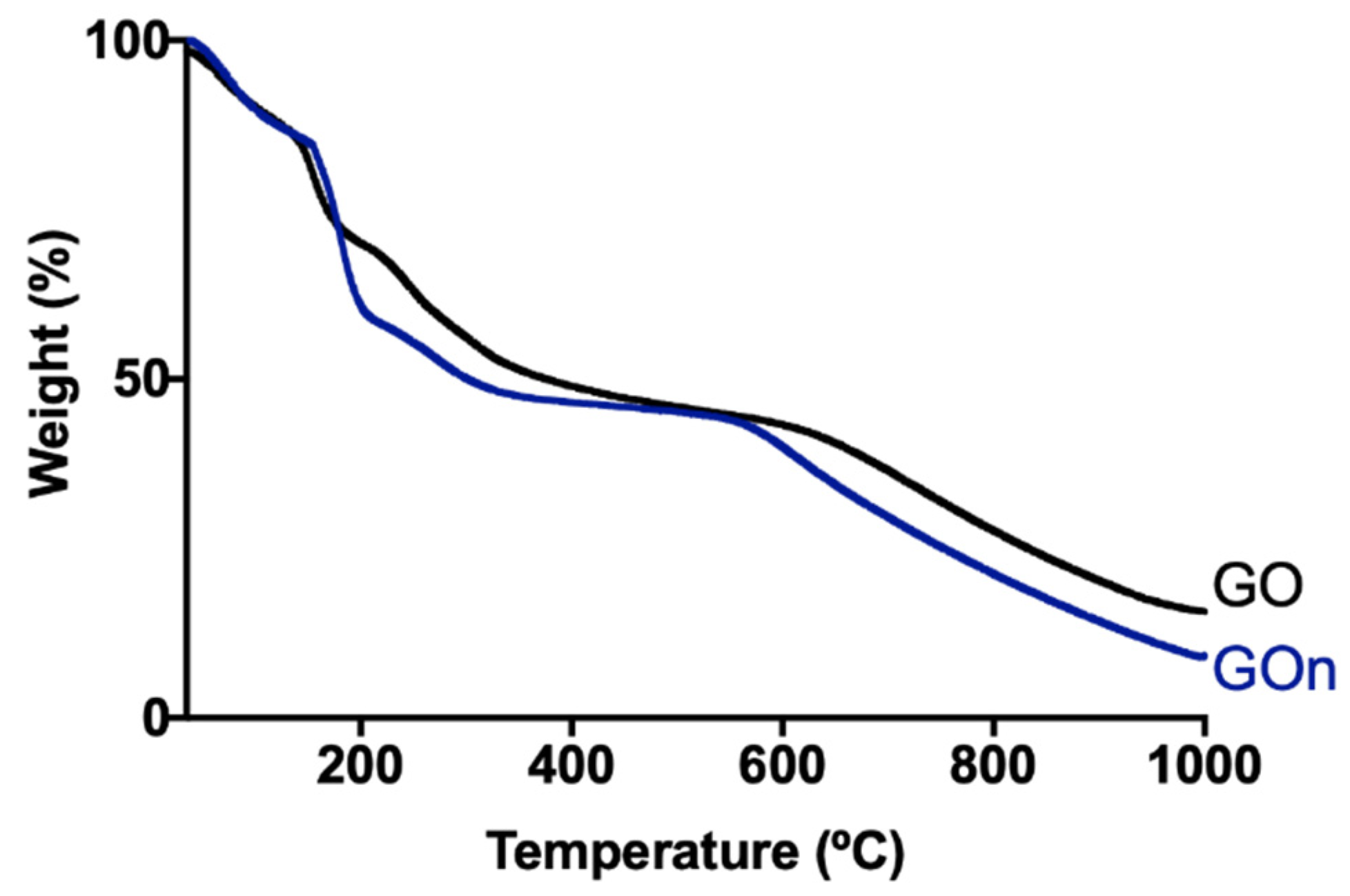
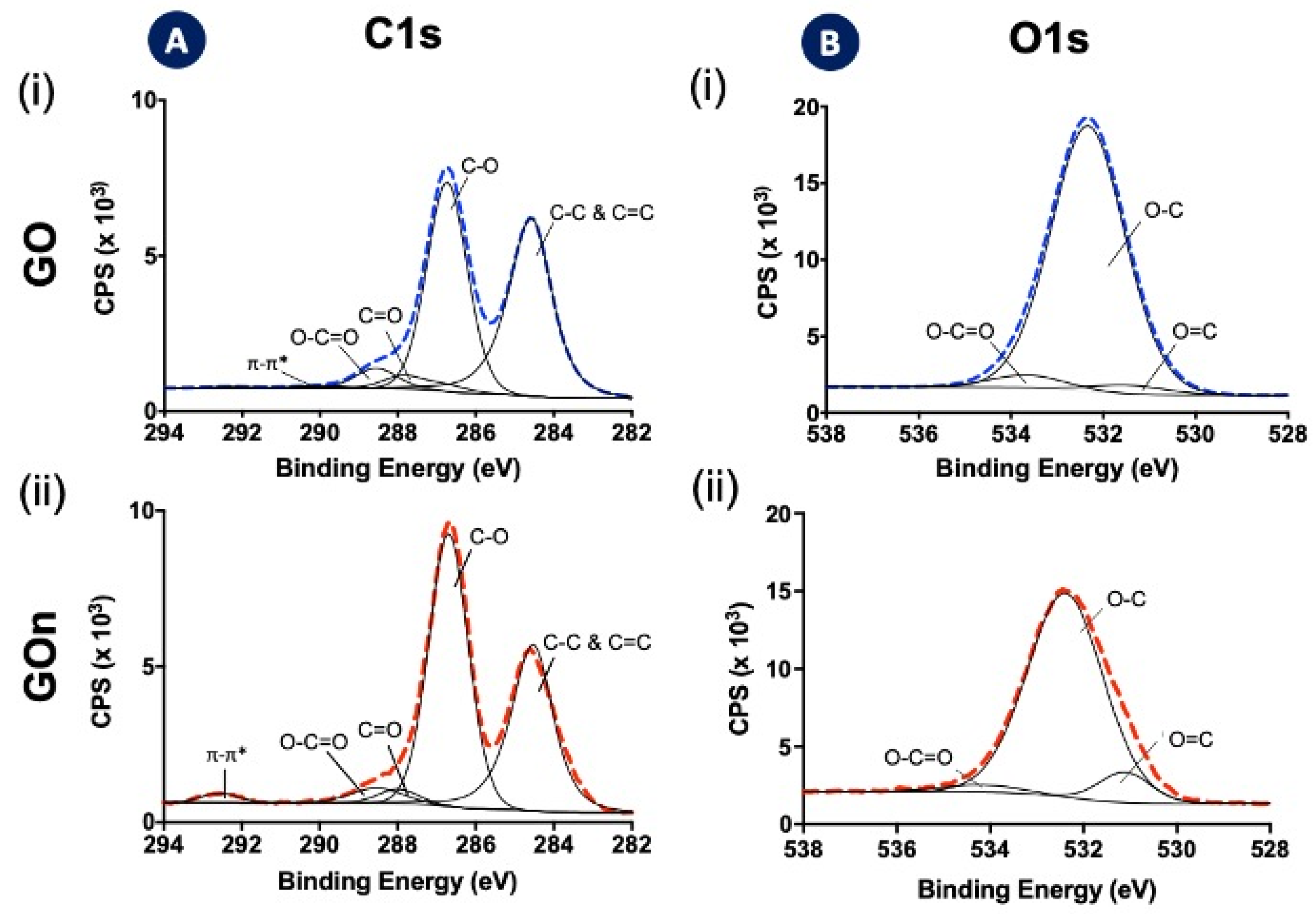
3.2. Chemical Properties
- sp2 and sp3 hybridizations of carbon (C–C and C=C, C1s at. % = 45.5 for GO and C1s at. % = 38.4 for GOn) in the graphitic backbone;
- single bonds between carbon and oxygen (C–O) in hydroxyls and ethers (C1s at. % = 44.9 for GO and C1s at. % = 53.2 for GOn);
- double bonds between carbon and oxygen (C=O), indicating the presence of carbonyl groups (C1s at. % = 4.2% for GO and C1s at. % = 2.8% for GOn);
- multiple bonds between carbon and oxygen (O=C−O), indicating the occurrence of carboxyls (C1s at. % = 4.5 for GO and C1s at. % = 3.9 for GOn); and
- O=C bonds present in carbonyl and carboxyl groups (O1s at. % = 3.1 for GO and O1s at. % = 5.7 for GOn);
- O–C bonds in hydroxyl groups and ethers (O1s at. % = 92.4 for GO and O1s at. % = 92.5 for GOn); and
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Wang, X.; Chen, T. Facile and green reduction of covalently PEGylated nanographene oxide via a ‘water-only’ route for high-efficiency photothermal therapy. Nanoscale Res. Lett. 2014, 9, 86. [Google Scholar] [CrossRef]
- Tufano, I.; Vecchione, R.; Netti, P.A. Methods to Scale Down Graphene Oxide Size and Size Implication in Anti-cancer Applications. Front. Bioeng. Biotechnol. 2020, 8, 613280. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Bogas, D.; Fernandes, J.R.; Timochenco, L.; Silva, F.; Meneses, J.; Goncalves, I.C.; Magalhaes, F.D.; Pinto, A.M. Near-Infrared Radiation-Based Mild Photohyperthermia Therapy of Non-Melanoma Skin Cancer with PEGylated Reduced Nanographene Oxide. Polymers (Basel) 2020, 12, 1840. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Alves, N.M.; Paiva, M.C. Graphene-polymer nanocomposites for biomedical applications. Polym. Adv. Technol. 2018, 29, 687–700. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.X.; Chen, M.G.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotech. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Fadeel, B.; Bussy, C.; Merino, S.; Vazquez, E.; Flahaut, E.; Mouchet, F.; Evariste, L.; Gauthier, L.; Koivisto, A.J.; Vogel, U.; et al. Safety Assessment of Graphene-Based Materials: Focus on Human Health and the Environment. ACS Nano 2018, 12, 10582–10620. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.L.; Song, B.; Liang, H.M.; Liu, J.; Feng, X.L.; Deng, B.; Sun, T.; Shao, L.Q. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, C.; Sui, X.; Riaz, M.A.; Xu, M.; Wei, L.; Chen, Y. Synthesis of graphene materials by electrochemical exfoliation: Recent progress and future potential. Carbon Energy 2019, 12, 173–199. [Google Scholar] [CrossRef]
- Farazas, A.; Mavropoulos, A.; Christofilos, D.; Tsiaoussis, I.; Tsipas, D. Ultrasound Assisted Green Synthesis and Characterization of Graphene Oxide. Int. J. Nanosci. Nanotechnol. 2018, 14, 11–17. [Google Scholar]
- Méndez-Romero, U.A.; Pérez-García, S.A.; Fan, Q.; Wang, E.; Licea-Jiménez, L. Lateral size reduction of graphene oxide preserving its electronic properties and chemical functionality. RSC Adv. 2020, 10, 229432–229440. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.F.; Newman, L.; Lozano, N.; Mukherjee, S.P.; Fadeel, B.; Bussy, C.; Kostarelos, K. A blueprint for the synthesis and characterisation of thin graphene oxide with controlled lateral dimensions for biomedicine. 2D Mater. 2018, 5, 035020. [Google Scholar] [CrossRef]
