The Green Synthesis of 2D Copper Nanosheets and Their Light Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
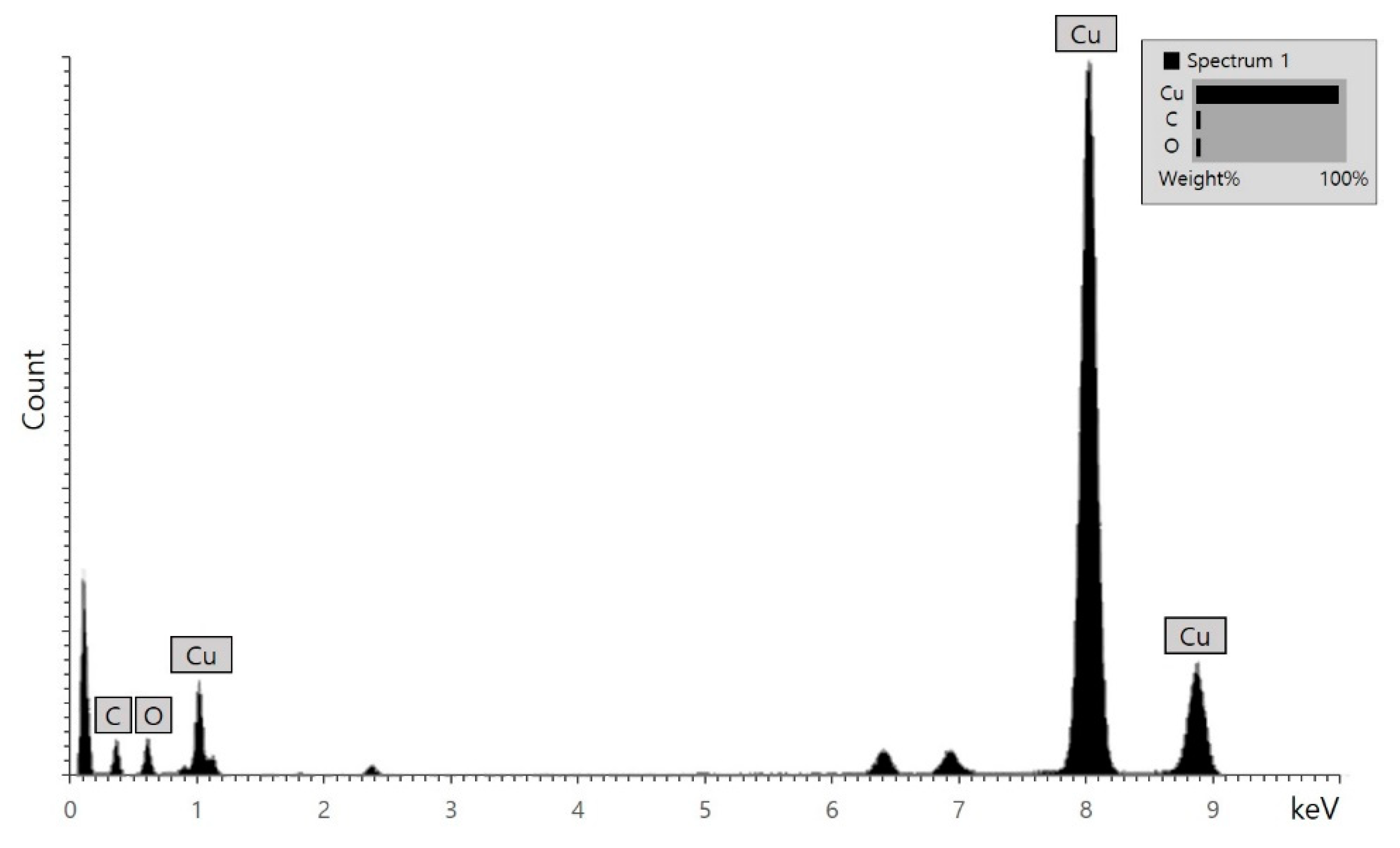
2.3. Characterization
3. Results and Discussion
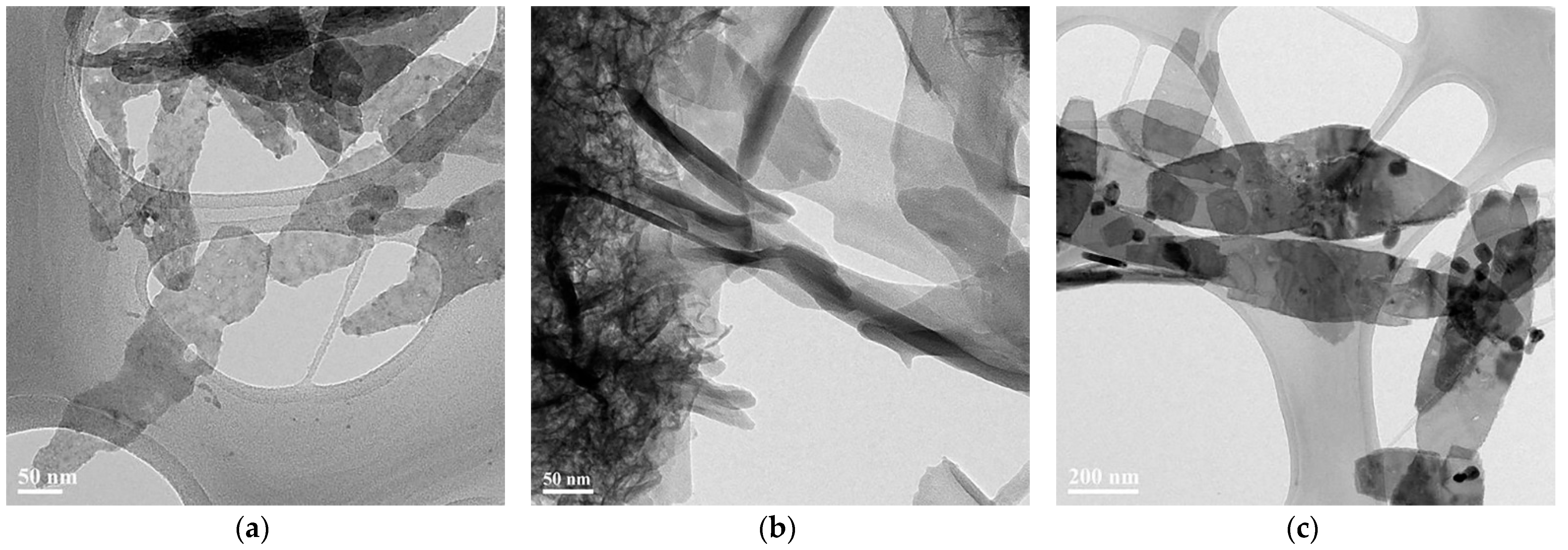
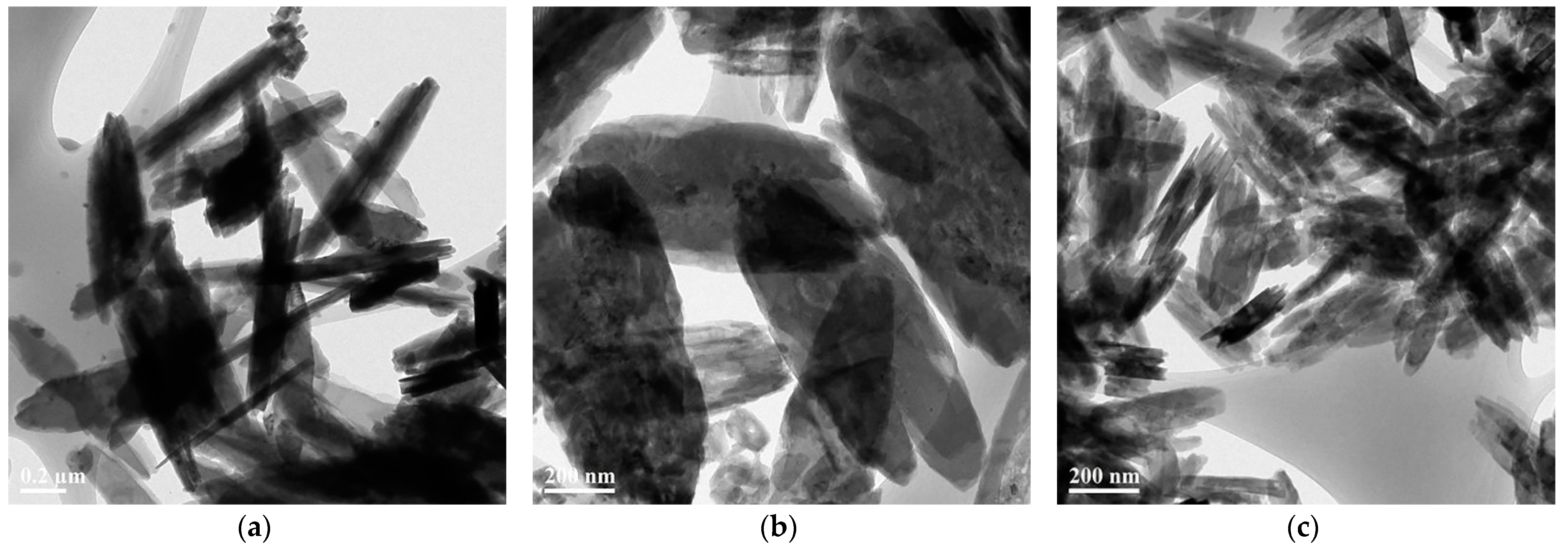
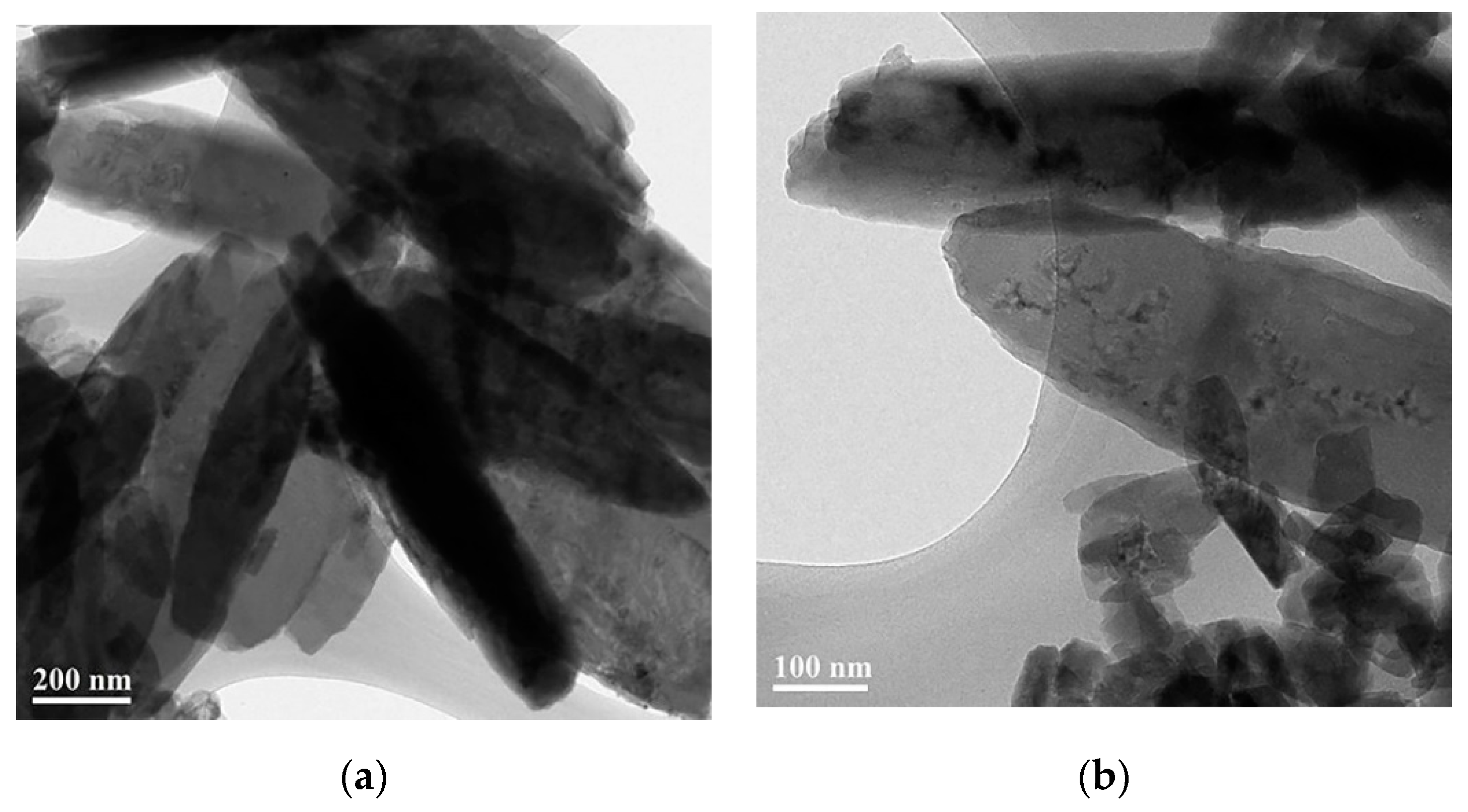
3.1. The Formation of 2D Copper Nanosheets
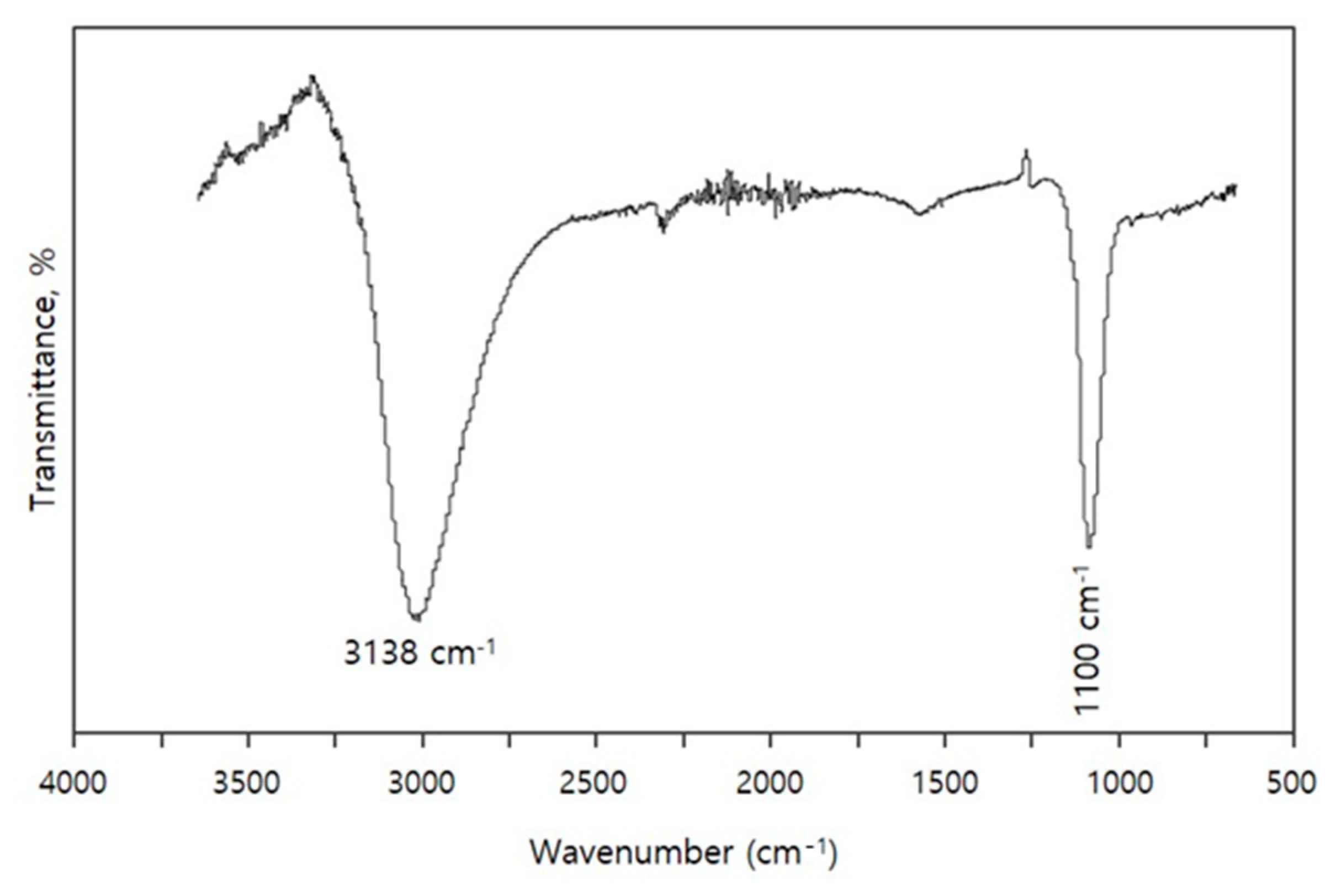
3.2. FTIR Study
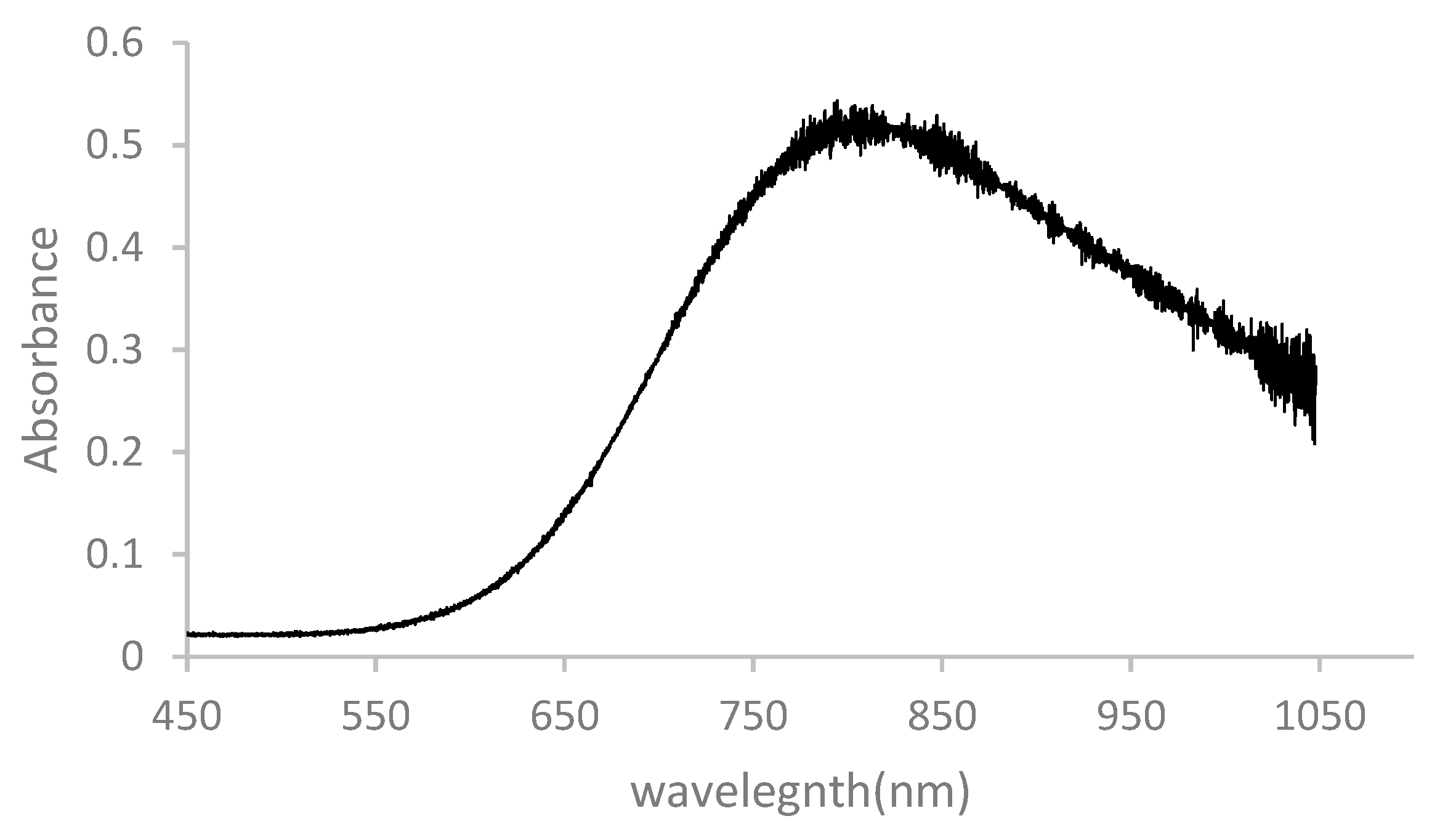
3.3. UV–Vis Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhanushali, S.; Ghosh, P.; Ganesh, A.; Cheng, W. 1D Copper Nanostructures: Progress, Challenges and Opportunities. Small 2015, 11, 1232–1252. [Google Scholar] [CrossRef]
- Ling, T.; Wang, J.J.; Zhang, H.; Song, S.T.; Zhou, Y.Z.; Zhao, J.; Du, X.W. Freestanding ultrathin metallic nanosheets: Materials, synthe-sis, and applications. Adv. Mater. 2015, 27, 5396–5402. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Xie, H.; Chen, W. Experimental investigation on thermal conductivity of nanofluids containing graphene oxide nanosheets. J. Appl. Phys. 2010, 107, 094317. [Google Scholar] [CrossRef]
