Heterostructural Mixed Oxides Prepared via ZnAlLa LDH or ex-ZnAl LDH Precursors—Effect of La Content and Its Incorporation Route
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of La-Doped Zn–Al Materials
2.2. Material Characterization
3. Results and Discussion
3.1. Physicochemical Parameters of ZnAl_W_xLa and ZnAl_I_xLa Materials
3.2. Structural and Textural Properties of ZnAl_W_xLa and ZnAl_I_xLa Materials
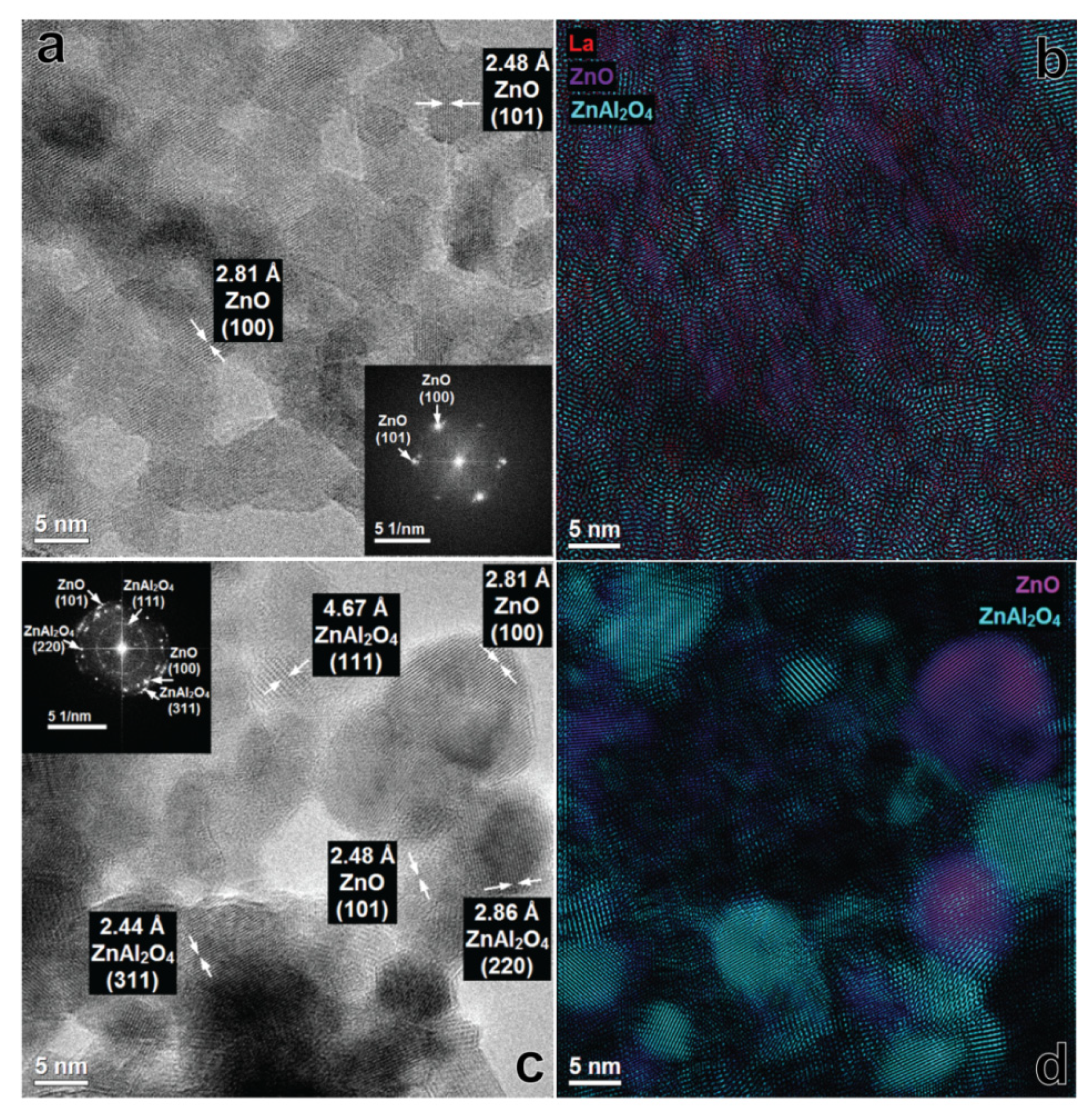
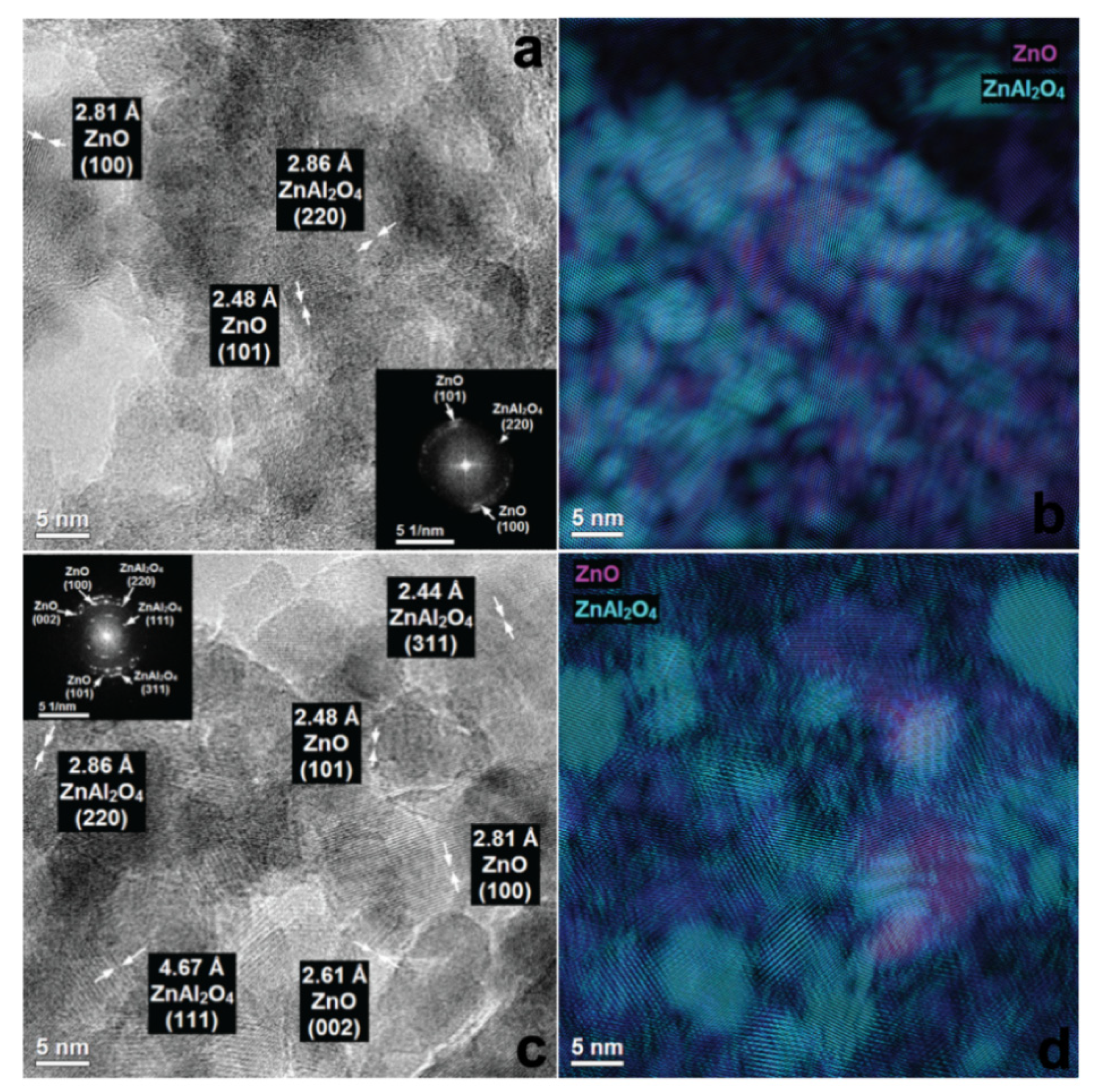
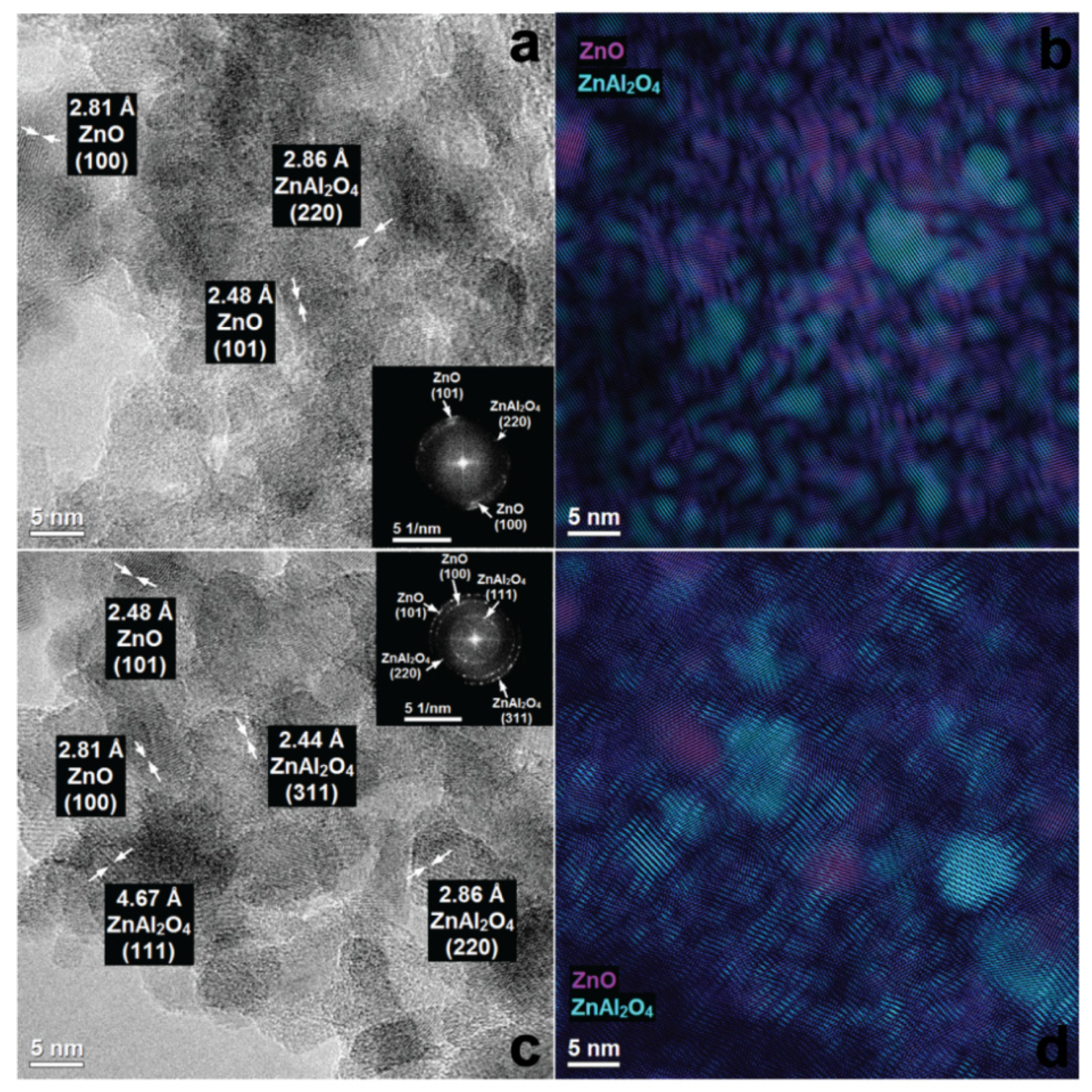
3.3. Suface Morphology of Mixed Oxides Obtained from ZnAlLa LDHs and ex-ZnAl LDHs
3.4. Evaluation of ZnO/Zn(AlLa)2O4 and La–ZnO/ZnAl2O4 Mixed Oxides in the High-Temperature Water–Gas Shift Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ertl, G.; Knözinger, H.; Weitkam, J. Preparation of Solid Catalysts; John Wiley & Sons: Weinheim, NY, USA, 2012. [Google Scholar]
- Xu, M.; Wie, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Meng, Y.; Pan, G.; Ni, Z.; Xia, S. Layered Double Hydroxides-based Photocatalysts and Visible-light-driven Photodegradation of Organic Pollutants: A Review. Chem. Eng. J. 2020, 392, 123684. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Hu, L.; Huang, S.; Jin, Z.; Zhang, M.; Huang, X.; Lu, J.; Ruana, S.; Zeng, Y.J. Multifunctional Zn–Al layered double hydroxides for surface-enhanced Raman scattering and surface-enhanced infrared absorption. Dalton Trans. 2019, 48, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, S.M.; Carrado, K.A.; Dutta, P.K. Handbook of Layered Materials; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Elhalil, A.; Elmoubarki, R.; Farnane, M.; Machrouhi, A.; Mahjoubi, F.Z.; Sadiq, M.; Qourzal, S.; Abdennouri, M.; Barka, N. Novel Ag-ZnO-La2O2CO3 photocatalysts derived from the Layered Double Hydroxide structure with excellent photocatalytic performance for the degradation of pharmaceutical compounds. J. Sci. Adv. Mater. Devices 2019, 4, 34–46. [Google Scholar] [CrossRef]
- Carriazo, D.; Domingo, C.; Martin, C.; Rives, V. Structural and Texture Evolution with Temperature of Layered Double Hydroxides Intercalated with Paramolybdate Anions. Inorg. Chem. 2006, 45, 1243. [Google Scholar] [CrossRef] [PubMed]
- Mei-Yu, G.; Dong-Mei, X.; Yu-Fei, S.; Guo, Y. ZnO/ZnAl2O4 prepared by calcination of ZnAl layered double hydroxides for ethanol sensing. Sens. Actuators B 2013, 188, 1148. [Google Scholar]
- Sun, Y.; Zhou, Y.; Wang, Z.; Ye, X. Structural and morphological transformations of Zn–Al layered double hydroxides through hydrothermal treatment. Appl. Surf. Sci. 2009, 255, 6372–6377. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Ladavos, A.K.; Armatas, G.S.; Petrakis, D.E.; Pomonis, P.J. Effect of composition on the conductivity of CTAB–butanol–octane–nitrate salts (Al(NO3)3 + Zn(NO3)2) microemulsions and on the surface and textural properties of resulting spinels ZnAl2O4. Appl. Surf. Sci. 2006, 252, 2159–2170. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, G.; Bian, T.; Zhou, C.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; Smith, L.J.; O’Hare, D.; Zhang, T. Defect-Rich Ultrathin ZnAl-Layered Double Hydroxide Nanosheets for Efficient Photoreduction of CO2 to CO with Water. Adv. Mater. 2015, 27, 7824–7831. [Google Scholar] [CrossRef] [PubMed]