- Luo, J.; Cote, L.J.; Tung, V.C.; Tan, A.T.; Goins, P.E.; Wu, J.; Huang, J. Graphene oxide nanocolloids. J. Am. Chem. Soc. 2010, 132, 17667–17669. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, B.; Ultav, G.; Korucu, H.; Yartaşi, A. Improvement of the Graphene Oxide Dispersion Properties with the Use of TOPSIS Based Taguchi Application. Period. Polytech. Chem. Eng. 2018, 62, 323–335. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, S.; Cho, D.H.; Kang, B.; Kwon, H.; Kim, Y.; Park, S.O.; Jung, G.Y.; Shin, E.; Kim, W.G.; et al. Direct exfoliation and dispersion of two-dimensional materials in pure water via temperature control. Nat. Commun. 2015, 6, 8294. [Google Scholar] [CrossRef]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiong, P.; Yang, X.; Wang, X. Novel PEG functionalized graphene nanosheets: Enhancement of dispersibility and thermal stability. Nanoscale 2011, 3, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Ain, Q.T.; Haq, S.H.; Alshammari, A.; Al-Mutlaq, M.A.; Anjum, M.N. The systemic effect of PEG-nGO-induced oxidative stress in vivo in a rodent model. Beilstein J. Nanotechnol. 2019, 10, 901–911. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Jasim, D.A.; Lozano, N.; Kostarelos, K. Synthesis of few-layered, high-purity graphene oxide sheets from different graphite sources for biology. 2D Mater. 2016, 3, 014006. [Google Scholar] [CrossRef]