- Pei, Y.; Huang, L.; Wang, J.; Han, L.; Li, S.; Zhang, S.; Zhang, H. Recent progress in the synthesis and applications of 2D metal nanosheets. Nanotechnology 2019, 30, 222001. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.M.; Pawar, B.S.; Hou, B.; Kim, J.; Ahmed, A.T.A.; Chavan, H.S.; Jo, Y.; Cho, S.; Inamdar, A.I.; Gunjakar, J.L.; et al. Self-assembled two-dimensional copper oxide nanosheet bundles as an efficient oxygen evolution reaction (OER) electrocatalyst for water splitting applications. J. Mater. Chem. A 2017, 5, 12747–12751. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, A.K.; Kim, H.J.; Han, I.; Tan, J.C. Optochemically responsive 2D nanosheets of a 3D metal-organic framework materi-al. Adv. Mater. 2017, 29, 1701463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Gurr, P.A.; Fu, Q.; Webley, P.A.; Qiao, G.G. Two-dimensional nanosheet-based gas separation membranes. J. Mater. Chem. A 2018, 6, 23169–23196. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, K.; Sun, H.; Yin, S. One-Step Synthesis of Single-Layer MnO2Nanosheets with Multi-Role Sodium Dodecyl Sulfate for High-Performance Pseudocapacitors. Small 2015, 11, 2182–2191. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Cui, M.; Cai, G.; Wang, J.; Cui, P.; Gong, X.; Lee, P.S. A High-Performance Lithium-Ion Capacitor Based on 2D Nanosheet Materials. Small 2017, 13. [Google Scholar] [CrossRef]
- Dubal, D.; Lokhande, C. Significant improvement in the electrochemical performances of nano-nest like amorphous MnO2 electrodes due to Fe doping. Ceram. Int. 2013, 39, 415–423. [Google Scholar] [CrossRef]
- Sun, P.; Wang, K.; Zhu, H. Recent developments in graphene-based membranes: Structure, mass-transport mechanism and po-tential applications. Adv. Mater. 2016, 28, 2287–2310. [Google Scholar] [CrossRef] [PubMed]
