Facilitated Synthesis of Mg2Ni Based Composites with Attractive Hydrogen Sorption Properties
Abstract
1. Introduction
2. Materials and Methods
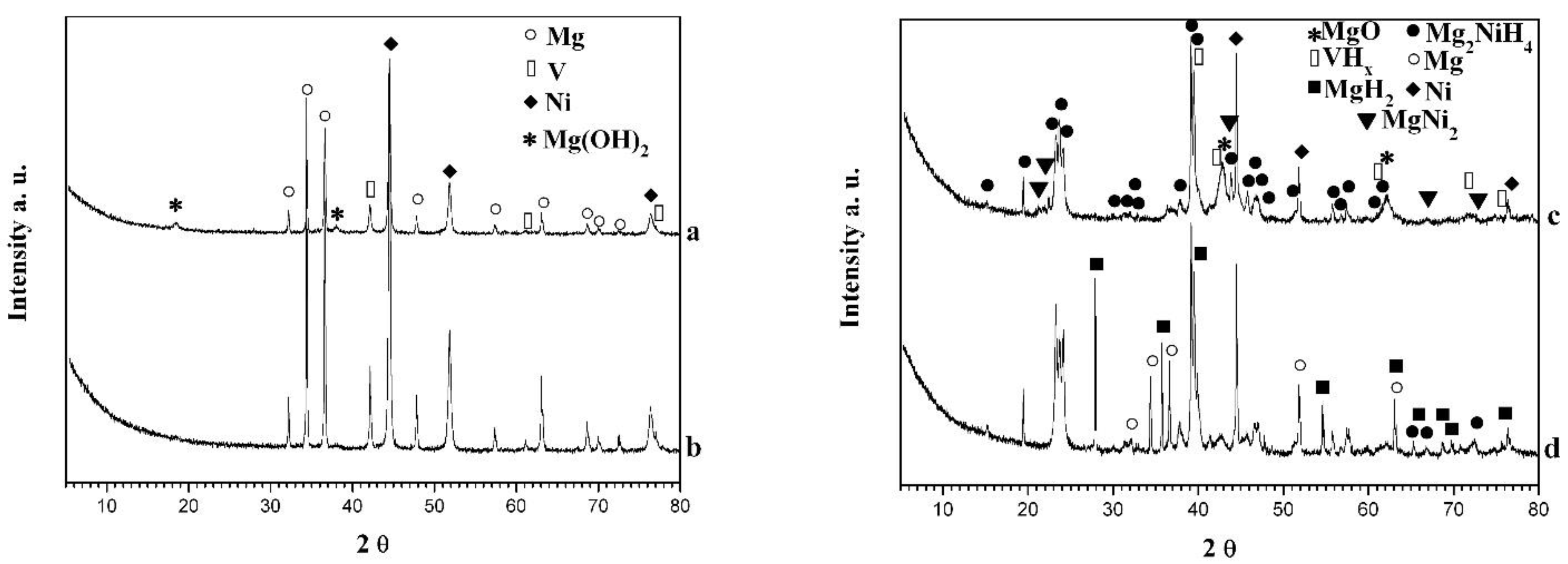
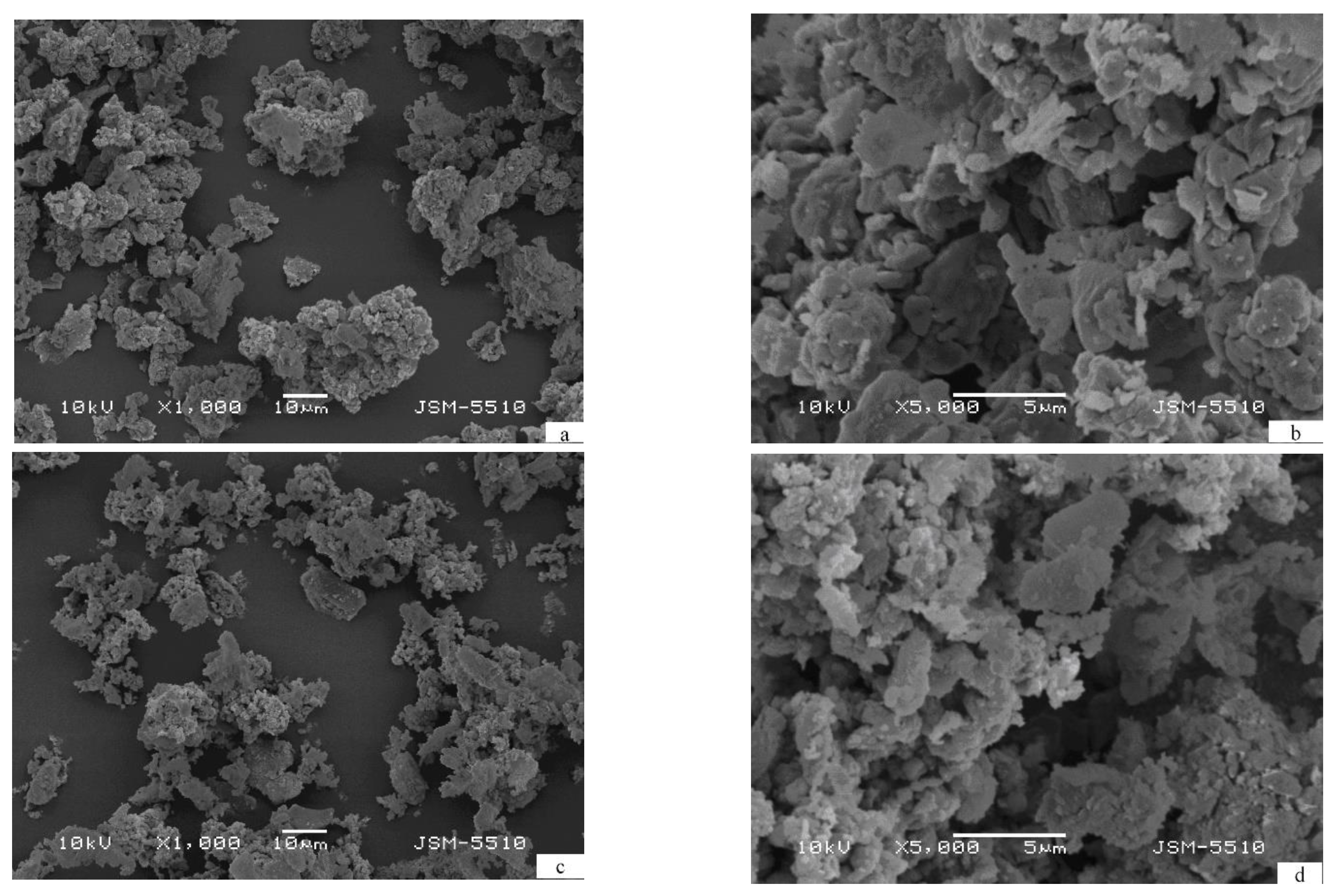
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blomqvist, H.; Rönnebro, E.; Noréus, D.; Kuji, T. Competing stabilisation mechanisms in Mg2NiH4. J. Alloys Compd. 2002, 330–332, 268–270. [Google Scholar] [CrossRef]
- Ronnebro, E.; Noreus, D. Surface sensitivity of Mg2NiH4 leading to a profound color change. Appl. Surf. Sci. 2004, 228, 115–119. [Google Scholar] [CrossRef]
- Ono, S.; Ishido, Y.; Imanari, K.; Tabata, T. Phase transformation and thermal expansion of Mg-Ni alloys in a hydrogen atmosphere. J. Less Common Met. 1982, 88, 57–61. [Google Scholar] [CrossRef]
- Hayakawa, H.; Ishido, Y.; Nomura, K.; Uruno, H.; Ono, S. Phase transformations among three polymorphs of Mg2NiH4. J. Less Common Met. 1983, 103, 277–283. [Google Scholar] [CrossRef]
- Li, L.; Akiyama, T.; Kabutomori, T.; Terao, K.; Yagi, J. In situ X-ray diffraction study of the hydriding combustion synthesis of Mg2NiH4. J. Alloys Compd. 1998, 281, 175–180. [Google Scholar] [CrossRef]
- Li, L.; Akiyama, T.; Yagi, J. Activation behaviors of Mg2NiH4 at different hydrogen pressures in hydriding combustion synthesis. Int. J. Hydrogen Energy 2001, 26, 1035–1040. [Google Scholar] [CrossRef]
- Li, L.; Akiyama, T.; Yagi, J. Reaction mechanism of hydriding combustion synthesis of Mg2NiH4. Intermetallics 1999, 7, 671–677. [Google Scholar] [CrossRef]
- Cermak, J.; David, B. Catalytic effect of Ni, Mg2Ni and Mg2NiH4 upon hydrogen desorption from MgH2. Int. J. Hydrogen Energy 2011, 36, 13614–13629. [Google Scholar] [CrossRef]
- Cermak, J.; Kral, L. Improvement of hydrogen storage characteristics of Mg/Mg2Ni by alloying: Beneficial effect of In. J. Power Sources 2012, 214, 208–215. [Google Scholar] [CrossRef]
- Cermak, J.; Kral, L. Beneficial effect of carbon on hydrogen desorption kinetics from Mg-Ni-In alloy. J. Alloys Compd. 2013, 546, 129–137. [Google Scholar] [CrossRef]
- Orimo, S.; Fujii, H. Hydriding properties of the Mg2Ni-H system synthesized by reactive mechanical grinding. J. Alloys Compd. 1996, 232, L16–L19. [Google Scholar] [CrossRef]