- Valle, B.; Aramburu, B.; Remiro, A.; Bilbao, J.; Gayubo, A.G. Effect of calcination/reduction conditions of Ni/La2O3–Al2O3 catalyst on its activity and stability for hydrogen production by steam reforming of raw bio-oil/ethanol. Appl. Catal. B Environ. 2014, 147, 402–410. [Google Scholar] [CrossRef]
- Bicki, R.; Antoniak-Jurak, K.; Michalska, K.; Franczyk, E.; Konkol, M.; Kowalik, P.; Pańczyk, M.; Ryczkowski, J.; Słowik, G.; Borowiecki, T. The effect of La2O3 and CeO2 modifiers on properties of Ni-Al catalysts for LNG prereforming. Int. J. Hydrogen Energy 2021, 46, 11664–11676. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Wang, C.; Qu, W.; Tian, Z.; Ma, H.; Wang, D.; Wang, B.; Xu, Z. The effect of lanthanum doping on activity of Zn-Al spinel for transesterification. Appl. Catal. B Environ. 2013, 136–137, 210–217. [Google Scholar] [CrossRef]
- Xie, X.; An, X.; Wang, X.; Wang, Z. Preparation, Characterization and Application of ZnAlLa-Hydrotalcite-like Compounds. J. Nat. Gas Chem. 2003, 12, 259–263. [Google Scholar]
- Tzompantzi, F.; Mendoza-Damian, G.; Rici, J.L.; Mantilla, A. Enhanced photoactivity for phenol mineralization on ZnAlLa mixed oxides prepared from calcined LDHs. Catal. Today 2014, 220–222, 56–60. [Google Scholar] [CrossRef]