- Dang, R.; Song, L.; Dong, W.; Li, C.; Zhang, X.; Wang, G.; Chen, X.-B. Synthesis and Self-Assembly of Large-Area Cu Nanosheets and Their Application as an Aqueous Conductive Ink on Flexible Electronics. ACS Appl. Mater. Interfaces 2013, 6, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Song, P.; Ruan, M.; Xu, W. Recent progress in two-dimensional nanomaterials: Synthesis, engineering, and applications. FlatChem 2019, 18. [Google Scholar] [CrossRef]
- Dehghanpour, S.; Mahmoudi, A.; Shadpour, S. Selective synthesis of copper microsheets and ultralong microwires via a surfac-tant assisted hydrothermal process. Russ. J. Gen. Chem. 2015, 85, 1167–1173. [Google Scholar] [CrossRef]
- Hou, C.; Zhu, J.; Liu, C.; Wang, X.; Kuang, Q.; Zheng, L. Formaldehyde-assisted synthesis of ultrathin Rh nanosheets for applica-tions in CO oxidation. CrystEngComm 2013, 15, 6127–6130. [Google Scholar] [CrossRef]
- Duan, H.; Yan, N.; Yu, R.; Chang, C.-R.; Zhou, G.; Hu, H.-S.; Rong, H.; Niu, Z.; Mao, J.; Asakura, H.; et al. Ultrathin rhodium nanosheets. Nat. Commun. 2014, 5, 3093. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Qian, H.; Mei, T.; Guo, J.; White, T. Facile synthesis of magnetic metal (Mn, Co, Fe, and Ni) oxide nanosheets. Mater. Lett. 2010, 64, 1095–1098. [Google Scholar] [CrossRef]
- Dubal, D.; Dhawale, D.; Salunkhe, R.; Jamdade, V.; Lokhande, C. Fabrication of copper oxide multilayer nanosheets for supercapacitor application. J. Alloy. Compd. 2010, 492, 26–30. [Google Scholar] [CrossRef]
- Shaik, A.H.; Chakraborty, J. A simple room temperature fast reduction technique for preparation of a copper nanosheet pow-der. RSC Adv. 2016, 6, 14952–14957. [Google Scholar] [CrossRef]
- Osgood, H.; Devaguptapu, S.V.; Xu, H.; Cho, J.; Wu, G. Transition metal (Fe, Co, Ni, and Mn) oxides for oxygen reduction and evolution bifunctional catalysts in alkaline media. Nano Today 2016, 11, 601–625. [Google Scholar] [CrossRef]
- Yin, A.-X.; Liu, W.-C.; Ke, J.; Zhu, W.; Gu, J.; Zhang, Y.-W.; Yan, C.-H. Ru Nanocrystals with Shape-Dependent Surface-Enhanced Raman Spectra and Catalytic Properties: Controlled Synthesis and DFT Calculations. J. Am. Chem. Soc. 2012, 134, 20479–20489. [Google Scholar] [CrossRef] [PubMed]