- Orimo, S.; Ikeda, K.; Fujii, H.; Fujikawa, Y.; Kitano, Y.; Yamamoto, K. Structural and hydriding properties of the Mg-Ni-H system with nano- and/or amorphous structures. Acta Mater. 1997, 45, 2271–2278. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T.; Mizera, J. The effect of MgNi2 intermetallic compound on nanostructurization and amorphization of Mg2Ni alloys processed by controlled mechanical milling. J. Alloys Compd. 2003, 354, 289–295. [Google Scholar] [CrossRef]
- Varin, R.A.; Czujko, T. Overview of processing of nanocrystalline hydrogen storage intermetallics by mechanical alloying/milling. Mater. Manuf. Process 2002, 17, 129–156. [Google Scholar] [CrossRef]
- Akiyama, T.; Isogai, H.; Yagi, Y. Hydriding combustion synthesis for the production of hydrogen storage alloy. J. Alloys Compd. 1997, 252, L1–L4. [Google Scholar] [CrossRef]
- Tessier, P.; Enoki, H.; Bououdina, M.; Akiba, E. Ball-milling of Mg2Ni under hydrogen. J. Alloys Compd. 1998, 268, 285–289. [Google Scholar] [CrossRef]
- Gennari, F.C.; Esquivel, M.R. Structural characterization and hydrogen sorption properties of nanocrystalline Mg2Ni. J. Alloys Compd. 2008, 459, 425–432. [Google Scholar] [CrossRef]
- Polanski, M.; Nielsen, T.K.; Kunce, I.; Norek, M.; Płociński, T.; Jaroszewicz, L.R.; Gundlach, C.; Jensen, T.R.; Bystrzycki, J. Mg2NiH4 synthesis and decomposition reactions. Int. J. Hydrogen Energy 2013, 38, 4003–4010. [Google Scholar] [CrossRef]
- Martínez-Coronado, R.; Retuerto, M.; Torres, B.; Martínez-Lope, M.J.; Fernández-Díaz, M.T.; Alonso, J.A. High-pressure synthesis, crystal structure and cyclability of the Mg2NiH4 hydride. Int. J. Hydrogen Energy 2013, 38, 5738–5745. [Google Scholar] [CrossRef]
- Hou, X.; Hu, R.; Zhang, T.; Kou, H.; Song, W.; Li, J. Hydrogen desorption performance of high-energy ball milled Mg2NiH4 catalyzed by multi-walled carbon nanotubes coupling with TiF3. Int. J. Hydrogen Energy 2014, 39, 19672–19681. [Google Scholar] [CrossRef]
- Baran, A.; Polański, M. Magnesium-Based Materials for Hydrogen Storage—A Scope Review. Materials 2020, 13, 3993. [Google Scholar] [CrossRef]
- Kohno, T.; Tsuruta, S.; Kanda, M. The hydrogen storage properties of new Mg2Ni alloy. J. Electrochem. Soc. 1996, 143, L198–L199. [Google Scholar] [CrossRef]