- Tzompantzi, F.; Carrera, Y.; Morales-Mendoza, G.; Valverde-Aguilar, G.; Mantill, A. ZnO–Al2O3–La2O3 layered double hydroxides as catalysts precursors for the esterification of oleic acid fatty grass at low temperature. Catal. Today 2013, 212, 164–168. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Bagherpour Sardsahra, F. Synthesis and characterization of novel spinel Zn1.114La1.264Al0.5O4.271 nanoparticles. J. Alloys Compd. 2016, 686, 384–393. [Google Scholar] [CrossRef]
- Khani, F.Y.; Bahadoran, S.; Soltanali, J.; Ahari, S. Hydrogen production by methanol steam reforming on a cordierite monolith reactor coated with Cu–Ni/LaZnAlO4 and Cu–Ni/γ-Al2O3 catalysts. Res. Chem. Intermed. 2018, 44, 925–942. [Google Scholar] [CrossRef]
- Khani, F.Y.; Bahadoran, N.; Safari, S.; Soltanali, S.; Taheri, A. Hydrogen production from steam reforming of methanol over Cu-based catalysts: The behavior of ZnxLaxAl1-xO4 and ZnO/La2O3/Al2O3 lined on cordierite monolith reactors. Int. J. Hydrogen Energy 2019, 44, 11824–11837. [Google Scholar] [CrossRef]