- Roger, I.; Symes, M.D. First row transition metal catalysts for solar-driven water oxidation produced by electrodeposition. J. Mater. Chem. A 2016, 4, 6724–6741. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Han, J.; Lee, D.S.; Bae, S.; Lee, S.H.; Lee, S.K.; Moon, B.J.; Choi, C.J.; Wang, G.; Kim, T.W. 2D Single-Crystalline Copper Nano-plates as a Conductive Filler for Electronic Ink Applications. Small 2018, 14, 1703312. [Google Scholar] [CrossRef]
- Liu, X.; Cui, S.; Sun, Z.; Ren, Y.; Zhang, X.; Du, P. Self-supported copper oxide electrocatalyst for water oxidation at low overpoten-tial and confirmation of its robustness by Cu K-edge X-ray absorption spectroscopy. J. Phys. Chem. C 2016, 120, 831–840. [Google Scholar] [CrossRef]
- Wang, X.; Tian, W.; Liao, M.; Bando, Y.; Golberg, D. Recent advances in solution-processed inorganic nanofilm photodetectors. Chem. Soc. Rev. 2014, 43, 1400–1422. [Google Scholar] [CrossRef]
- Fujioka, N.; Morimoto, Y.; Arai, T.; Kikuchi, M. Discrimination between normal and malignant human gastric tissues by Fourier transform infrared spectroscopy. Cancer Detect. Prev. 2004, 28, 32–36. [Google Scholar] [CrossRef]
- Lu, S.; Chen, S.; Zheng, Z.; Zhang, H.; Zhao, C.; Wen, S. Saturable absorption in graphene at 800-nm band. Optoelectron. Devices Integr. IV 2012, 8555, 855512. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Wang, S.; Wern, C.; Yi, S. The Green Synthesis of 2D Copper Nanosheets and Their Light Absorption. Materials 2021, 14, 1926. https://doi.org/10.3390/ma14081926
Lee S, Wang S, Wern C, Yi S. The Green Synthesis of 2D Copper Nanosheets and Their Light Absorption. Materials. 2021; 14(8):1926. https://doi.org/10.3390/ma14081926
Chicago/Turabian StyleLee, Suhyun, Suming Wang, Chien Wern, and Sung Yi. 2021. "The Green Synthesis of 2D Copper Nanosheets and Their Light Absorption" Materials 14, no. 8: 1926. https://doi.org/10.3390/ma14081926