- Cui, N.; Luo, J.L. Electrochemical study of hydrogen diffusion behavior in Mg2Ni-type hydrogen storage alloy electrodes. Int. J. Hydrogen Energy 1999, 24, 37–42. [Google Scholar] [CrossRef]
- Cui, N.; Luo, J.L.; Chuang, K.T. Nickel–metal hydride (Ni–MH) battery using MgNi-type hydrogen storage alloy. J. Alloys Compd. 2000, 302, 218–226. [Google Scholar] [CrossRef]
- Takatoshi, T.; Issei, Y.; Qiwu, Z.; Fumio, S. Discharge properties of Mg2Ni-Ni alloy synthesized by mechanical alloying. Adv. Powder Technol. 2005, 16, 649–658. [Google Scholar]
- Pedneault, S.; Huot, J.; Roué, L. Nanostructured Mg2Ni materials prepared by cold rolling and used as negative electrode for Ni-MH batteries. J. Power Sources 2008, 185, 566–569. [Google Scholar] [CrossRef]
- Zaїdi, W.; Bonnet, J.-P.; Zhang, J.; Cuevas, F.; Latroche, M.; Couillaud, S.; Bobet, J.-L.; Sougrati, M.T.; Jumas, J.-C.; Aymard, L. Reactivity of complex hydrides Mg2FeH6, Mg2CoH5 and Mg2NiH4 with lithium ion: Far from equilibrium electrochemically driven conversion reactions. Int. J. Hydrogen Energy 2013, 38, 4798–4800. [Google Scholar] [CrossRef]
- Huaiyu, S.; Xingguo, L. Effect of nanostructure and partial substitution on gas absorption and electrochemical properties in Mg2Ni-based alloys. J. Alloys Compd. 2016, 667, 191–197. [Google Scholar]
- Qi, Y.; Li, X.; Yuan, Z.; Cai, Y.; Guo, S.; Zhang, Y. Structure and hydrogen storage performances of La–Mg–Ni–Cu alloys prepared by melt spinning. Int. J. Hydrogen Energy 2019, 44, 5399–5407. [Google Scholar] [CrossRef]
- Gergova, K.; Petrov, N.; Eser, S. Adsorption properties and microstructure of activated carbons produced from agricultural by- products from steam pyrolysis. Carbon 1994, 32, 693–702. [Google Scholar] [CrossRef]
- Bobet, J.-L.; Grigorova, E.; Khrussanova, M.; Khristov, M.; Peshev, P. Hydrogen sorption properties of the nanocomposite 90 wt% Mg2Ni-10 wt% V. J. Alloys Compd. 2003, 356–357, 593–597. [Google Scholar] [CrossRef]
- Grigorova, E.; Khristov, M.; Khrussanova, M.; Bobet, J.-L.; Peshev, P. Effect of additives on the hydrogen sorption properties of mechanically alloyed composites based on Mg and Mg2Ni. Int. J. Hydrogen Energy 2005, 30, 1099–1105. [Google Scholar] [CrossRef]