- Antoniak-Jurak, K.; Kowalik, P.; Michalska, K.; Próchniak, W.; Bicki, R. Zn-Al Mixed Oxides Decorated with Potassium as Catalysts for HT-WGS: Preparation and Properties. Catalysts 2020, 10, 1094. [Google Scholar] [CrossRef]
- Antoniak-Jurak, K.; Kowalik, P.; Michalska, K.; Franczyk, E.; Mrozek, A. WGS reaction empirical kinetics over novel potassium promoted ZnAlLa mixed oxides catalyst. Chem. Eng. Res. Des. 2020, 164, 293–298. [Google Scholar] [CrossRef]
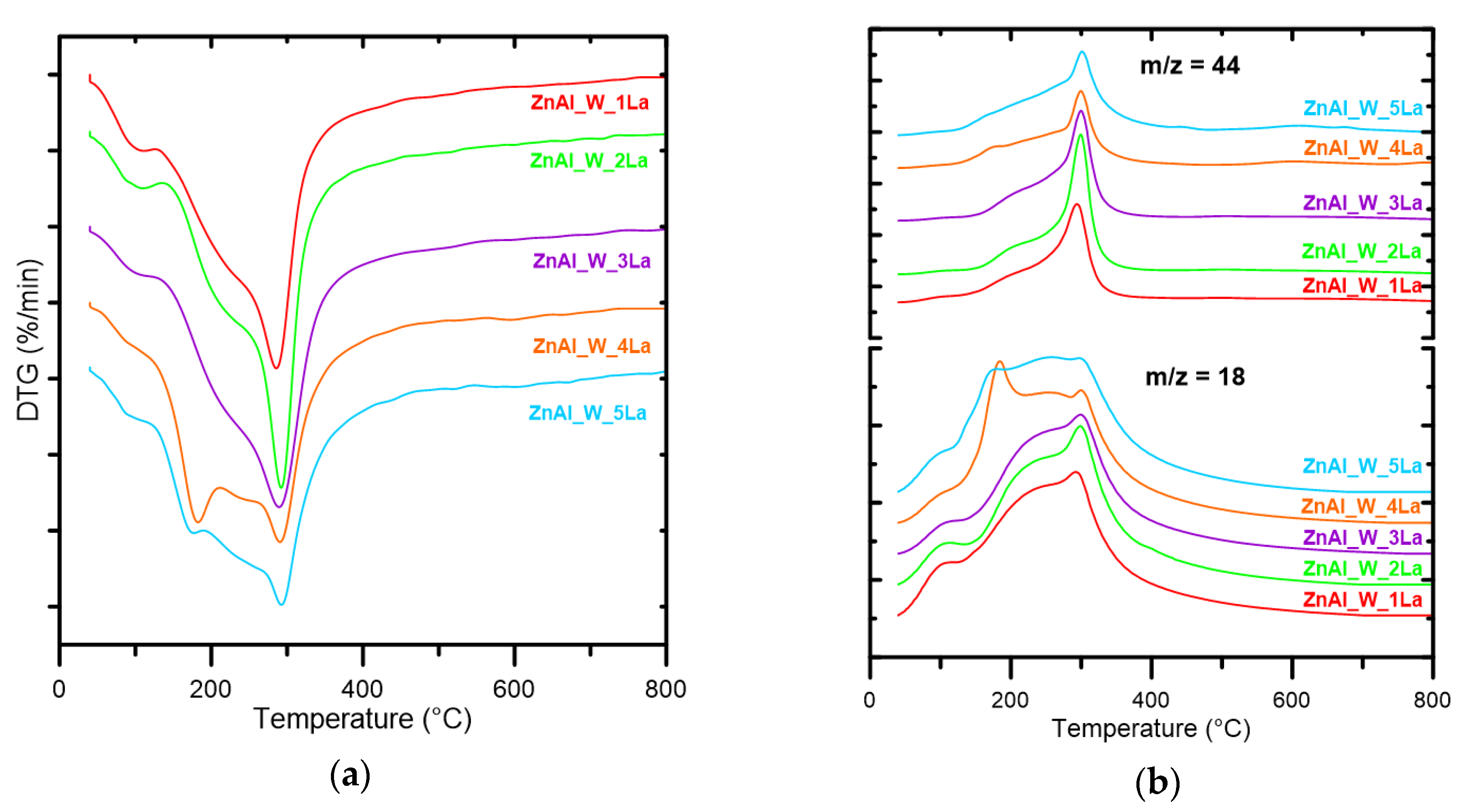

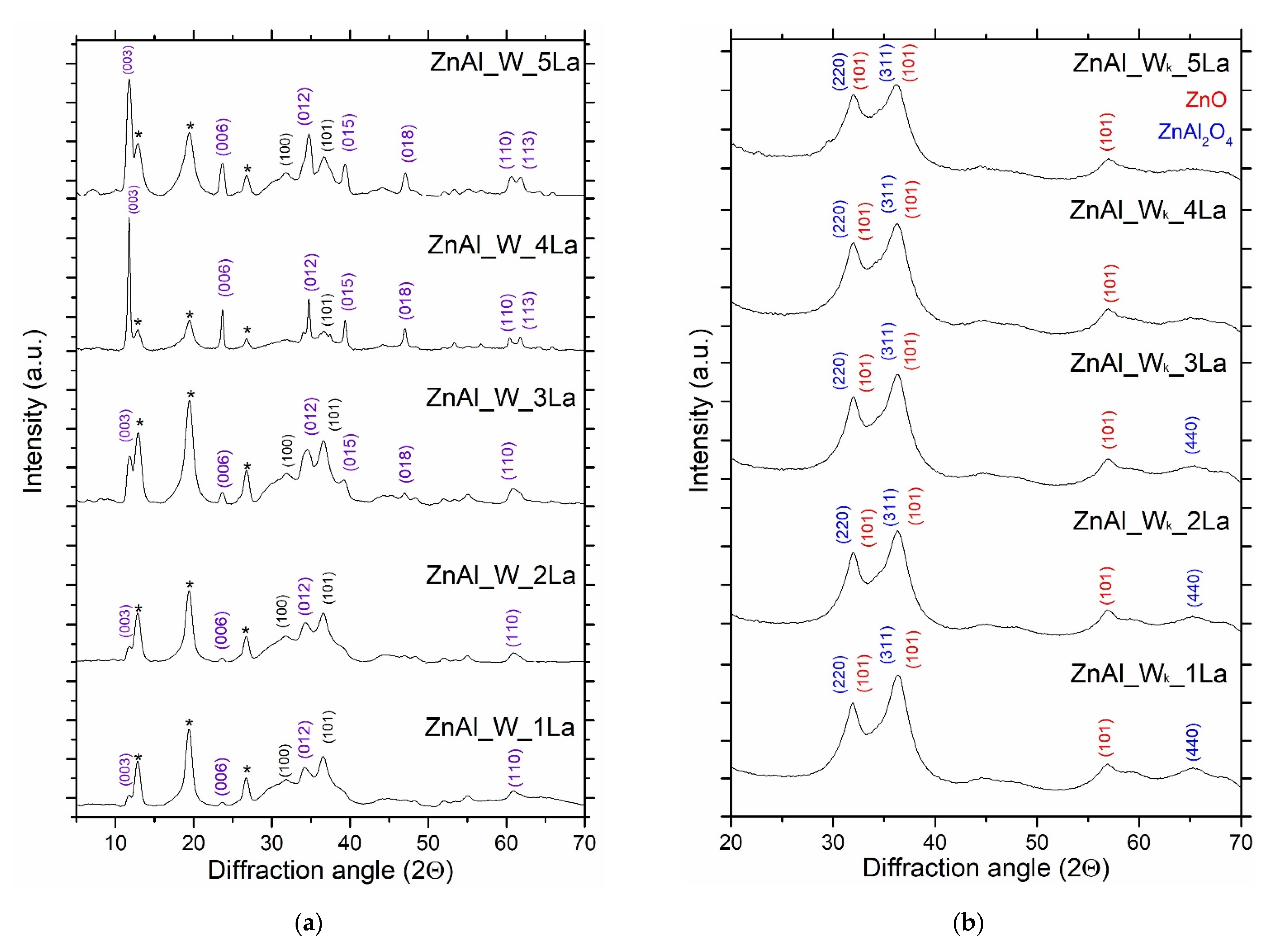
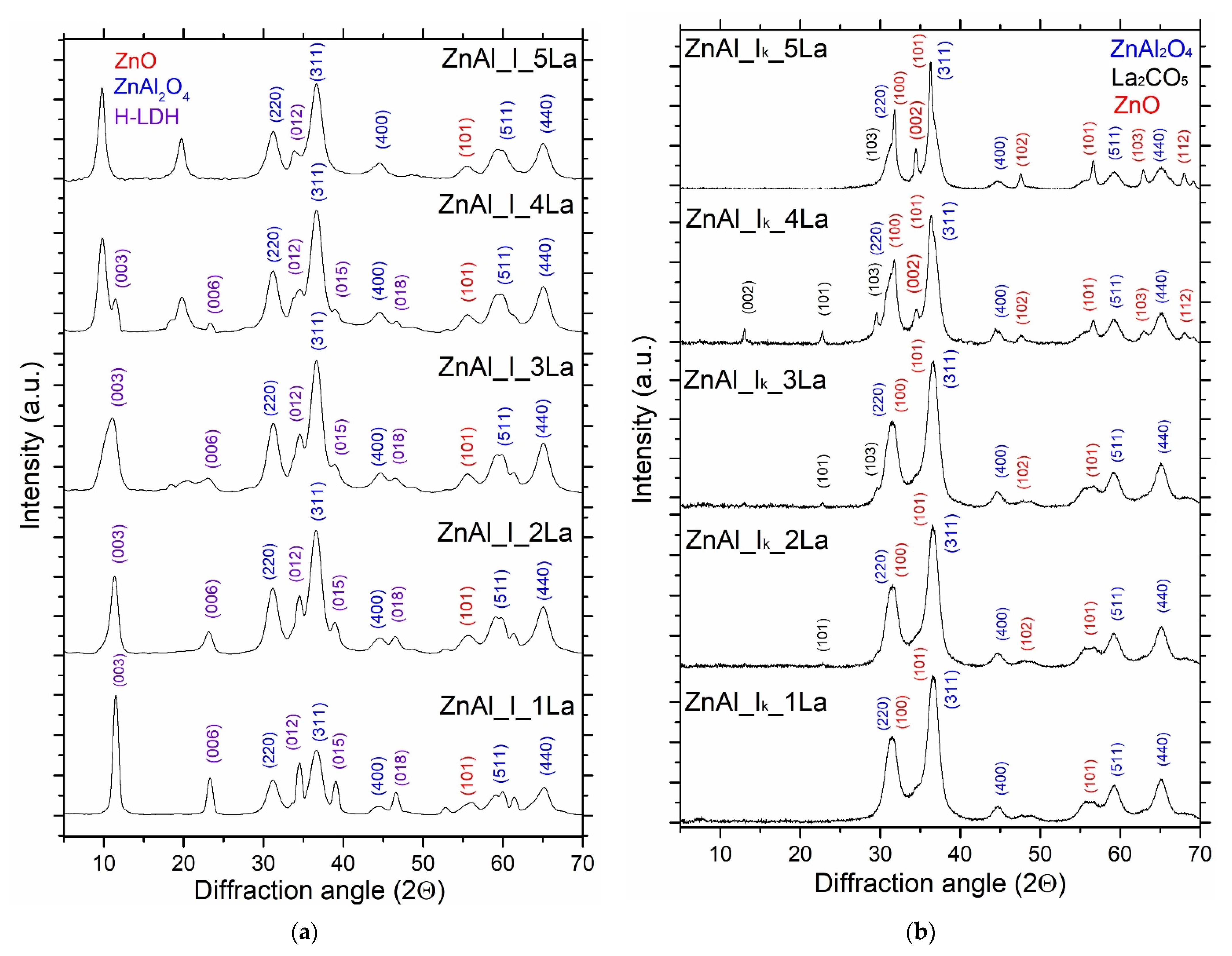
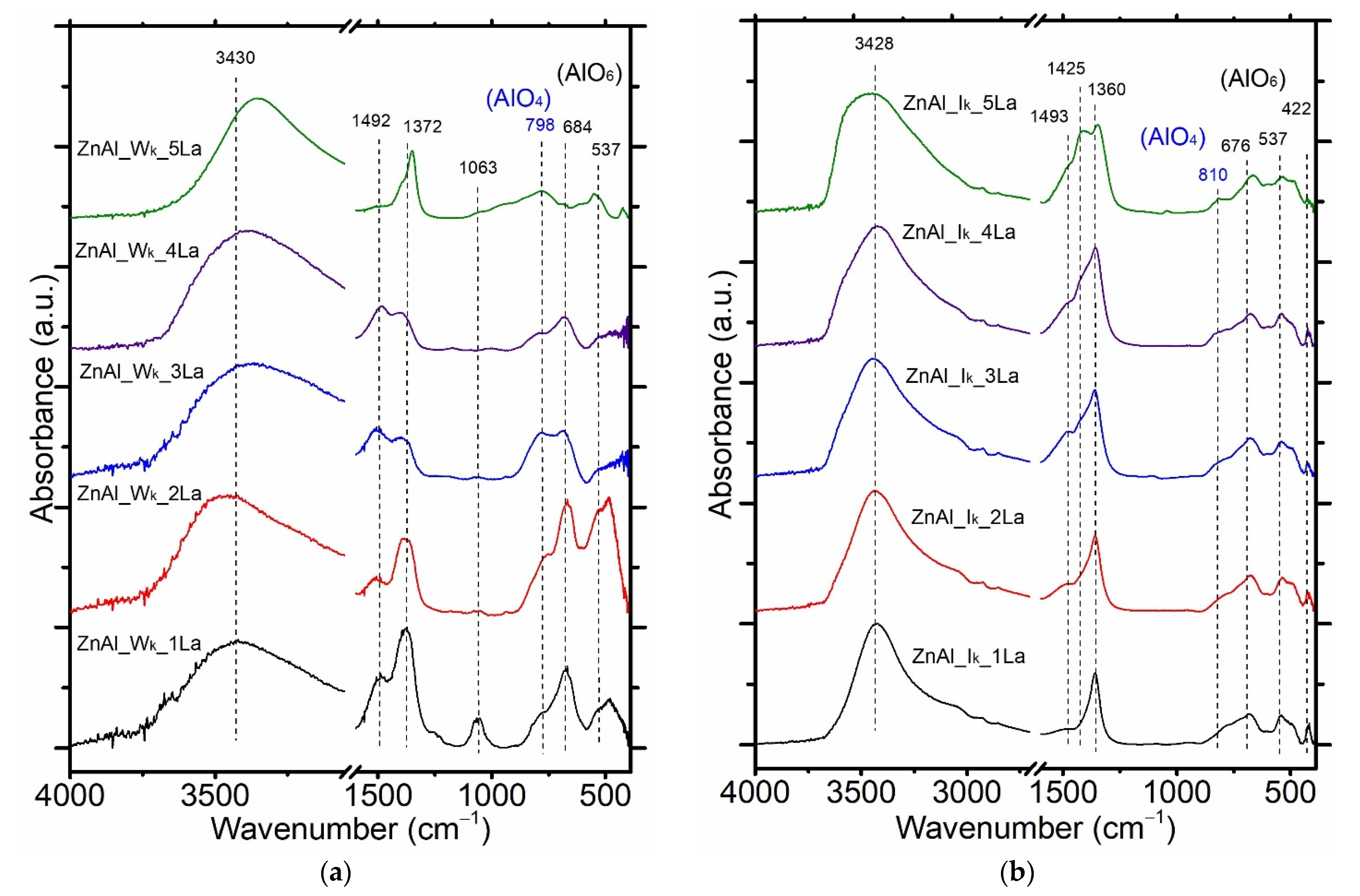
| Sample | TG (%) | Tmax Decomposition Rate (°C) | Sample | TG (%) | Tmax Decomposition Rate (°C) |
|---|---|---|---|---|---|
| ZnAl_W_1La | 24.5 | 289 | ZnAl_Ik_1La | 12.7 | 164 |
| ZnAl_W_2La | 24.7 | 284 | ZnAl_Ik_2La | 13.6 | 153 |
| ZnAl_W_3La | 24.6 | 291 | ZnAl_Ik_3La | 13.7 | 199 |
| ZnAl_W_4La | 25.4 | 284 | ZnAl_Ik_4La | 14.9 | 206 |
| ZnAl_W_5La | 25.5 | 284 | ZnAl_Ik_5La | 16.9 | 240 |
| Sample | wt% La a | (Zn/Al)mol a | SBET (m2/g) | TPV b (cm3/g) | MPVBJH c (cm3/g) |