- Wojtoniszak, M.; Chen, X.; Kalenczuk, R.J.; Wajda, A.; Lapczuk, J.; Kurzewski, M.; Drozdzik, M.; Chu, P.K.; Borowiak-Palen, E. Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf. B Biointerfaces 2012, 89, 79–85. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, J. Probing the Thermal Deoxygenation of Graphene Oxide Using High-Resolution In Situ X-ray-Based Spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar] [CrossRef]
- Araújo, M.P.; Soares, O.S.G.P.; Fernandes, A.J.S.; Pereira, M.F.R.; Freire, C. Tuning the surface chemistry of graphene flakes: New strategies for selective oxidation. RSC Adv. 2017, 7, 14290–14301. [Google Scholar] [CrossRef]
- Some, S.; Kim, Y.; Yoon, Y.; Yoo, H.; Lee, S.; Park, Y.; Lee, H. High-Quality Reduced Graphene Oxide by a Dual-Function Chemical Reduction and Healing Process. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman spectroscopy of graphene-based materials and its applications in related devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of Graphite Oxide and Reduced Graphene Oxide Obtained from Different Graphite Precursors and Oxidized by Different Methods Using Raman Spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef] [PubMed]

| GBM | Surface Charge (mV) |
|---|---|
| GO | −29.7 ± 1.2 |
| GOn | −39.9 ± 2.2 |
| Elemental at. %/Chemical Group | Binding Energy (eV) | GO (%) | GOn (%) | |
|---|---|---|---|---|
| C/O ratio | - | 1.94 | 2.16 | |
| C1s | - | 62.1 | 66.3 | |
| O1s | - | 32.0 | 30.7 | |
| C 1s (at. %) | C–C and C=C | 284.5 | 45.5 | 38.4 |
| C–O | 286.7 | 44.9 | 53.2 | |
| C=O | 287.9 | 4.2 | 2.8 | |
| O=C–O | 288.5 | 4.5 | 3.9 | |