- Grigorova, E.; Nihtianova, D.; Tsyntsarski, B.; Stoycheva, I. Investigation of hydrogen storage characteristics of MgH2 based materials with addition of Ni and activated carbon. Inorg. Open Access 2020, 8, 12. [Google Scholar] [CrossRef]
- Bliznakov, S.; Drenchev, N.; Drenchev, B.; Delchev, P.; Solson, P.; Spassov, T. Electrochemical properties of nanocrystalline Mg2Ni-type alloys prepared by mechanical alloying. J. Alloys Compd. 2005, 404–406, 682–686. [Google Scholar] [CrossRef]
- Zlatanova, Z.; Spassov, T.; Eggeler, G.; Spassova, M. Synthesis and hydriding/dehydriding properties of Mg2Ni-AB (AB = TiNi or TiFe) nanocomposites. Int. J. Hydrogen Energy 2011, 36, 7559–7566. [Google Scholar] [CrossRef]
- Tanguy, B.; Soubeyroux, J.L.; Pezat, M.; Portier, J.; Hagemuller, P. Amélioration des conditions de synthèse de l’hydrure de magnésium à l’aide de l’adjuvants. Mater. Res. Bull. 1976, 11, 1441–1447. [Google Scholar] [CrossRef]
- Eva Software Bruker. Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/x-ray-diffraction/xrd-software/eva.html.
- Kumar, S.; Jain, A.; Ichikawa, T.; Kojima, Y.; Dey, G.K. Development of vanadium based hydrogen storage material: A review. Renew. Sustain. Energy Rev. 2017, 72, 791–800. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Hydrogen storage properties of the mechanically milled MgH2-V nanocomposite. J. Alloys Compd. 1999, 291, 295–299. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigorova, E.; Tzvetkov, P.; Todorova, S.; Markov, P.; Spassov, T. Facilitated Synthesis of Mg2Ni Based Composites with Attractive Hydrogen Sorption Properties. Materials 2021, 14, 1936. https://doi.org/10.3390/ma14081936
Grigorova E, Tzvetkov P, Todorova S, Markov P, Spassov T. Facilitated Synthesis of Mg2Ni Based Composites with Attractive Hydrogen Sorption Properties. Materials. 2021; 14(8):1936. https://doi.org/10.3390/ma14081936
Chicago/Turabian StyleGrigorova, Eli, Petar Tzvetkov, Stanislava Todorova, Pavel Markov, and Tony Spassov. 2021. "Facilitated Synthesis of Mg2Ni Based Composites with Attractive Hydrogen Sorption Properties" Materials 14, no. 8: 1936. https://doi.org/10.3390/ma14081936
APA StyleGrigorova, E., Tzvetkov, P., Todorova, S., Markov, P., & Spassov, T. (2021). Facilitated Synthesis of Mg2Ni Based Composites with Attractive Hydrogen Sorption Properties. Materials, 14(8), 1936. https://doi.org/10.3390/ma14081936