|---|---|---|---|---|---|
| ZnAl_Wk_1La | 0.9 | 0.68 | 158 | 0.34 | 0.34 |
| ZnAl_Wk_2La | 1.7 | 0.67 | 162 | 0.3 | 0.3 |
| ZnAl_Wk_3La | 2.4 | 0.7 | 160 | 0.29 | 0.29 |
| ZnAl_Wk_4La | 4.3 | 0.66 | 162 | 0.3 | 0.3 |
| ZnAl_Wk_5La | 8.5 | 0.66 | 154 | 0.32 | 0.32 |
| ZnAl_Ik_1La | 0.9 | 0.7 | 100 | 0.25 | 0.25 |
| ZnAl_Ik_2La | 1.9 | 0.68 | 98 | 0.27 | 0.27 |
| ZnAl_Ik_3La | 2.8 | 0.67 | 90 | 0.35 | 0.35 |
| ZnAl_Ik_4La | 4.6 | 0.69 | 89 | 0.27 | 0.27 |
| ZnAl_Ik_5La | 8.7 | 0.68 | 76 | 0.22 | 0.22 |
| Samples | Average HT-WGS Rate Constants k (Ndm3∙gcat−1∙h−1∙at−0.95) | |||
|---|---|---|---|---|
| 350 °C | 370 °C | 400 °C | 420 °C | |
| ZnAl_Wk_1La | 1.5 | 4.8 | 7.9 | 11.3 |
| ZnAl_Wk_2La | 1.9 | 3.1 | 8 | 11.9 |
| ZnAl_Wk_3La | 1.9 | 4.6 | 9.9 | 12 |
| ZnAl_Wk_4La | 2.2 | 4.8 | 10.5 | 12.6 |
| ZnAl_Wk_5La | 2.5 | 5 | 10.3 | 13.3 |
| ZnAl_Ik_1La | 2.8 | 5.2 | 9.9 | 12.5 |
| ZnAl_Ik_2La | 2.9 | 5.7 | 11.5 | 14.1 |
| ZnAl_Ik_3La | 2.3 | 4.9 | 11 | 13.8 |
| ZnAl_Ik_4La | 2.4 | 4.4 | 7.8 | 8.3 |