| π–π* | 292.6 | 0.93 | 1.8 | |
| O 1s (at. %) | C=O | 531.2 | 3.1 | 5.7 |
| C–O | 532.5 | 92.4 | 92.5 | |
| O=C–O | 534 | 4.5 | 1.9 | |
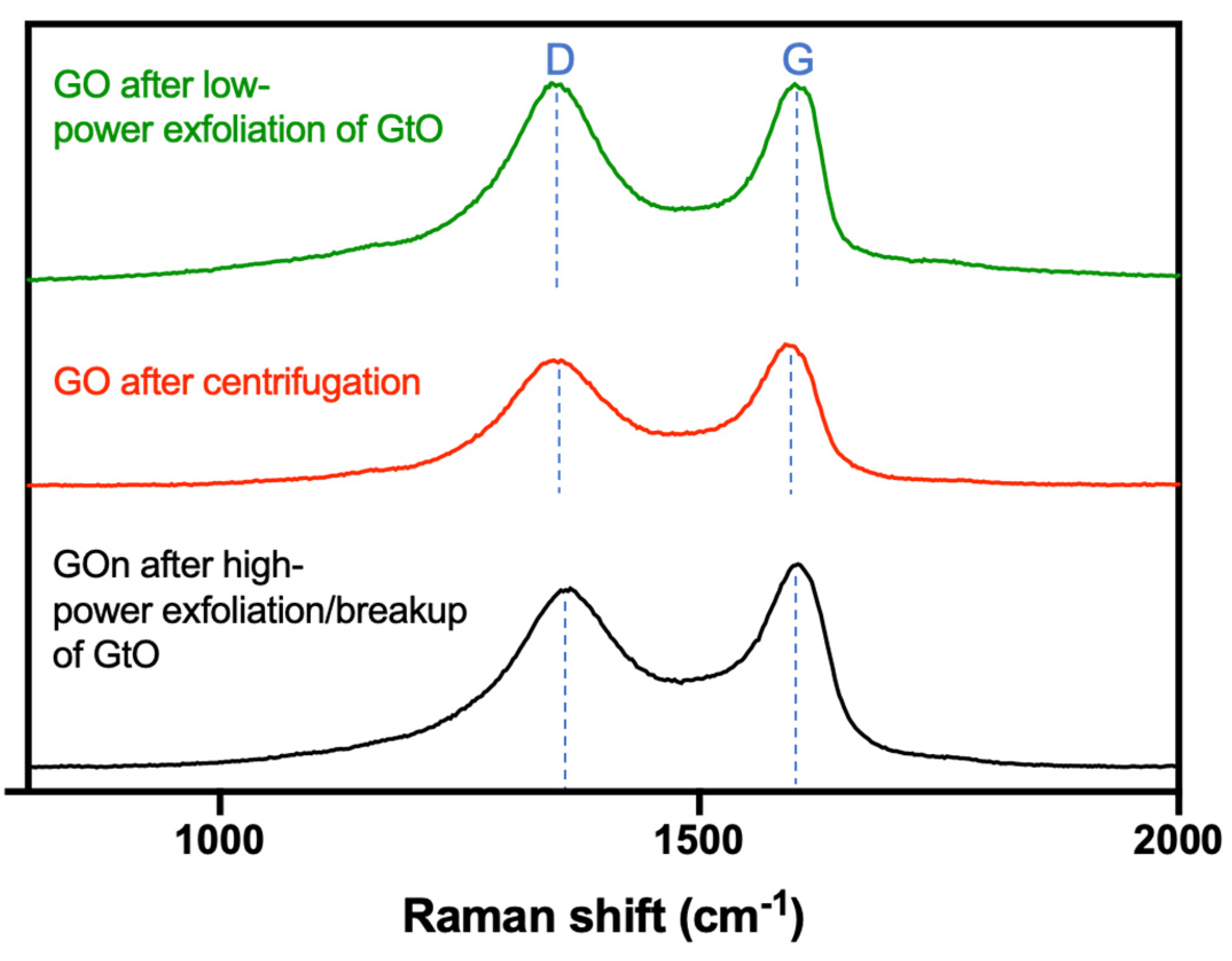
| Samples | D Band | G Band | ID/IG Band |
|---|---|---|---|
| GO after low-power exfoliation of GtO | 1355 | 1605 | 0.998 |
| GO after centrifugation | 1358 | 1600 | 0.968 |
| GO after high-power exfoliation/breakup of GtO | 1365 | 1602 | 0.880 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timochenco, L.; Costa-Almeida, R.; Bogas, D.; Silva, F.A.L.S.; Silva, J.; Pereira, A.; Magalhães, F.D.; Pinto, A.M. High-Yield Production of Nano-Lateral Size Graphene Oxide by High-Power Ultrasonication. Materials 2021, 14, 1916. https://doi.org/10.3390/ma14081916
Timochenco L, Costa-Almeida R, Bogas D, Silva FALS, Silva J, Pereira A, Magalhães FD, Pinto AM. High-Yield Production of Nano-Lateral Size Graphene Oxide by High-Power Ultrasonication. Materials. 2021; 14(8):1916. https://doi.org/10.3390/ma14081916
Chicago/Turabian StyleTimochenco, Licínia, Raquel Costa-Almeida, Diana Bogas, Filipa A. L. S. Silva, Joana Silva, André Pereira, Fernão D. Magalhães, and Artur M. Pinto. 2021. "High-Yield Production of Nano-Lateral Size Graphene Oxide by High-Power Ultrasonication" Materials 14, no. 8: 1916. https://doi.org/10.3390/ma14081916
APA StyleTimochenco, L., Costa-Almeida, R., Bogas, D., Silva, F. A. L. S., Silva, J., Pereira, A., Magalhães, F. D., & Pinto, A. M. (2021). High-Yield Production of Nano-Lateral Size Graphene Oxide by High-Power Ultrasonication. Materials, 14(8), 1916. https://doi.org/10.3390/ma14081916