| ZnAl_Ik_5La | 2.3 | 3.5 | 5.6 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniak-Jurak, K.; Kowalik, P.; Próchniak, W.; Bicki, R.; Słowik, G. Heterostructural Mixed Oxides Prepared via ZnAlLa LDH or ex-ZnAl LDH Precursors—Effect of La Content and Its Incorporation Route. Materials 2021, 14, 2082. https://doi.org/10.3390/ma14082082
Antoniak-Jurak K, Kowalik P, Próchniak W, Bicki R, Słowik G. Heterostructural Mixed Oxides Prepared via ZnAlLa LDH or ex-ZnAl LDH Precursors—Effect of La Content and Its Incorporation Route. Materials. 2021; 14(8):2082. https://doi.org/10.3390/ma14082082
Chicago/Turabian StyleAntoniak-Jurak, Katarzyna, Paweł Kowalik, Wiesław Próchniak, Robert Bicki, and Grzegorz Słowik. 2021. "Heterostructural Mixed Oxides Prepared via ZnAlLa LDH or ex-ZnAl LDH Precursors—Effect of La Content and Its Incorporation Route" Materials 14, no. 8: 2082. https://doi.org/10.3390/ma14082082
APA StyleAntoniak-Jurak, K., Kowalik, P., Próchniak, W., Bicki, R., & Słowik, G. (2021). Heterostructural Mixed Oxides Prepared via ZnAlLa LDH or ex-ZnAl LDH Precursors—Effect of La Content and Its Incorporation Route. Materials, 14(8), 2082. https://doi.org/10.3390/ma14082082







